Abstract
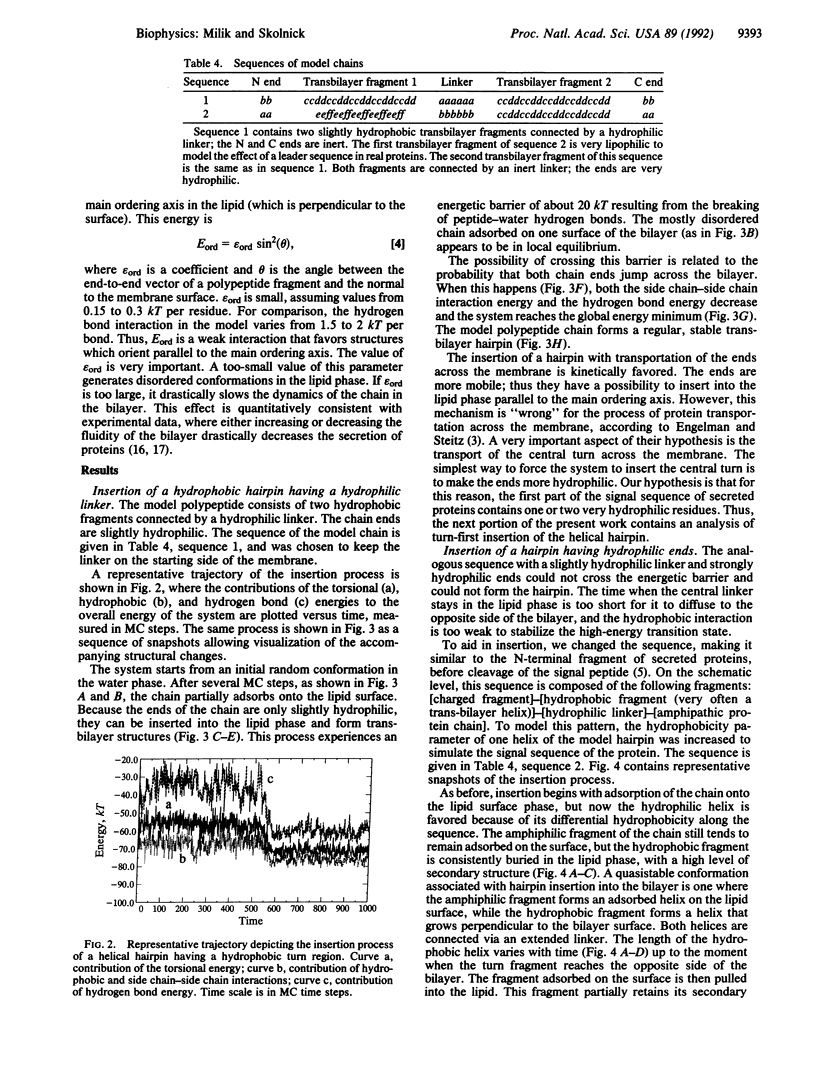
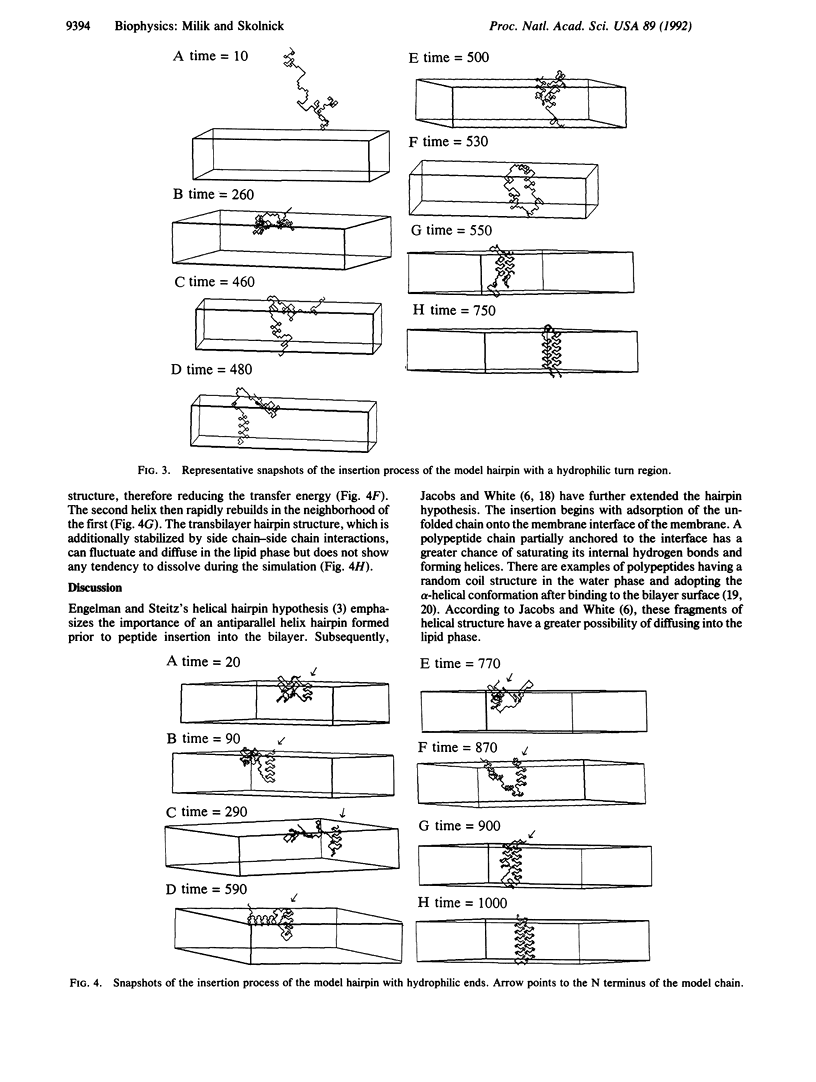
The Monte Carlo dynamics method was used to examine the process of protein insertion into model cell membranes. The water and lipid environments were taken into account via an effective medium approximation based on coordinate-dependent hydrophobic and hydrogen bond potentials. The polypeptide chain was represented in a full-backbone atom representation as a chain of diamond lattice vectors. The simulations support the idea that to a good approximation insertion may be depicted as a spontaneous thermodynamic process. The mechanism of membrane insertion of a simple lattice protein chain exhibits many features of theoretical predictions and is in good accord with experimental data. In the model, insertion begins with adsorption of the chain onto the interface, followed by the formation of helical fragments. These fragments, having partially saturated internal hydrogen bonds, can be transported into the lipid phase and then form transbilayer structures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs M. S., Gierasch L. M. Molecular mechanisms of protein secretion: the role of the signal sequence. Adv Protein Chem. 1986;38:109–180. doi: 10.1016/s0065-3233(08)60527-6. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Godzik A., Skolnick J., Kolinski A. Simulations of the folding pathway of triose phosphate isomerase-type alpha/beta barrel proteins. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2629–2633. doi: 10.1073/pnas.89.7.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. E., White S. H. Mixtures of a series of homologous hydrophobic peptides with lipid bilayers: a simple model system for examining the protein-lipid interface. Biochemistry. 1986 May 6;25(9):2605–2612. doi: 10.1021/bi00357a049. [DOI] [PubMed] [Google Scholar]
- Jacobs R. E., White S. H. The nature of the hydrophobic binding of small peptides at the bilayer interface: implications for the insertion of transbilayer helices. Biochemistry. 1989 Apr 18;28(8):3421–3437. doi: 10.1021/bi00434a042. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Thermodynamics and kinetics of protein incorporation into membranes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3691–3695. doi: 10.1073/pnas.80.12.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdunski C. J., Benedetti H. Insertion and translocation of proteins into and through membranes. FEBS Lett. 1990 Aug 1;268(2):408–414. doi: 10.1016/0014-5793(90)81295-y. [DOI] [PubMed] [Google Scholar]
- Pagès J. M., Piovant M., Varenne S., Lazdunski C. Mechanistic aspects of the transfer of nascent periplasmic proteins across the cytoplasmic membrane in Escherichia coli. Eur J Biochem. 1978 May 16;86(2):589–602. doi: 10.1111/j.1432-1033.1978.tb12343.x. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Engelman D. M. Membrane protein folding and oligomerization: the two-stage model. Biochemistry. 1990 May 1;29(17):4031–4037. doi: 10.1021/bi00469a001. [DOI] [PubMed] [Google Scholar]
- Sargent D. F., Schwyzer R. Membrane lipid phase as catalyst for peptide-receptor interactions. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5774–5778. doi: 10.1073/pnas.83.16.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer R. Estimated conformation, orientation, and accumulation of dynorphin A-(1-13)-tridecapeptide on the surface of neutral lipid membranes. Biochemistry. 1986 Jul 29;25(15):4281–4286. doi: 10.1021/bi00363a016. [DOI] [PubMed] [Google Scholar]
- Skolnick J., Kolinski A. Simulations of the folding of a globular protein. Science. 1990 Nov 23;250(4984):1121–1125. doi: 10.1126/science.250.4984.1121. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Brunner J., Baker A., Schatz G. A 42K outer-membrane protein is a component of the yeast mitochondrial protein import site. Nature. 1989 Sep 21;341(6239):205–209. doi: 10.1038/341205a0. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. A chimeric mitochondrial precursor protein with internal disulfide bridges blocks import of authentic precursors into mitochondria and allows quantitation of import sites. J Cell Biol. 1988 Dec;107(6 Pt 1):2037–2043. doi: 10.1083/jcb.107.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. Point mutations destabilizing a precursor protein enhance its post-translational import into mitochondria. EMBO J. 1988 Apr;7(4):1147–1151. doi: 10.1002/j.1460-2075.1988.tb02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. Point mutations destabilizing a precursor protein enhance its post-translational import into mitochondria. EMBO J. 1988 Apr;7(4):1147–1151. doi: 10.1002/j.1460-2075.1988.tb02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Wickner W. Mechanisms of membrane assembly: general lessons from the study of M13 coat protein and Escherichia coli leader peptidase. Biochemistry. 1988 Feb 23;27(4):1081–1086. doi: 10.1021/bi00404a001. [DOI] [PubMed] [Google Scholar]