Abstract
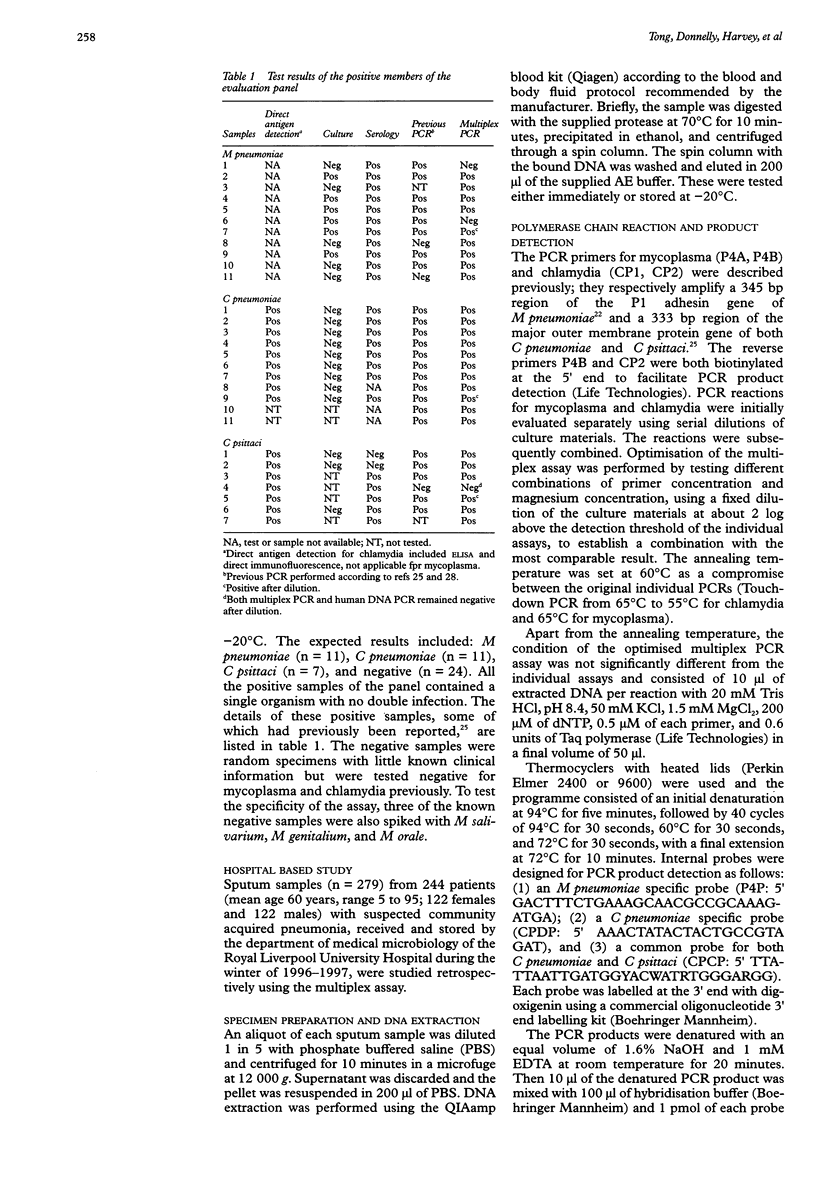
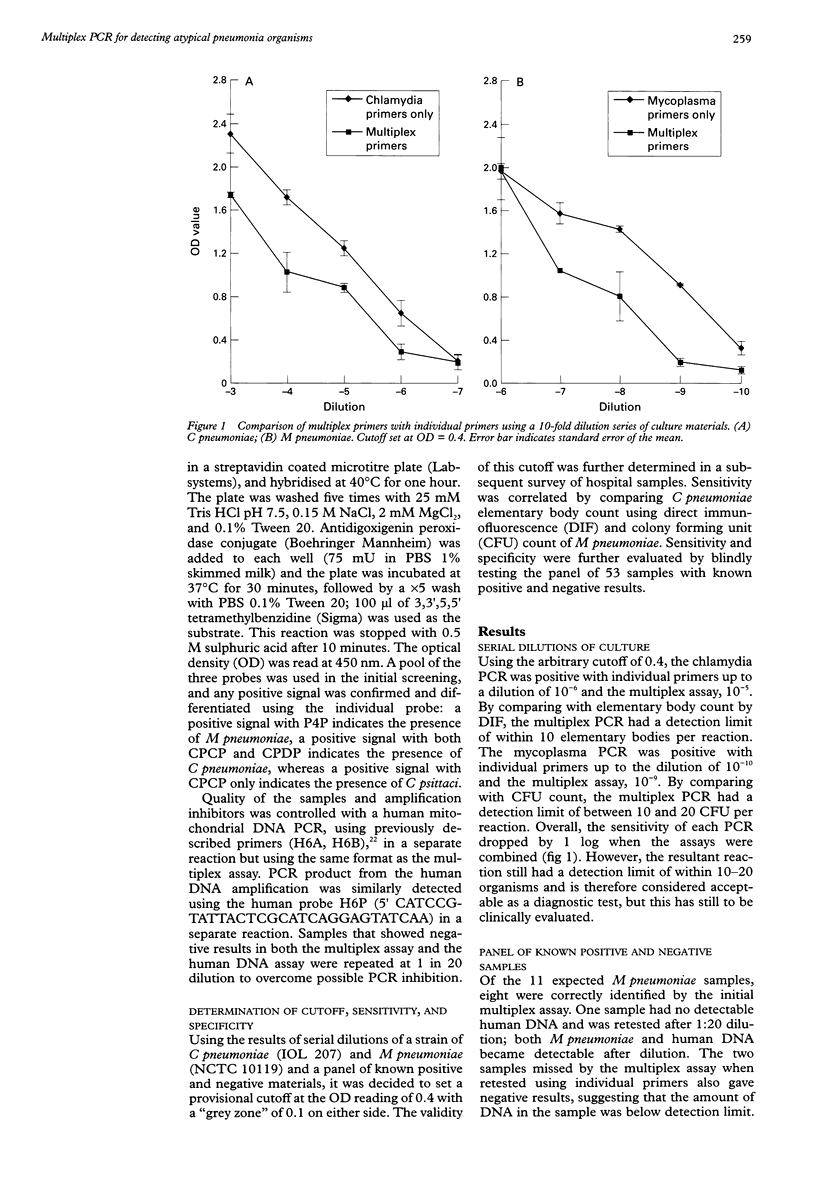
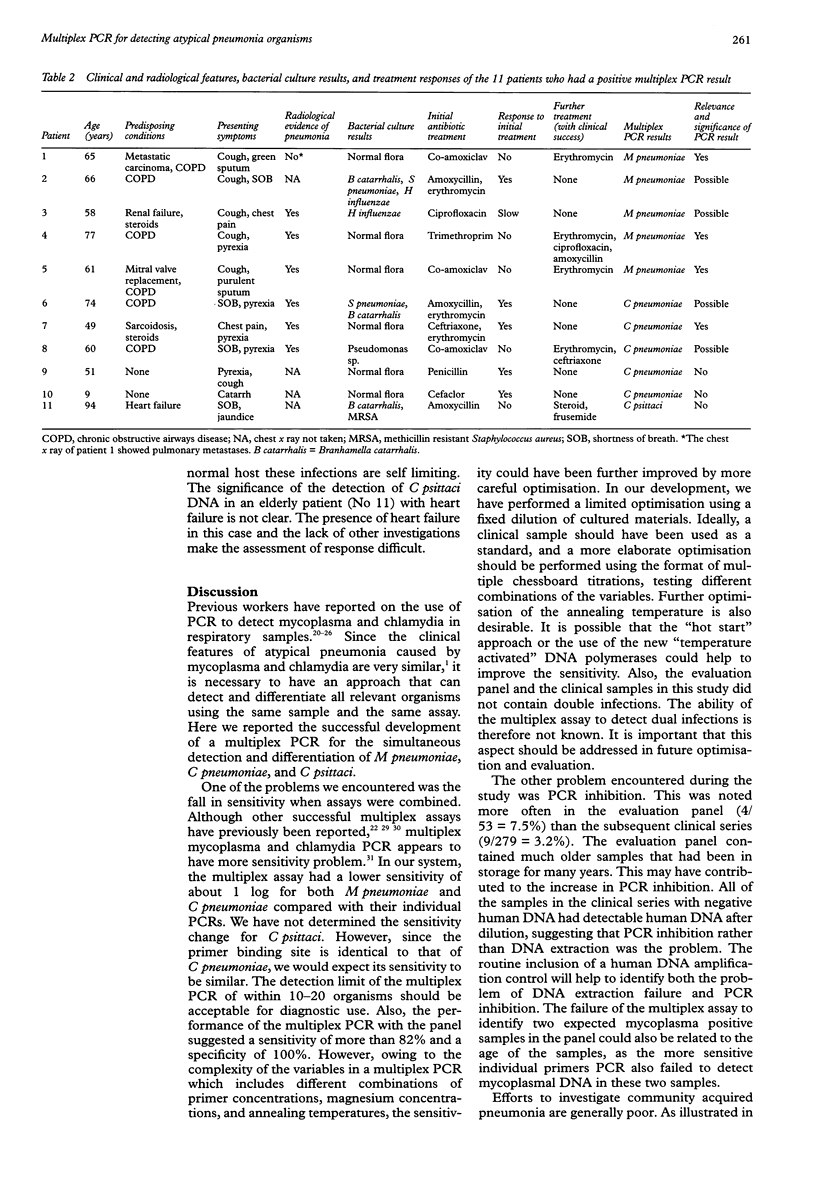
AIMS: To develop a multiplex polymerase chain reaction (PCR) for the simultaneous detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Chlamydia psittaci in respiratory samples. METHODS: Oligonucleotide primers for the amplification of the DNA of these three organisms were optimised for use in combination in the same reaction. PCR products were detected by hybridisation with pooled internal probes using an enzyme linked immunosorbent assay. Those with positive signals were further differentiated using species specific probes. Quality of DNA extraction and PCR inhibition were controlled by amplification of a human mitochondrial gene. A panel of 53 respiratory samples with known results was evaluated blindly. This was followed by a retrospective study on sputa collected from 244 patients with suspected community acquired pneumonia. RESULTS: The multiplex assay had a lower sensitivity than PCR with individual primers by about one log. The resultant sensitivity was considered acceptable for diagnostic use. Of the panel of 53 samples, nine of 11 M pneumoniae, 11 of 11 C pneumoniae, six of seven C psittaci, and 24 of 24 negative samples were correctly identified. Of the 244 patients with pneumonia, seven (2.9%) had detectable M pneumoniae, six (2.5%) had C pneumoniae, and one (0.4%) had C psittaci. The case notes from 11 patients were studied. The PCR finding was of possible significance in at least eight of these patients. CONCLUSIONS: This multiplex PCR assay has the potential to be used as a diagnostic and epidemiological tool. Further prospective studies are needed to establish its clinical value.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. E., Major R., Palmer S. R. Ornithosis in poultry workers. Lancet. 1981 Mar 21;1(8221):632–634. doi: 10.1016/s0140-6736(81)91552-x. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Mundy L. M. Community-acquired pneumonia. N Engl J Med. 1995 Dec 14;333(24):1618–1624. doi: 10.1056/NEJM199512143332408. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Dallo S. F., Tully J. G., Rose D. L. Isolation and characterization of Mycoplasma genitalium strains from the human respiratory tract. J Clin Microbiol. 1988 Nov;26(11):2266–2269. doi: 10.1128/jcm.26.11.2266-2269.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassiri M., Mårdh P. A., Domeika M. Multiplex AMPLICOR PCR screening for Chlamydia trachomatis and Neisseria gonorrhoeae in women attenting non-sexually transmitted disease clinics. The European Chlamydia Epidemiology Group. J Clin Microbiol. 1997 Oct;35(10):2556–2560. doi: 10.1128/jcm.35.10.2556-2560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman J., Söderberg S., Forsberg J., Birgander L. S., Allard A., Persson K., Jidell E., Kumlin U., Juto P., Waldenström A. High prevalence of Chlamydia pneumoniae DNA in peripheral blood mononuclear cells in patients with cardiovascular disease and in middle-aged blood donors. J Infect Dis. 1998 Jul;178(1):274–277. doi: 10.1086/517452. [DOI] [PubMed] [Google Scholar]
- Bourke S. J. Chlamydial respiratory infections. BMJ. 1993 May 8;306(6887):1219–1220. doi: 10.1136/bmj.306.6887.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadieux N., Lebel P., Brousseau R. Use of a triplex polymerase chain reaction for the detection and differentiation of Mycoplasma pneumoniae and Mycoplasma genitalium in the presence of human DNA. J Gen Microbiol. 1993 Oct;139(10):2431–2437. doi: 10.1099/00221287-139-10-2431. [DOI] [PubMed] [Google Scholar]
- Campbell L. A., Perez Melgosa M., Hamilton D. J., Kuo C. C., Grayston J. T. Detection of Chlamydia pneumoniae by polymerase chain reaction. J Clin Microbiol. 1992 Feb;30(2):434–439. doi: 10.1128/jcm.30.2.434-439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsey T., Darougar S., Treharne J. D. Prevalence in human beings of antibodies to Chlamydia IOL-207, an atypical strain of chlamydia. J Infect. 1986 Mar;12(2):145–152. doi: 10.1016/s0163-4453(86)93608-x. [DOI] [PubMed] [Google Scholar]
- Foy H. M., Grayston J. T., Kenny G. E., Alexander E. R., McMahan R. Epidemiology of Mycoplasma pneumoniae infection in families. JAMA. 1966 Sep 12;197(11):859–866. [PubMed] [Google Scholar]
- Gaydos C. A., Eiden J. J., Oldach D., Mundy L. M., Auwaerter P., Warner M. L., Vance E., Burton A. A., Quinn T. C. Diagnosis of Chlamydia pneumoniae infection in patients with community-acquired pneumonia by polymerase chain reaction enzyme immunoassay. Clin Infect Dis. 1994 Jul;19(1):157–160. doi: 10.1093/clinids/19.1.157. [DOI] [PubMed] [Google Scholar]
- Gaydos C. A., Fowler C. L., Gill V. J., Eiden J. J., Quinn T. C. Detection of Chlamydia pneumoniae by polymerase chain reaction-enzyme immunoassay in an immunocompromised population. Clin Infect Dis. 1993 Oct;17(4):718–723. doi: 10.1093/clinids/17.4.718. [DOI] [PubMed] [Google Scholar]
- Gnarpe J., Lundbäck A., Sundelöf B., Gnarpe H. Prevalence of Mycoplasma pneumoniae in subjectively healthy individuals. Scand J Infect Dis. 1992;24(2):161–164. doi: 10.3109/00365549209052607. [DOI] [PubMed] [Google Scholar]
- Holland S. M., Gaydos C. A., Quinn T. C. Detection and differentiation of Chlamydia trachomatis, Chlamydia psittaci, and Chlamydia pneumoniae by DNA amplification. J Infect Dis. 1990 Oct;162(4):984–987. doi: 10.1093/infdis/162.4.984. [DOI] [PubMed] [Google Scholar]
- Hyman C. L., Roblin P. M., Gaydos C. A., Quinn T. C., Schachter J., Hammerschlag M. R. Prevalence of asymptomatic nasopharyngeal carriage of Chlamydia pneumoniae in subjectively healthy adults: assessment by polymerase chain reaction-enzyme immunoassay and culture. Clin Infect Dis. 1995 May;20(5):1174–1178. doi: 10.1093/clinids/20.5.1174. [DOI] [PubMed] [Google Scholar]
- Kai M., Kamiya S., Yabe H., Takakura I., Shiozawa K., Ozawa A. Rapid detection of Mycoplasma pneumoniae in clinical samples by the polymerase chain reaction. J Med Microbiol. 1993 Mar;38(3):166–170. doi: 10.1099/00222615-38-3-166. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Shor A., Campbell L. A., Fukushi H., Patton D. L., Grayston J. T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993 Apr;167(4):841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- Macfarlane J. T., Colville A., Guion A., Macfarlane R. M., Rose D. H. Prospective study of aetiology and outcome of adult lower-respiratory-tract infections in the community. Lancet. 1993 Feb 27;341(8844):511–514. doi: 10.1016/0140-6736(93)90275-l. [DOI] [PubMed] [Google Scholar]
- Macfarlane J., Prewett J., Rose D., Gard P., Cunningham R., Saikku P., Euden S., Myint S. Prospective case-control study of role of infection in patients who reconsult after initial antibiotic treatment for lower respiratory tract infection in primary care. BMJ. 1997 Nov 8;315(7117):1206–1210. doi: 10.1136/bmj.315.7117.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M., Matsuzono Y., Togashi T., Kajii N. DNA diagnosis of central nervous system infection by Mycoplasma pneumoniae. Pediatrics. 1992 Aug;90(2 Pt 1):250–253. [PubMed] [Google Scholar]
- Orle K. A., Gates C. A., Martin D. H., Body B. A., Weiss J. B. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J Clin Microbiol. 1996 Jan;34(1):49–54. doi: 10.1128/jcm.34.1.49-54.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeling R. W., Brunham R. C. Chlamydiae as pathogens: new species and new issues. Emerg Infect Dis. 1996 Oct-Dec;2(4):307–319. doi: 10.3201/eid0204.960406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillis M. Mycoplasma pneumoniae. Lancet. 1991 May 4;337(8749):1101–1101. doi: 10.1016/0140-6736(91)91752-g. [DOI] [PubMed] [Google Scholar]
- Sillis M. The limitations of IgM assays in the serological diagnosis of Mycoplasma pneumoniae infections. J Med Microbiol. 1990 Dec;33(4):253–258. doi: 10.1099/00222615-33-4-253. [DOI] [PubMed] [Google Scholar]
- Steinhoff D., Lode H., Ruckdeschel G., Heidrich B., Rolfs A., Fehrenbach F. J., Mauch H., Höffken G., Wagner J. Chlamydia pneumoniae as a cause of community-acquired pneumonia in hospitalized patients in Berlin. Clin Infect Dis. 1996 Jun;22(6):958–964. doi: 10.1093/clinids/22.6.958. [DOI] [PubMed] [Google Scholar]
- Thom D. H., Grayston J. T., Wang S. P., Kuo C. C., Altman J. Chlamydia pneumoniae strain TWAR, Mycoplasma pneumoniae, and viral infections in acute respiratory disease in a university student health clinic population. Am J Epidemiol. 1990 Aug;132(2):248–256. doi: 10.1093/oxfordjournals.aje.a115654. [DOI] [PubMed] [Google Scholar]
- Tong C. Y., Sillis M. Detection of Chlamydia pneumoniae and Chlamydia psittaci in sputum samples by PCR. J Clin Pathol. 1993 Apr;46(4):313–317. doi: 10.1136/jcp.46.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wort S. J., Rogers T. R. Community acquired pneumonia in elderly people. Current British guidelines need revision. BMJ. 1998 Jun 6;316(7146):1690–1690. doi: 10.1136/bmj.316.7146.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreghitt T. G., Taylor C. E. Incidence of respiratory tract chlamydial infections and importation of psittacine birds. Lancet. 1988 Mar 12;1(8585):582–582. doi: 10.1016/s0140-6736(88)91368-2. [DOI] [PubMed] [Google Scholar]
- de Barbeyrac B., Bernet-Poggi C., Fébrer F., Renaudin H., Dupon M., Bébéar C. Detection of Mycoplasma pneumoniae and Mycoplasma genitalium in clinical samples by polymerase chain reaction. Clin Infect Dis. 1993 Aug;17 (Suppl 1):S83–S89. doi: 10.1093/clinids/17.supplement_1.s83. [DOI] [PubMed] [Google Scholar]



