Abstract
Species habitat associations are often complex, making it difficult to assess their influence on populations. Among coral reef fishes, habitat requirements vary among species and with ontogeny, but the relative importance of nursery and adult-preferred habitats on future abundances remain unclear. Moreover, adult populations may be influenced by recruitment of juveniles and assessments of habitat importance should consider relative effects of juvenile abundance. We conducted surveys across 16 sites and 200 km of reef to identify the microhabitat preferences of juveniles, sub-adults and adults of the damselfish Pomacentrus moluccensis. Microhabitat preferences at different life-history stages were then combined with 6 years of juvenile abundance and microhabitat availability data to show that the availability of preferred juvenile microhabitat (corymbose corals) at the time of settlement was a strong predictor of future sub-adult and adult abundance. However, the influence of nursery microhabitats on future population size differed spatially and at some locations abundance of juveniles and adult microhabitat (branching corals) were better predictors of local populations. Our results demonstrate that while juvenile microhabitats are important nurseries, the abundance of coral-dependent fishes is not solely dependent on these microhabitats, especially when microhabitats are readily available or following large influxes of juveniles.
Keywords: recruitment, population size, ontogeny, specialization, habitat conservation
1. Introduction
Identifying the habitat requirements of species and how this affects their abundance is a central tenet of ecology. At the species level, suitable habitats provide shelter and sustenance to promote survival and growth, while at the community level, variation in habitat complexity can moderate key processes such as competition and predation. However, habitat degradation and fragmentation through climate change and other anthropogenic stressors are altering species interactions and contributing to local extinctions [1–3]. Consequently, recognizing and conserving essential habitats has become a key component of ecosystem-based management [4,5]. Availability of suitable habitat is especially important for ecological specialists, who use only a small proportion of available resources [6]. Indeed, studies on birds [7], butterflies [8] and coral reef fishes [9] have all demonstrated that habitat loss has a greater impact upon habitat specialists than generalists.
Habitat preferences can, however, change with ontogeny [10], which may alter how a species responds to habitat changes at different life-history stages [11]. For example, juveniles of many marine fishes can occupy very different habitats compared with their adult conspecifics, with such nursery areas being critically important for effective conservation and fisheries management [12]. While bottlenecks in juvenile habitat may limit the abundance of adult populations [13,14], the relative importance of juvenile versus adult habitats for regulating total population size is often unclear [15]. Moreover, in the marine environment, the supply rate of recruits may have a greater influence on adult abundances than habitat or density regulating processes [16,17]. Consequently, an appreciation of both ontogenetic shifts in habitat use and supply rates of recruits is needed to understand fluctuations in population size [18,19].
Experimental work has demonstrated that density-dependent mortality regulates populations, and that availability of suitable refuge microhabitats can be a key mediator of predation and mortality rates within fish populations [20,21]. While these and other experiments have helped elucidate the extent of small-scale mechanisms for density-dependent regulation of fish populations, very few studies have examined the importance of microhabitat-related drivers of population size over spatial scales that are relevant to management [22,23]. While rare, long-term field observations over large spatial scales have provided key information on natural rates of recruitment, changes in adult abundance and microhabitat availability [24–27]. What remains to be established, however, is the extent to which the supply of new recruits interacts with the availability of preferred microhabitats through ontogeny to shape the future size of fish populations.
Here, we assess the relative importance of juvenile abundance and microhabitat availability for shaping population sizes of a microhabitat specialist, the coral-dwelling damselfish Pomacentrus moluccensis. We examine patterns in juvenile, sub-adult and adult abundance over 6 years across the Ningaloo region in Western Australia, alongside assessments of microhabitat associations and specialization at these different life-history stages. Throughout the study period, abnormally warm water and high cyclone activity in some years produced strong temporal and spatial variability in coral microhabitat availability [28], which provided an ideal system to test how microhabitat availability may influence the abundance of adults relative to the supply of new recruits over space and time.
2. Material and methods
(a). Recruitment patterns
Abundance of juvenile (less than 2 cm total length, TL) P. moluccensis was surveyed across 16 sites spread along 200 km of the World Heritage Ningaloo coast of Western Australia. Five sites were in the northern zone, and six in the southern zone, which experience different temperature regimes and currents [29,30]. Three sites were located in the transitional middle zone around Point Cloates [31], while a further two sites were situated within Exmouth Gulf (electronic supplementary material, figure S1). All sites were on the wave-sheltered shallow (1–4 m) coral back reef, where P. moluccensis juveniles are most abundant [32].
Juvenile P. moluccensis abundance was recorded at each site once per annum for 6 years (2010–2015) during February/March, immediately after the new moon to ensure surveys were completed within the same lunar phase within and among years. Being at the end of the Austral summer, surveys were completed shortly after the peak recruitment season in our study region [33]. At each site, fish counts were recorded within six to nine 1 × 30 m haphazardly placed transects, with at least 5 m between each transect. This covered an area many times greater than mean distances (3.3 m) that juvenile P. moluccensis typically move [34]. Per cent benthic cover of live coral growth forms (branching, corymbose, encrusting, foliose, plate, massive), rubble, sand, dead coral, erect fleshy macroalgae and hard pavement was estimated within each of the same transects, using planform visual assessments that are comparable with line-intercept transects [35].
For all analyses, sites were used as replicates, with fish abundance and microhabitat occurrence expressed as mean abundance per transect. Spatial and temporal variation in juvenile P. moluccensis was assessed using permutational analysis of covariance (PERMANCOVA), from a resemblance matrix constructed using a modified Gower, base 2 measure and 9999 permutations [36]. PERMANCOVA was used instead of ANCOVA because data were not normally distributed. Spatial zone (Gulf, North, Middle and South) and year (2010–2015) were entered as fixed factors. Per cent cover of corymbose coral, a preferred microhabitat type of juvenile P. moluccensis at Ningaloo [32], was included as a covariate. Significant results for a given factor or interaction term were explored further using pairwise PERMANOVA. Statistical analysis was completed with Primer (v. 6.1.12) and Permanova+ (v. 1.0.02).
(b). Microhabitat associations
Microhabitat associations of juvenile (less than 2 cm TL), sub-adult (2–4 cm TL) and adult (more than 4 cm TL) P. moluccensis were assessed annually along three to six transects at each site from 2013 to 2015. Life-history categories were based on length from age growth curves for P. moluccensis [37]. Visual size estimates by observers were calibrated at the start of each day by estimating and subsequently measuring the length of rubble and small corals, with differences between estimated and actual sizes being less than 1% (0.2 cm) and insignificant (paired t-test, t410 = 1.68, p = 0.09). Microhabitat associations were determined by recording the type of benthos (categories noted above) directly beneath each fish when first observed. Microhabitat availability was estimated within the same transects using planform visual assessments of the aforementioned categories. Microhabitat specialization for the three fish life-history stages were then assessed at each site where 10 or more fish were observed using relativized electivity indices that consider microhabitat use relative to availability [38]. Mean electivity indices for each microhabitat category (±95% confidence interval (CI)) were calculated using annual surveys of sites as replicates. Indices with 95% CI that were more than zero suggest positive selection/preference for a given microhabitat type. Microhabitat niche breadth for each life-history stage was also calculated for each site using the proportional similarity index of Feinsinger et al. [39]. Values close to zero indicate smaller niche breadths and greater specialization, while values that approach one suggest a broader range of microhabitat associations.
(c). Predicting adult abundances
We explored the relative importance of microhabitat and abundance of juvenile P. moluccensis for shaping adult and sub-adult population sizes using a full-subsets approach and general additive models (GAM). Explanatory variables considered for adult analysis were: per cent cover of preferred adult microhabitat (branching corals; electronic supplementary material, table S1) at the time of surveys (2013–2015), juvenile abundance 2 and 3 years prior to each survey year, and juvenile microhabitat (corymbose corals) 2 and 3 years prior to each survey year (corresponding with time lag expected for juveniles to reach adult TL, and maturity [37,40]). Explanatory variables considered for sub-adults were: per cent cover of preferred sub-adult microhabitat (branching corals; electronic supplementary material, table S1) at the time of survey (2013–2015), and juvenile abundance and/or juvenile-preferred microhabitat (corymbose corals) 1 year prior to each survey year. For both analyses, spatial zone (Gulf, North, Middle and South) was considered as an explanatory variable. All possible combinations of explanatory variables were considered and best models chosen as those with the fewest variables within two Akaike information criterion corrected for small sample size (AICc) units of the model with the lowest AICc value [41]. AICc weights were calculated for each model, and support for each explanatory variable was obtained by summing AICc weights across all models containing that variable [41]. Statistical analysis was completed in R (v. 3.2.1, R Core Development Team) using the function ‘gamm’ from library mgcv [42] and ‘gamm4’ from the library gamm4 [43].
3. Results
(a). Microhabitat associations
More than 60% of juvenile P. moluccensis observed during 2013–2015 were closely associated with corymbose corals, even though these corals typically represented less than 10% of the total available habitat cover (figure 1a). Electivity indices (electronic supplementary material, table S1) suggest juvenile fish preferentially associated with live corymbose corals, with the high proportion of juveniles on either corymbose or branching corals translating to a low realized niche and high level of specialization (figure 1). Both sub-adult and adult fish were more closely associated with branching corals, and electivity indices suggest these larger fish favour this microhabitat over others (figure 1b,c). However, sub-adult and adult fish were observed to associate with a much broader suite of microhabitats than juveniles, such that their niche space expanded with increasing body size (figure 1).
Figure 1.
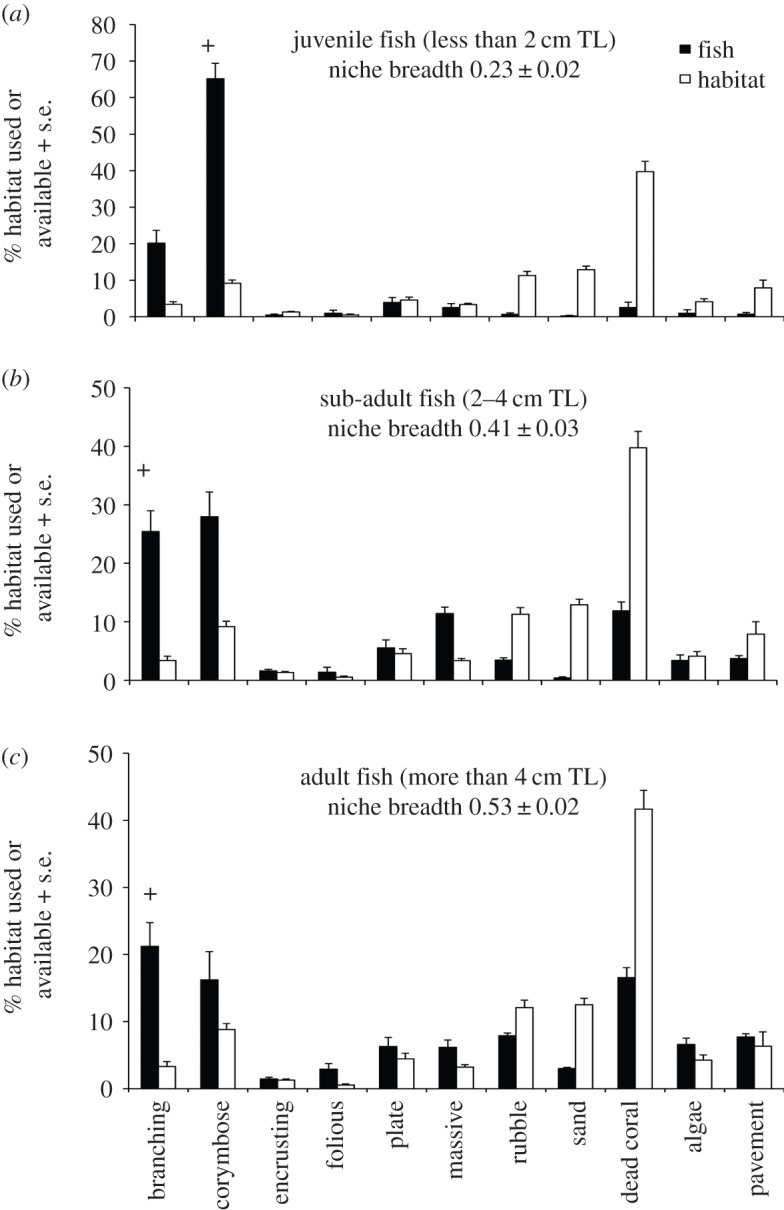
(a–c) Habitat associations and selection by different life-history stages of Pomacentrus moluccensis. Plus (+) symbol indicates all instances of positive selection for a given habitat type indicated by electivity indices.
(b). Recruitment patterns
Annual abundances of juvenile P. moluccensis fluctuated by an order of magnitude during the 6 year survey period in a pattern that was not consistent among spatial zones (zone × year interaction F15,70 = 2.9, p = 0.001; figure 2). Within Exmouth Gulf, juvenile abundance was highest in 2012 at greater than 30 fish per 30 m2, while the highest numbers of juveniles were observed in 2011 within the Middle and Southern zones, and in 2013 within the Southern zone. Juvenile numbers were generally low (less than 10) throughout the study period in the Northern zone. Juvenile P. moluccensis abundance was positively related to per cent cover of corymbose coral (F1,70 = 16.4, p < 0.001), although this microhabitat variable explained only 7% of the total variance in juvenile abundance over space and time. Indeed, there were instances where juvenile fish abundance was high but corymbose coral cover was very low and vice versa (electronic supplementary material, figure S2).
Figure 2.
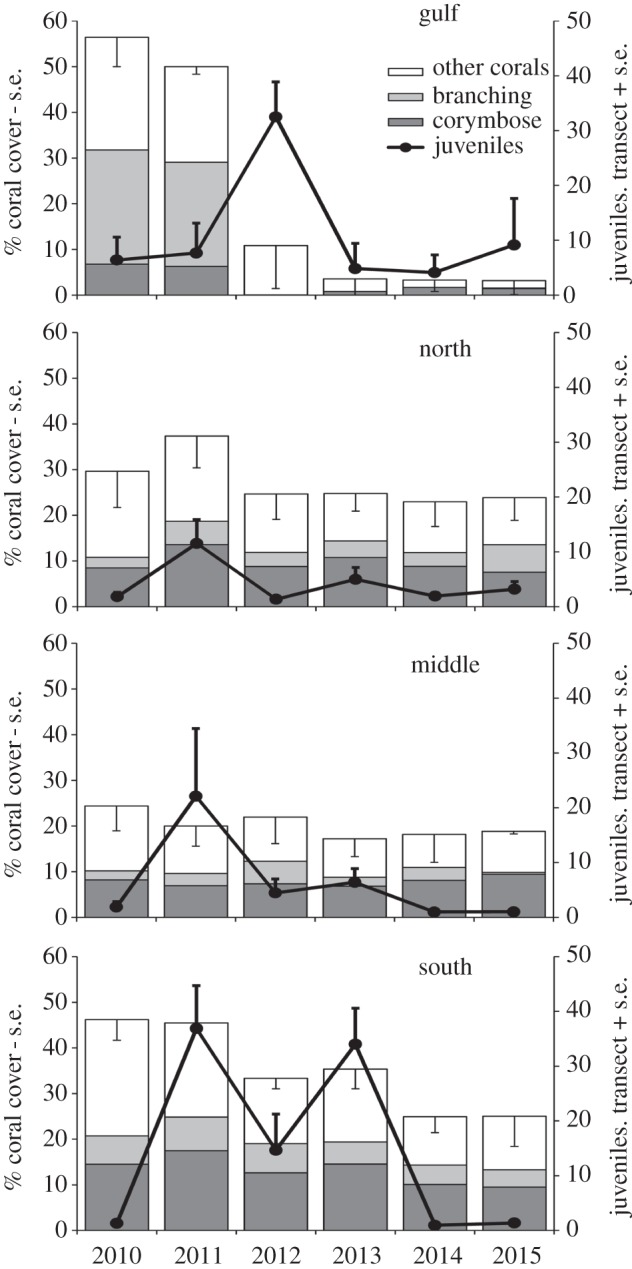
Temporal changes in abundance of juvenile Pomacentrus moluccensis (lines) and per cent cover of live coral habitat (bars) across four spatial zones of Ningaloo Reef, Western Australia. Standard errors for total coral cover and juvenile fish calculated from 2–6 sites in each zone.
(c). Predicting sub-adult and adult abundances
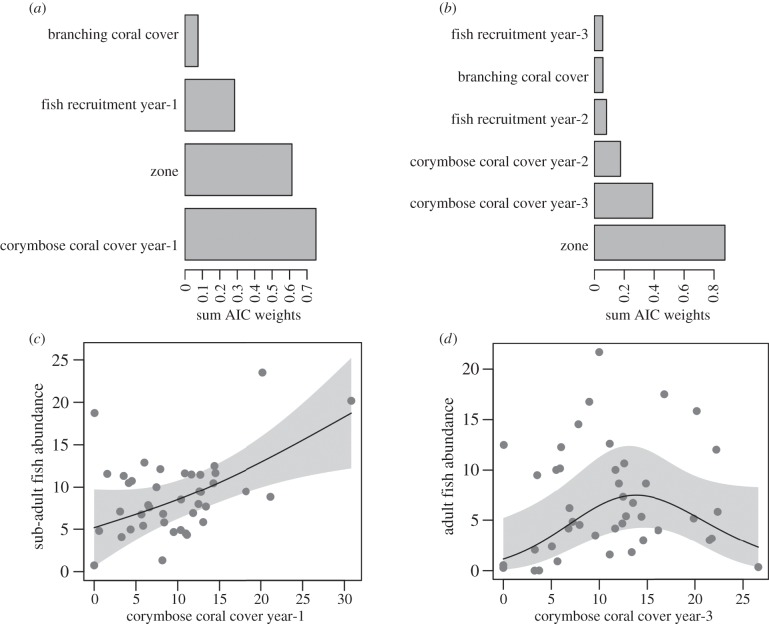
Availability of preferred juvenile microhabitat at the approximate time of larval settlement into benthic habitats had a stronger influence upon the abundance of sub-adult and adult P. moluccensis than either the abundance of juveniles or availability of adult microhabitats, though this pattern was not always consistent among study zones. Spatial variation among the four zones, combined with per cent cover of juvenile-preferred microhabitat (corymbose corals) 1 year prior to sub-adult surveys, explained 40% of the variation in sub-adult abundance (table 1). Abundance of sub-adults was high within the South and Gulf zones, although there was considerable variation in estimates from the Gulf (electronic supplementary material, figure S3). Across all zones, corymbose coral cover a year before survey was a particularly good predictor of sub-adult abundance, which increased linearly with the juvenile-preferred microhabitat (figure 3a,b). The relationship between corymbose corals and sub-adults was strongest in the northern and southern zones; however, in the Gulf, abundance of juveniles from the previous year was also a strong predictor of sub-adults and within the middle zone cover of preferred sub-adult microhabitat (branching corals) at the time of survey was positively related to sub-adult abundance (electronic supplementary material, table S2 and figure S4).
Table 1.
Best five models (GAM) for predicting abundance of adult and sub-adult Pomacentrus moluccensis. (Top models (<2 ΔAIC) are indicated in italics. Numbers in parentheses indicate the lag in years between the level of a variable (e.g. per cent cover of live corymbose coral) and current abundances of adult and sub-adult fish.)
| model | d.f. | r2 | ΔAICc | AICc wt | |
|---|---|---|---|---|---|
| sub-adults | zone, % corymbose coral (−1) | 8 | 0.40 | 0 | 0.51 |
| zone | 6 | 0.26 | 3.04 | 0.11 | |
| % corymbose coral (−1), juveniles (−1) | 7 | 0.30 | 3.29 | 0.10 | |
| juveniles (−1) | 5 | 0.19 | 4.10 | 0.06 | |
| % corymbose coral (−1) | 5 | 0.19 | 4.20 | 0.06 | |
| adults | zone | 6 | 0.26 | 0 | 0.36 |
| zone, % corymbose coral (−3) | 8 | 0.34 | 0.57 | 0.27 | |
| zone, % corymbose coral (−2) | 8 | 0.31 | 2.57 | 0.10 | |
| % corymbose coral (−3) | 5 | 0.12 | 4.55 | 0.04 | |
| % corymbose coral (−2) | 5 | 0.11 | 5.03 | 0.03 |
Figure 3.
Relative importance of variables in predicting future abundances of sub-adult (a) and adult (b) Pomacentrus moluccensis. Partial plots show variance in sub-adult (c) and adult (d) abundances with respect to per cent cover of live corymbose coral 1 year and 3 years before surveys, respectively. Shaded areas are 95% confidence limits.
Spatial zones were the best single predictor of adult fish abundance (figure 3c), owing to higher adult densities observed in the South and Gulf versus other zones (electronic supplementary material, figure S3). However, cover of corymbose coral 3 years before each survey year was also a good predictor, adult abundance increased linearly until corymbose coral cover was more than 15% (figure 3d). Notably, juvenile fish abundance in preceding years and the availability of adult microhabitat were relatively poor predictors of adult abundance when data from all zones were considered. The relationship between corymbose corals and adults was strongest in the Middle zone, while in other zones other variables better explained variation in adult abundance. In particular, within the Gulf, there was a positive relationship between juveniles 3 years before surveys and adult fish (electronic supplementary material, table S2). This relationship is primarily driven by high numbers of juveniles in 2012 and a corresponding high number of adults in 2015 (electronic supplementary material, figure S5). Within the Gulf and Middle zones cover of adult microhabitat (branching corals) had also declined substantially over the 5 years of surveying and was positively related to adult abundance (electronic supplementary material, table S3 and figure S5).
4. Discussion
Nursery habitats are often advocated for conservation of species [44], but there is little empirical understanding of how these habitats contribute to adult populations. Identifying microhabitat associations and assessing their importance relative to supply of new recruits is therefore critical for evaluating factors that may influence population levels and prioritizing areas for conservation management. Here, we demonstrate that juvenile microhabitat availability at the time of larval settlement is a good predictor of sub-adult population sizes in a widely distributed and abundant coral-associated reef fish. However, the importance of juvenile microhabitat to future populations varies spatially, and in some areas adult microhabitat or changes in the abundance of juveniles are better predictors of sub-adult and adult fish abundance.
The availability of specific coral microhabitats has previously been shown to have a profound influence on the abundance and distribution patterns of many coral-dependent fishes [18,45,46]. But it is not always clear how ontogenetic shifts in coral use influence contemporary populations because the ‘nursery’ microhabitat at the time when adults were juveniles have not been expressly considered. By considering the availability of corymbose corals at the time of recruitment, we demonstrated a strong influence of nursery microhabitat availability on the future abundance of older conspecifics. However, unlike previous studies that have investigated the influence of microhabitat availability on coral specialists [45,46], we found relations between juvenile fish abundance and per cent cover of corymbose corals were weak. In particular, in the Gulf, abnormally warm water and cyclonic activity during the Austral summer of 2010/2011 caused extensive corymbose coral mortality [28,47], yet there was a large pulse of juvenile P. moluccensis the subsequent year, demonstrating abundance of juveniles may act independently of microhabitat. Juveniles from this 2012 pulse clearly contributed to future sub-adult and adult abundance, even though their preferred microhabitat had gone, suggesting some level of habitat plasticity. Pomacentrus moluccensis are known to associate with more than 30 coral species [48] and it is assumed that juveniles from the Gulf associated with less-preferred microhabitats in 2012. The ecological consequences of associating with sub-optimal microhabitats are not clear, though it is likely that predation becomes more intense in the absence of suitable microhabitat [19,49,50], and it is probable that adult P. moluccensis abundances would have been higher without the loss of corymbose coral in 2012.
As P. moluccensis grow they used a broader range of microhabitats and became less dependent on corymbose corals. The number of fish that survive to these older stages will be partially dependent on predation of juveniles and sub-adults, which may be extensive [51]. Indeed, some predators are known to target juveniles within corymbose corals, resulting in substantial mortality [52]. Older fish are also subject to predation and tend to associate with microhabitats that provide refuge space that matches their larger body size [46]. The importance of juvenile microhabitat to fish may therefore diminish, as fish get larger and older. Indeed, live corymbose coral cover at the time of recruitment is only relevant to adult abundance when it is below 15%. This is consistent with models that suggest availability of juvenile microhabitat has the greatest influence on adult populations when it is scarce [15]. Conversely, the availability of adult microhabitat, branching coral, may be a good predictor of adults when juvenile habitat is readily available and adult microhabitat is sparse [15], as was observed at sites within the Middle zone. Hence, it is unlikely that a single factor drives population sizes in a cosmopolitan manner [19,22,53]; a pluralistic scenario is most likely, where different explanatory variables underpin local population sizes according to the interaction between levels of recruitment, preferred habitat availability, and the existing abundance of conspecifics. In particular, heterogeneous patterns of habitat loss and strong recruitment pulses can alter the relative importance of microhabitats and juvenile abundance for shaping future adult populations.
5. Conclusion
Nursery habitats have long been recognized as important for the health and survival of juvenile life-history stages [49]. Here, we extend this importance to include coral microhabitats in the future population sizes of a coral reef fish. Juvenile P. moluccensis were found predominantly in corymbose corals, and we find a positive relationship between juvenile microhabitat availability and sub-adult population sizes. This relationship is, however, not always consistent among different areas and strong pulses of juvenile recruits can leave their mark on adult abundances even when cover of preferred juvenile microhabitats is very low. Relationships between adults and juvenile microhabitats were also weak, particularly when the cover of corymbose corals was high. Our findings demonstrate that while juvenile microhabitats are key to the persistence of some coral reef fish, adult microhabitats and historic abundance of juveniles may also be important, and the relative influence of these drivers can vary both spatially and temporally.
Supplementary Material
Acknowledgements
We thank staff at the Department of Parks and Wildlife Exmouth for field support. Early versions of the manuscript were improved through comments and discussion with A. Kendrick and H. Raudino.
Data accessibility
Raw data are stored by the Department of Parks and Wildlife Western Australia and can be accessed by contacting the lead author or MarineDataRequests@dec.wa.gov.au.
Authors' contributions
S.K.W., M.D., T.H.H., C.J.F. and P.T conceived, designed and collected data for the study. Statistical analyses were carried out by B.T.R. and S.K.W. All authors drafted the manuscript and gave final approval for publication
Competing interests
The authors have no competing interests.
Funding
Funding for the project was provided by AIMS 2013 Appropriation funding 3.3.5, Western Australian Marine Science Institute, WA Department of Parks and Wildlife and The Australian National University.
References
- 1.Dulvy NK, Sadovy Y, Reynolds JD. 2003. Extinction vulnerability in marine populations. Fish Fish. 4, 25–64. ( 10.1046/j.1467-2979.2003.00105.x) [DOI] [Google Scholar]
- 2.McCormick MI. 2012. Lethal effects of habitat degradation on fishes through changing competitive advantage. Proc. R. Soc. B 279, 3899–3904. ( 10.1098/rspb.2012.0854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis JMJ. 2003. Climate change and habitat destruction: a deadly anthropogenic cocktail. Proc. R. Soc. Lond. B 270, 467–473. ( 10.1098/rspb.2002.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pikitch EK, et al. 2004. Ecosystem-based fishery management. Science (Washington) 305, 346–347. ( 10.1126/science.1098222) [DOI] [PubMed] [Google Scholar]
- 5.Thrush SF, Dayton PK. 2010. What can ecology contribute to ecosystem-based management? Annu. Rev. Mar. Sci. 2, 419–441. ( 10.1146/annurev-marine-120308-081129) [DOI] [PubMed] [Google Scholar]
- 6.Vázquez DP, Simberloff D. 2002. Ecological specialization and susceptibility to disturbance: conjectures and refutations. Am. Nat. 159, 606–623. ( 10.1086/339991) [DOI] [PubMed] [Google Scholar]
- 7.Devictor V, Julliard R, Jiguet F. 2008. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117, 507–514. ( 10.1111/j.2008.0030-1299.16215.x) [DOI] [Google Scholar]
- 8.Charrette NA, Cleary DFR, Mooers A. 2006. Range-restricted, specialist Bornean butterflies are less likely to recover from ENSO-induced disturbance. Ecology 87, 2330–2337. ( 10.1890/0012-9658(2006)87%5B2330:RSBBAL%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 9.Munday PL. 2004. Habitat loss, resource specialization, and extinction on coral reefs. Glob. Change Biol. 10, 1642–1647. ( 10.1111/j.1365-2486.2004.00839.x) [DOI] [Google Scholar]
- 10.Werner EE, Gilliam JF. 1984. The ontogenetic niche and species interactions in size-structured populations. Annu. Rev. Ecol. Syst. 15, 393–425. ( 10.1146/annurev.es.15.110184.002141) [DOI] [Google Scholar]
- 11.Shima JS, Osenberg CW, St Mary CM. 2008. Quantifying site quality in a heterogeneous landscape: recruitment of a reef fish. Ecology 89, 86–94. ( 10.1890/07-0021.1) [DOI] [PubMed] [Google Scholar]
- 12.Nagelkerken I, Sheaves M, Baker R, Connolly RM. 2015. The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fish. 16, 362–371. ( 10.1111/faf.12057) [DOI] [Google Scholar]
- 13.Graham NAJ, Wilson SK, Jennings S, Polunin NVC, Robinson J, Bijoux JP, Daw TM. 2007. Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 21, 1291–1300. ( 10.1111/j.1523-1739.2007.00754.x) [DOI] [PubMed] [Google Scholar]
- 14.Sundblad G, Bergstrom U, Sandstrom A, Eklov P. 2014. Nursery habitat availability limits adult stock sizes of predatory coastal fish. ICES J. Mar. Sci. 71, 672–680. ( 10.1093/icesjms/fst056) [DOI] [Google Scholar]
- 15.Halpern BS, Gaines SD, Warner RR. 2005. Habitat size, recruitment, and longevity as factors limiting population size in stage-structured species. Am. Nat. 165, 82–94. ( 10.1086/426672) [DOI] [PubMed] [Google Scholar]
- 16.Roughgarden J, Gaines S, Possingham H. 1988. Recruitment dynamics in complex life cycles. Science 241, 1460–1466. ( 10.1126/science.11538249) [DOI] [PubMed] [Google Scholar]
- 17.Doherty P, Fowler A. 1994. An empirical test of recruitment limitation in a coral reef fish. Science 263, 935–939. ( 10.1126/science.263.5149.935) [DOI] [PubMed] [Google Scholar]
- 18.Schmitt RJ, Holbrook SJ. 2000. Habitat-limited recruitment of coral reef damselfish. Ecology 81, 3479–3494. ( 10.1890/0012-9658(2000)081%5B3479:HLROCR%5D2.0.CO;2) [DOI] [Google Scholar]
- 19.Shima JS. 2001. Recruitment of a coral reef fish: roles of settlement, habitat, and postsettlement losses. Ecology 82, 2190–2199. ( 10.2307/2680225) [DOI] [Google Scholar]
- 20.Beukers JS, Jones GP. 1998. Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oecologia 114, 50–59. ( 10.1007/s004420050419) [DOI] [PubMed] [Google Scholar]
- 21.Hixon MA, Jones GP. 2005. Competition, predation, and density-dependent mortality in demersal marine fishes. Ecology 86, 2847–2859. ( 10.1890/04-1455) [DOI] [Google Scholar]
- 22.Jones GP. 1991. Postrecruitment processes in the ecology of coral reef fish populations: a multifactorial perspective. In The ecology of fishes on coral reefs (ed. Sale P.), pp. 294–330. San Diego, CA: Academic Press. [Google Scholar]
- 23.Steele MA, Forrester GE. 2005. Small-scale field experiments accurately scale up to predict density dependence in reef fish populations at large scales. Proc. Natl Acad. Sci. USA 102, 13 513–13 516. ( 10.1073/pnas.0504306102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrester GE, Steele MA, Samhouri JF, Evans B, Vance RR. 2008. Spatial density dependence scales up but does not produce temporal density dependence in a reef fish. Ecology 89, 2980–2985. ( 10.1890/07-1546.1) [DOI] [PubMed] [Google Scholar]
- 25.Caley MJ, Carr MH, Hixon MA, Hughes TP, Jones GP, Menge BA. 1996. Recruitment and the local dynamics of open marine populations. Annu. Rev. Ecol. Syst. 27, 477–500. ( 10.1146/annurev.ecolsys.27.1.477) [DOI] [Google Scholar]
- 26.Hixon MA, Anderson TW, Buch KL, Johnson DW, McLeod JB, Stallings CD. 2012. Density dependence and population regulation in marine fish: a large-scale, long-term field manipulation. Ecol. Monogr. 82, 467–489. ( 10.1890/11-1525.1) [DOI] [Google Scholar]
- 27.Sponaugle S, Walter KD, Grorud-Colvert K, Paddack MJ. 2012. Influence of marine reserves on reef fish recruitment in the upper Florida Keys. Coral Reefs 31, 641–652. ( 10.1007/s00338-012-0915-y) [DOI] [Google Scholar]
- 28.Moore JAY, et al. 2012. Unprecedented mass bleaching and loss of coral across 12° of latitude in Western Australia in 2010–11. PLoS ONE 7, e51807 ( 10.1371/journal.pone.0051807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowe RJ, Ivey GN, Brinkman RM, Jones NL. 2012. Seasonal circulation and temperature variability near the North West Cape of Australia. J. Geophys. Res. 117, C04010. ( 10.1029/2011JC007653) [DOI] [Google Scholar]
- 30.Fulton CJ, Depczynski M, Holmes TH, Noble MM, Radford B, Wernberg T, Wilson SK. 2014. Sea temperature shapes seasonal fluctuations in seaweed biomass within the Ningaloo coral reef ecosystem. Limnol. Oceanogr. 59, 156–166. ( 10.4319/lo.2014.59.1.0156) [DOI] [Google Scholar]
- 31.Woo M, Pattiaratchi C, Schroeder W. 2006. Dynamics of the Ningaloo Current off Point Cloates, Western Australia. Mar. Freshw. Res. 57, 291–301. ( 10.1071/MF05106) [DOI] [Google Scholar]
- 32.Wilson SK, Depczynski M, Fisher R, Holmes TH, O'Leary RA, Tinkler P. 2010. Habitat associations of juvenile fish at Ningaloo Reef, Western Australia: the importance of coral and algae. PLoS ONE 5, e15185 ( 10.1371/journal.pone.0015185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIlwain JL. 2003. Fine-scale temporal and spatial patterns of larval supply to a fringing reef in Western Australia. Mar. Ecol. Prog. Ser. 252, 207–222. ( 10.3354/meps252207) [DOI] [Google Scholar]
- 34.Brunton BJ, Booth DJ. 2003. Density- and size-dependent mortality of a settling coral-reef damselfish (Pomacentrus moluccensis Bleeker). Oecologia 137, 377–384. ( 10.1007/s00442-003-1377-2) [DOI] [PubMed] [Google Scholar]
- 35.Wilson SK, Graham NAJ, Polunin NVC. 2007. Appraisal of visual assessments of habitat complexity and benthic composition on coral reefs. Mar. Biol. 151, 1069–1076. ( 10.1007/s00227-006-0538-3) [DOI] [Google Scholar]
- 36.Anderson M, Gorley RN, Clarke RK. 2008. PERMANOVA+ for primer: guide to software and statistical methods.
- 37.Abesamis RA, Jadloc CRL, Russ GR. 2015. Varying annual patterns of reproduction in four species of coral reef fish in a monsoonal environment. Mar. Biol. 162, 1993–2006. ( 10.1007/s00227-015-2725-6) [DOI] [Google Scholar]
- 38.Vanderploeg HA, Scavia D. 1979. Calculation and use of selectivity coefficients of feeding: zooplankton grazing. Ecol. Model. 7, 135–149. ( 10.1016/0304-3800(79)90004-8) [DOI] [Google Scholar]
- 39.Feinsinger P, Spears EE, Poole RW. 1981. A simple measure of niche breadth. Ecology 62, 27–32. ( 10.2307/1936664) [DOI] [Google Scholar]
- 40.Fowler AJ, Doherty PJ. 1992. Validation of annual growth increments in the otoliths of two species of damselfish from the southern Great Barrier Reef. Mar. Freshw. Res. 43, 1057–1068. ( 10.1071/MF9921057) [DOI] [Google Scholar]
- 41.Burnham KP, Anderson DR. 2003. Model selection and multimodel inference: a practical information-theoretic approach. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 42.Wood S. 2015. gamm4: generalized additive mixed models using mgcv and lme4. R package version 1.8.6.
- 43.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-7. This Comput. Program R Package URL Package HttpCRAN R-Proj. Orgpackage Lme4.
- 44.Beck MW, et al. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51, 633–641. ( 10.1641/0006-3568(2001)051%5B0633:TICAMO%5D2.0.CO;2) [DOI] [Google Scholar]
- 45.Munday PL, Jones GP, Caley MJ. 1997. Habitat specialisation and the distribution and abundance of coral-dwelling gobies. Mar. Ecol. Prog. Ser. 152, 227–239. ( 10.3354/meps152227) [DOI] [Google Scholar]
- 46.Holbrook SJ, Forrester GE, Schmitt RJ. 2000. Spatial patterns in abundance of a damselfish reflect availability of suitable habitat. Oecologia 122, 109–120. ( 10.1007/PL00008826) [DOI] [PubMed] [Google Scholar]
- 47.Depczynski M, et al. 2013. Bleaching, coral mortality and subsequent survivorship on a West Australian fringing reef. Coral Reefs 32, 233–238. ( 10.1007/s00338-012-0974-0) [DOI] [Google Scholar]
- 48.Coker DJ, Wilson SK, Pratchett MS. 2014. Importance of live coral habitat for reef fishes. Rev. Fish Biol. Fish. 24, 89–126. ( 10.1007/s11160-013-9319-5) [DOI] [Google Scholar]
- 49.Dahlgren C, Kellison G, Adams A, Gillanders B, Kendall M, Layman C, Ley J, Nagelkerken I, Serafy J. 2006. Marine nurseries and effective juvenile habitats: concepts and applications. Mar. Ecol. Prog. Ser. 312, 291–295. ( 10.3354/meps312291) [DOI] [Google Scholar]
- 50.Almany GR. 2004. Differential effects of habitat complexity, predators and competitors on abundance of juvenile and adult coral reef fishes. Oecologia 141, 105–113. ( 10.1007/s00442-004-1617-0) [DOI] [PubMed] [Google Scholar]
- 51.Almany GR, Webster MS. 2006. The predation gauntlet: early post-settlement mortality in reef fishes. Coral Reefs 25, 19–22. ( 10.1007/s00338-005-0044-y) [DOI] [Google Scholar]
- 52.Holmes TH, Wilson SK, Vanderklift M, Babcock R, Fraser M. 2012. The role of Thalassoma lunare as a predator of juvenile fish on a sub-tropical coral reef. Coral Reefs 31, 1113–1123. ( 10.1007/s00338-012-0934-8) [DOI] [Google Scholar]
- 53.Tolimieri N. 2015. Density dependence and independence and the population dynamics of coral reef fishes. In Ecology of fishes on coral reefs (ed. Mora C.), pp. 227–235. Cambridge, UK: Cambridge University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are stored by the Department of Parks and Wildlife Western Australia and can be accessed by contacting the lead author or MarineDataRequests@dec.wa.gov.au.