Abstract
Several studies have shown associations between shorter telomere length in blood and weakened immune function, susceptibility to infections, and increased risk of morbidity and mortality. Recently, we have shown that malaria accelerates telomere attrition in blood cells and shortens lifespan in birds. However, the impact of infections on telomere attrition in different body tissues within an individual is unknown. Here, we tested whether malarial infection leads to parallel telomere shortening in blood and tissue samples from different organs. We experimentally infected siskins (Spinus spinus) with the avian malaria parasite Plasmodium ashfordi, and used real-time quantitative polymerase chain reaction (PCR) to measure telomere length in control and experimentally infected siskins. We found that experimentally infected birds showed faster telomere attrition in blood over the course of infection compared with control individuals (repeatedly measured over 105 days post-infection (DPI)). Shorter telomeres were also found in the tissue of all six major organs investigated (liver, lungs, spleen, heart, kidney, and brain) in infected birds compared with controls at 105 DPI. To the best of our knowledge, this is the first study showing that an infectious disease results in synchronous telomere shortening in the blood and tissue cells of internal organs within individuals, implying that the infection induces systemic stress. Our results have far-reaching implications for understanding how the short-term effects of an infection can translate into long-term costs, such as organ dysfunction, degenerative diseases, and ageing.
Keywords: malaria, cost of infection, telomere length, Spinus spinus, systemic stress, ageing
1. Introduction
Telomere length has been proposed to be involved in organismal health and ageing [1–3]. Several lines of evidence suggest that loss of genome integrity and accumulation of DNA damage, including telomere shortening, are important factors involved in tissue degeneration during ageing [4]. Telomeres comprise nucleoprotein complexes that cap and protect the ends of chromosomes. The DNA component of telomeres consists of tandem repeats of (TTTAAG)n that shorten as a function of replication in most human somatic cells as they age [5]. Thus, telomere length is an indicator of the replicative age of a cell. Only in germ cells, stem cells, and white blood cells, telomere attrition is counteracted by the action of the enzyme telomerase. However, persistent chronic stress owing to intrinsic and extrinsic factors (e.g. chronic and inflammatory diseases) inhibits telomerase activity [6–8].
Gradual telomere loss appears to be a normal part of the ageing process in many species. However, accelerated telomere attrition is a feature of mammalian pathology [9], including cancer [4], immune dysfunction [10], stroke [11], cardiovascular disease [12], and diabetes [13]. Recent studies have also reported that infections may increase the rate of telomere attrition in mice [14] and birds [1]. In addition, there is compelling evidence for connections between telomere shortening and some degenerative diseases [15,16], whereas telomere lengthening reverses tissue degeneration [17]. It is, however, difficult to determine causality between disease and telomere length, and studies of infections have the potential to reveal the effect of specific exposures/events on telomere dynamics.
Some studies of humans have provided preliminary evidence that infection may enhance allostatic load (the wear and tear on the body that increases over the time an individual is exposed to repeated or chronic stress) and reduce physiological function as a consequence of accelerated biological ageing [18,19]. One important aspect is to reveal whether the telomere shortening effect of infections is manifested not only in blood cells, but also in other tissue cells throughout the body. If there is a generally increased rate of telomere attrition that affects the cells in several organs (see also [20]), this will be a strong indication that even limited short-term costs induced by an infectious disease can translate into long-term costs (see discussion in [21]). So far, studies investigating the association between infectious disease and telomere attrition have in most cases mainly examined blood cells [1,9–12,14]. As blood cells are rapidly regenerated during an infection (not least in malaria), these cells may be more sensitive to a direct effect of the disease on telomere length and may not represent what is going on in other body tissues. Thus, it is still largely unknown how a disease may affect the rate of telomere attrition in other body tissues, including major organs within an individual.
In this study, we investigated whether an infectious disease (avian malaria) affects the rate of telomere attrition in blood cells as well as the cells of six internal organs (liver, lungs, heart, kidney, spleen, and brain). We experimentally infected siskins (Spinus spinus), a small passerine bird in the finch family, with the avian malaria parasite Plasmodium ashfordi (mitochondrial lineage GRW2), using methods described elsewhere [22]. Malaria is a disease, caused by protozoan parasites, which has been linked with negative consequences on host fitness [1,23–25]. The parasite can cause mortality in birds during the short acute stage of the infection [26,27]; however, most birds that survive the acute stage develop long-lasting low-level chronic infections [27,28]. Therefore, this host–parasite system makes it suitable for studies aiming to understand the links between chronic infections and systemic stress via accelerated telomere attrition.
2. Methods
(a). Experimental set-up
The experimental part of this study was carried out at the Biological Station of the Zoological Institute of the Russian Academy of Sciences on the Curonian Spit in the Baltic Sea (55°05′N, 20°44′E) in 2012. All birds were caught with mist nets and large ‘Rybachy’-type traps.
All experimental and control birds were juveniles; they were initially screened to ensure they were uninfected with haemosporidian parasites, both by microscopic examination of blood films and polymerase chain reaction (PCR)-based analysis of blood samples [29] before experimental infection. In total, 44 siskins (22 control and 22 experimental birds) were included in this experiment. Experimental siskins (12 males and 10 females) were inoculated with avian red blood cells containing a single infection of P. ashfordi (GRW2) following the protocol described elsewhere [22]. This parasite was originally isolated from a common cuckoo (Cuculus canorus) in 2011 and frozen in liquid nitrogen as described by Palinauskas et al. [30]. The strain was multiplied in common crossbills (Loxia curvirostra), which were used as donors of infected red blood cells. Control siskins (15 males and seven females) were inoculated with uninfected red blood cells from common crossbills at the same time as experimental birds.
The freshly prepared mixture of infected blood was inoculated by injecting it into the pectoral muscles of recipient birds. We ran the experiment over 105 days without any antimalarial treatment. Blood from all birds was sampled every 5th day until 31 days post-infection (DPI) and then at 105 DPI. At each sampling occasion, about 30 µl of whole blood was taken in heparinized microcapillaries by puncturing the brachial vein and stored in SET buffer (containing sodium chloride and sodium dodecyl sulfate) and kept frozen at −80°C for future molecular analysis. After the acute phase of infection (when birds reached the peak parasite intensity levels; figure 1), birds usually developed a chronic low-level infection [28] with some variation between the individuals [31]. Parasitaemia (parasite density) was monitored by microscopy and the highest parasitaemia of the experimental birds reached levels where 50–80% of the red blood cells contained parasites (figure 1), whereas none of the control birds developed malaria infection. Body mass and haematocrit values were measured at the time of inoculation. Five of the infected birds (three males and two females) died during the course of infection and the remaining 17 birds were followed until they reached the chronic phase of infection (105 days). Neither blood telomere length (at the time of inoculation) nor peak parasitaemia of the five birds that died differed from the rest of the infected birds (all p > 0.6). However, two birds died during peak parasitaemia, whereas three died just after.
Figure 1.
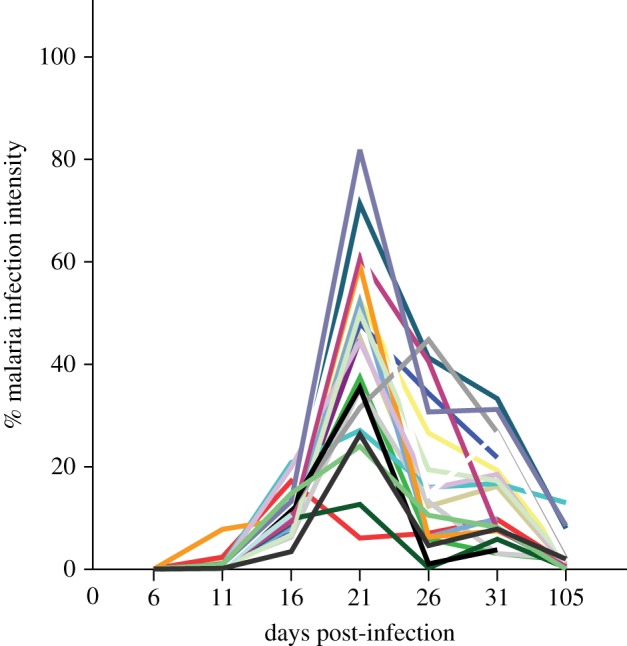
Malaria parasite intensity (Plasmodium ashfordi) over the course of infection measured by microscopy showing that individuals differ in their peak parasite intensity and that all birds had passed the peak parasite intensity (and thus the acute phase of infection) before day 105 post-inoculation. Each line represents one individual of the experimentally infected birds. (Online version in colour.)
On 105 DPI, six experimental and seven control siskins were decapitated and dissected. The brain, heart, liver, lungs, kidney, and spleen were isolated, and a part of each organ was fixed with absolute ethanol for telomere analysis. Ethanol-fixed samples were kept frozen and sent to Lund on dry ice together with blood samples where they were kept at −80°C until DNA extraction.
(b). Molecular analyses
DNA was extracted using standard phenol/chloroform methods [32] from blood and body tissues and diluted to a concentration of 1 ng µl−1. Real-time quantitative PCRs (qPCRs) were performed on an Mx3000P q-PCR system (Stratagene) to quantify telomere length as described in [33], using the primers tel1b and tel2b [33,34]. To obtain an accurate measurement of the total DNA content in the samples, a second reaction was carried out with host-specific primers sfsr/3Fb and sfsr/3Rb [31,35] that amplify a single-copy nuclear sequence (114 bp long), which is ultra-conserved across vertebrates [36]. Each reaction of 25 µl included 5 µl DNA (1 ng µl−1), 12.5 µl Supermix (Platinum SYBR-green q-PCR SuperMix-UDG, Invitrogen), 0.1 µl ROX, 1 µl (10 µM) of the sfsr/3 primers, 0.3 µl (10 µM) of the tel primers, and ddH2O. We ran 30 thermal cycles for telomere measurement and 40 thermal cycles for total DNA content. Before thermal cycling the samples were incubated at 50°C for 2 min and 95°C for 10 min, followed by thermal cycling (95°C for 15 s, then sfsr/3 58°C for 30 s; tel 59°C for 30 s and finally, 72°C for 30 s). Each DNA sample was run in duplicate, and a reference sample (one siskin sample of 1 ng µl−1) was also run in duplicate on each plate as a golden sample to control interplate variability [33,34]. Standard curves were produced by diluting one random siskin DNA sample with ddH2O in a five-step dilution series (25, 5, 1, 0.2, and 0.04 ng).
We discarded and re-ran qPCR plates that produced standard curves which were outside 85–115% qPCR efficiency. We ran the telomere repeat and single-copy nuclear sequence primers on separate plates. To control for variation between plates, we first adjusted both the telomere measurement and the total DNA content measurement by dividing them by the values obtained from the ‘golden sample’ run on each plate. We then calculated a relative telomere length (T/S ratio) value, by dividing the (plate-adjusted) qPCR value for the telomere length (T) with the (plate-adjusted) qPCR value for the single-copy nuclear sequence (S) following the method described in [33]. Our method showed very high within-plate repeatability, intraclass correlation (ICC = 0.98) as well as between plate repeatability (ICC = 0.97) for both telomere and single-copy gene measurements. Furthermore, repeatability of the final telomere length (T/S ratio) measurement was also very high (ICC = 0.97).
(c). Statistics
Statistical analyses were performed using R v. 3.1.3. We ran linear-mixed-effects models (LME) fitted by maximum-likelihood (package nlme) to investigate the effect of malaria infection on telomere length in blood cells. We included telomere length as a response variable, sex and malaria infection status as fixed factors, body mass, haematocrit value (at time of inoculation), and sampling time (DPI) as covariates, and bird ID as a random factor in the model with all two-way interactions. The non-significant interactions were eliminated from the model (using stepwise backward elimination). As there was a significant interaction between malaria infection status and DPI affecting telomere length (table 1), we ran separate LME (as described above) for both experimental and control birds. A Pearson correlation test was used to analyse whether peak parasite intensity during the acute phase correlated with the telomere loss in blood over 105 DPI. Peak parasite intensity (% infected erythrocytes) and degree of telomere loss (% telomere length loss compared with pre-infection) were logit transformed before being included in the correlation test.
Table 1.
Factors influencing telomere length in blood samples of control and experimentally infected siskins (Spinus spinus) measured over the course of the malarial infection. The linear-mixed-effect model included; sex and malaria infection status as fixed factors, body mass, haematocrit value at time of infection, and days post-infection (DPI) as covariates, and bird ID as a random factor in the model with all two-way interactions. Non-significant interactions were excluded from the model (using stepwise backward elimination).
| source | estimate | standard error (s.e.) | d.f. | F | p |
|---|---|---|---|---|---|
| response variable: telomere length | |||||
| sex | 0.012 | 0.082 | 1,22 | 1.76 | 0.200 |
| body mass | 1.521 | 1.167 | 1,22 | 6.85 | 0.016 |
| haematocrit value | 0.651 | 1.554 | 1,22 | 0.002 | 0.964 |
| DPI | 0.001 | 0.001 | 1,184 | 2.27 | 0.133 |
| malaria infection | 0.003 | 0.087 | 1,184 | 3.65 | 0.069 |
| malaria infection × DPI | −0.006 | 0.001 | 1,184 | 12.66 | 0.0005 |
We used the LME to investigate the effect of malaria infection on telomere length (at 105 DPI) in different tissues within individuals comparing infected and control individuals (fitting tissue telomere length as the response variable, sex, malaria infection status, and tissue as fixed factors, and bird ID as a random factor in the model with all two-way interactions). The non-significant interactions were eliminated from the model (using stepwise backward elimination). For detailed analysis of each tissue separately, we conducted t-tests to compare telomere length between infected and control individuals. We used Pearson correlations to obtain a matrix of all pairwise correlations between the telomere lengths of blood and the six vital organs, including 13 birds (seven control and six experimental birds) that were dissected at 105 DPI.
3. Results
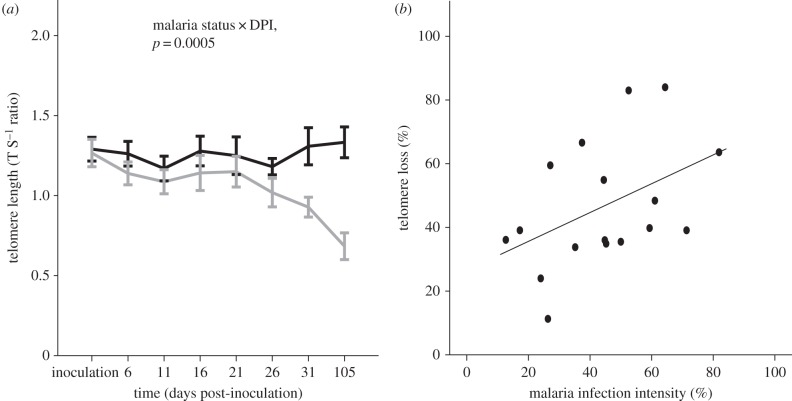
Experimental individuals that survived the experiment (n = 17) exhibited a greater degree of telomere shortening in blood cells compared with control individuals, in which telomere length remained unaffected over the 105 day observation period (n = 22; figure 2a). This was confirmed by the interaction between the variables malaria infection × DPI significantly explaining variation in blood cell telomere shortening (LME, F1,184 = 12.6, p = 0.0005, table 1 and figure 2a). Infected individuals experienced accelerated blood cell telomere shortening over 105 DPI (LME, F1,138 = 40.18, p < 0.0001, figure 2a), with no effect of sex (LME, F1,20 = 0.75, p = 0.40) or the interaction sex × DPI (LME, F1,138 = 0.07, p = 0.78). In contrast, control birds showed no significant change in blood cell telomere length over 105 days post-inoculation (LME, F1,152 = 0.78, p = 0.38) with no effect of sex or the interaction sex × DPI (all p > 0.63). There was a tendency for a positive correlation between acute phase peak parasite intensity and percentage telomere loss over 105 DPI (r = 0.46, p = 0.059, n = 17, figure 2b).
Figure 2.
(a) Telomere length in blood cells of control (n = 22) and malaria-infected (n = 17) siskins Spinus spinus. Infected birds (grey line) showed a higher rate of telomere attrition compared with control birds (black line), as there was a significant interaction of infection × DPI (LME, F1,178 = 12.6, p = 0.0005), (b) there was also a non-significant tendency of a positive relationship between malaria infection intensity and telomere loss in blood over 105 days post-infection (r = 0.46, p = 0.059, n = 17).
We then investigated the effects of malarial infection on telomere length in tissue samples from six major organs (brain, heart, liver, lungs, kidney, and spleen) in birds sacrificed at the end of the experiment (105 DPI). There was a significant effect of malaria on tissue telomere length (LME, F1,76 = 21.9, p < 0.0001, figure 3 and table 2) when adjusting for type of organ tissue (p < 0.0001), with no effect of sex (p = 0.160). When analysing the different organs separately, we found that the mean telomere length of the six organs investigated was 45–56% shorter in experimental when compared with control birds at 105 DPI. In blood cells, the mean telomere length of experimental birds at 105 DPI was 41% shorter in infected when compared with control birds. Infected individuals had significantly shorter telomeres than uninfected controls at 105 DPI in all body tissues investigated (blood, t1,10.4 = 2.9, p = 0.015; liver, t1,8.5 = 6.930, p = 0.0001; lung, t1,11 = 4.01, p = 0.002; heart, t1,8.8 = 5.02, p = 0.001; kidney, t1,11 = 2.38, p = 0.036; spleen, t1,11 = 4.01, p = 0.002; brain, t1,11 = 3.7, p = 0.003; figure 3), and this was also true if we applied a sequential Bonferroni correction to control for multiple testing.
Figure 3.
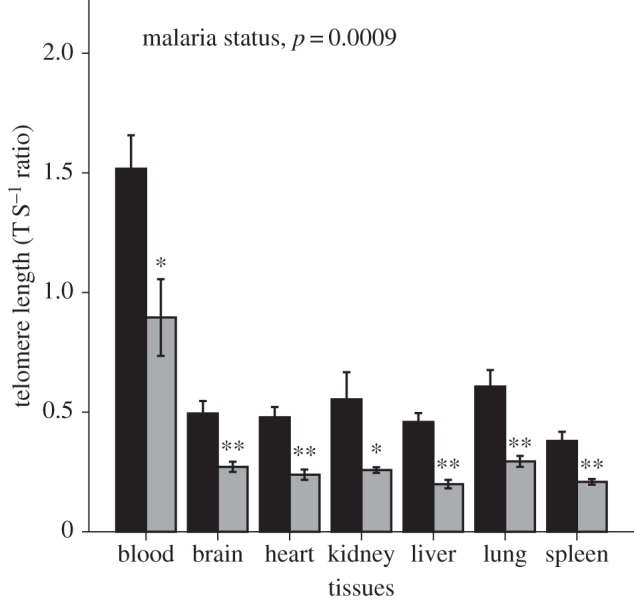
Effects of malaria infection in the birds sacrificed at 105 days post-infection (DPI; controls n = 7; infected n = 6) on telomere length measured in blood and tissue samples of six major organs (liver, lungs, heart, kidney, spleen, and brain). At 105 DPI, the experimentally infected birds (grey bars) showed shorter telomere length than control birds (black bars) in blood as well as all different organ tissues (glm, F1,74 = 23.5, p < 0.0001).
Table 2.
Factors predicting telomere length in blood and different body tissues at 105 days post-infection in siskins, where birds were either controls or experimentally infected with avian malaria. A linear-mixed model was used to investigate the effect of malaria infection on telomere length (at 105 DPI) in different tissues within an individual (fitting tissue telomere length as the response variable), sex, malaria infection status, and tissue as fixed factors, and bird ID as a random factor in the model with all 2-way interactions. Non-significant interactions were excluded from the model (using stepwise backward elimination).
| source | estimate | s.e. | d.f. | F | p |
|---|---|---|---|---|---|
| response variable: tissues telomere length | |||||
| sex | 0.69 | 0.063 | 1,76 | 2.02 | 0.160 |
| malaria infection | −0.030 | 0.063 | 1,76 | 21.86 | <0.0001 |
| tissues | −0.095 | 0.016 | 1,76 | 36.22 | <0.0001 |
We then compared telomere lengths between tissue samples of different organs and blood of an individual, in correlations across individuals. In these pairwise analyses, we found that telomere lengths were significantly correlated between all body tissues (r = 0.64–0.97). When comparing blood telomere length with each of the other investigated tissues, all six correlations were positive (r = 0.30–0.52), but none reached significance (electronic supplementary material, table 1 and figure S1 and S2).
4. Discussion
This experimental study of small songbirds (siskins) revealed that malarial infection results in parallel telomere shortening in blood and tissue cells of internal organs within individuals. Malarial infection increased the rate of telomere attrition in blood cells, and this pattern was mirrored in cells of all the six major organs investigated (i.e. liver, lungs, heart, kidney, spleen, and brain) as we found consistently shorter telomere length in infected versus control birds at 105 days after inoculation.
Experimental and control birds did not show any significant telomere difference at the time of inoculation in blood cells. However, malaria-infected birds showed a significantly higher rate of telomere shortening in blood cells over the experimental period and had substantially shorter telomeres (45% shorter) compared with controls at 105 DPI. A causal link between an infectious disease and telomere attrition of blood cells has previously been shown in mice (Mus musculus musculus) infected with Salmonella enterica [14] as well as in our previous study of great reed warblers (Acrocephalus arundinaceus) infected with avian malaria P. ashfordi (GRW2) [1]. Hence, there seems to be a consistent pattern of a faster rate of telomere shortening in blood cells subsequent to infection in all these three studies, despite differences in aetiology of infection as well as parasite and host species involved. These data are congruent with some findings in humans where human immunodeficiency virus (HIV) and hepatitis C (HCV) infections have been found to accelerate biological ageing in blood cells, as indicated by the ageing markers, telomere length, and CDKN2A [18,19].
Our data on accelerated telomere shortening in the blood of infected individuals are of particular interest in relation to malaria infection, because the malaria parasites infect red blood cells and might therefore induce high cell stress as well as rapid proliferation of new red and white blood cells from stem cells in the bone marrow [37]. A recent study using the same experimentally infected and control siskin individuals [38], used blood transcriptome analysis to show that telomerase expression is reduced in infected individuals at peak parasitaemia (i.e. day 21 post-infection, figure 1). Interestingly, our data show that infected birds experienced rapid telomere loss from just after peak parasitaemia in blood cells (day 21, figure 2a), implying that malaria infection can inhibit telomerase activity and that this has negative consequences through increasing the rate of telomere attrition.
Owing to the specific negative effects of malaria infection on blood cells (including red blood cells that are nucleated in birds), a higher rate of telomere attrition in blood samples may, however, not necessarily be reflected in cells of other organs and thus have limited consequences for the general physiology of the malaria-infected individual. In this study, we demonstrated that the cells of six internal organs (beside blood cells) show significantly shorter telomeres in malaria-infected individuals, at a magnitude of 45–56% shorter telomere lengths than in control birds at 105 DPI, suggesting that infected birds were exposed to systemic stress (an inflammatory state affecting the whole body). This finding suggests that DNA damage of permanent cells, possibly caused by reactive oxygen species (ROS) activity of the immune system, can reduce the telomere lengths in tissue not directly affected by the infecting pathogen. Another possible mechanism behind telomere length shortening in different tissues could be an inadequate supply of oxygen to organ tissues owing to the formation of phanerozoites in endothelial cells of capillaries that block the blood supply [39]. This may then lead to anaerobic conditions and impaired metabolism, including acidosis in organ tissues and might accelerate telomere shortening. Irrespective of the mechanism, our results suggest that the infection has increased the allostatic load and accelerated biological ageing in cells throughout the body.
This type of experimental study cannot be carried out in humans. Yet, an increasing number of observational studies in humans have found a pattern of shorter telomeres in cells of dysfunctional organs [15,17]. The interpretations of these studies are however limited by the fact that it is not possible to know to what extent organ dysfunction is caused by short telomeres or if short telomeres mainly reflect cell stress and oxidative damage in organs that are dysfunctional for other reasons. In this respect, our study is important as it points at a causal relationship between infectious disease and telomere shortening in several organs of the body, and this may help us to understand the negative long-term effects of seemingly mild infectious diseases as well as degenerative organ-related diseases in humans and other animals.
Our results suggest that telomere length, and thus telomere attrition, is synchronized among different organs within an individual, as we found a significant positive correlation between the telomere length of six different tissues within an individual. However, blood telomere length and telomere length of the different tissues were not significantly correlated, although all showed positive relationships (r = 0.30–0.52). The telomere length of blood cells was considerably longer than in any of the other investigated organ tissues (figure 3). These results are in agreement with a previous study that also found longer telomeres in blood cells than in cells of different organ tissues within individuals [20], although note that the previous study did not analyse telomere lengths in relation to any effects of disease. Longer telomeres in blood cells could be due to longer telomere length in hematopoietic stem cells or better telomere maintenance in blood cells owing to telomerase activity.
One potential issue with our study could be that the observed correlation between blood cells and different organ tissue telomere lengths arises owing to the presence of blood in the organ tissues. However, the marked difference (figure 3) between telomere lengths of blood cells and different organ tissues strongly implies that if there is any contribution from blood cells on the telomere lengths measured in the organ tissues it must be very limited, and thus most of the contribution to the measured telomere lengths must come from the organ tissue cells. This is further strengthened by the fact that we found no significant positive correlation between individuals' telomere length measures of blood cells and any of the different organ tissues.
We know of no previous study that has investigated the effect of experimental infection on telomere lengths in cells of various tissues beside blood cells. These results suggest that infectious diseases might have a general effect on the body, a finding that has far-reaching implications for understanding how short-term effects of an infection can translate into long-term costs, such as organ dysfunction, degenerative diseases, and ageing. There is now a need for more studies of other host–pathogen systems to reveal to what extent this pathogen-induced telomere attrition throughout the body tissues is a general pattern, as well as for carrying out detailed (physiological) studies on the mechanisms behind this whole-body effect in the host when contracting an infection.
Supplementary Material
Acknowledgements
We thank the staff of the Biological Station ‘Rybachy’, for assistance in the field. The director of the Biological Station ‘Rybachy’, Casimir V. Bolshakov, is acknowledged for generously providing facilities for the experimental research.
Ethics
Experimental procedures of this study were approved by the International Research Cooperation Agreement between the Biological Station Rybachy of the Zoological Institute of the Russian Academy of Sciences and Institute of Ecology of Nature Research Centre (25 May 2010). All efforts were made to minimize handling time and potential suffering of birds. None of the experimental birds suffered apparent injury during experiments.
Data accessibility
Data are available from Dryad: http://dx.doi.org/10.5061/dryad.3477v.
Authors' contributions
Conceived and designed the study: M.A., V.P., G.V., S.B., and DH. Experiment and data collection: V.P., A.M., and E.P. Analysed the data: M.A., V.P., and NZ. Statistical analyses: M.A. Wrote the paper: M.A., V.P., G.V., A.M., E.P., N.Z., A.F., S.B., and D.H.
Competing interests
We have no competing interests.
Funding
This work was supported by grants from the Swedish Society for Medical Research (SSMF) to M.A. Swedish Research Council to D.H. (VR: 621-2013-4357), S.B. (VR: 621-2013-4839) and partly by CAnMove (a Linnaeus research excellence project funded by the Swedish Research Council and Lund University, VR: 349-2007-8690). Global Grant to G.V. (VPI-3.1.-ŠMM-07-K-01-047). A.M. and E.P. were supported by Russian Foundation for Basic Research (grant no. 15-04-00417) and by the Zoological Institute RAS (registered research project no. 01201351182).
References
- 1.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 2.Blasco MA. 2007. Telomere length, stem cells and aging. Nat. Chem. Biol. 3, 640–649. ( 10.1038/nchembio.2007.38) [DOI] [PubMed] [Google Scholar]
- 3.Campisi J. 2001. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11, S27–S31. ( 10.1016/S0962-8924(01)82148-6) [DOI] [PubMed] [Google Scholar]
- 4.Hoeijmakers JHJ. 2009. Molecular origins of cancer DNA damage, aging, and cancer. New Engl. J. Med. 361, 1475–1485. ( 10.1056/NEJMra0804615) [DOI] [PubMed] [Google Scholar]
- 5.Blackburn EH. 2010. Telomeres and telomerase: the means to the end (Nobel Lecture). Angew. Chem. 49, 7405–7421. ( 10.1002/anie.201002387) [DOI] [PubMed] [Google Scholar]
- 6.Choi J, Fauce SR, Effros RB. 2008. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain, Behav. Immun. 22, 600–605. ( 10.1016/j.bbi.2007.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. 2004. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17 312–17 315. ( 10.1073/pnas.0407162101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiels PG, Ritzau-Reid K. 2015. Biological ageing, inflammation and nutrition: how might they impact on systemic sclerosis? Curr. Aging Sci. 8, 123–130. ( 10.2174/187460980801150727110353) [DOI] [PubMed] [Google Scholar]
- 9.Atzmon G, et al. 2010. Evolution in health and medicine Sackler colloquium: genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc. Natl Acad. Sci. USA 107 (Suppl 1), 1710–1717. ( 10.1073/pnas.0906191106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaszubowska L. 2008. Telomere shortening and ageing of the immune system. J. Physiol. Pharmacol. 59 (Suppl 9), 169–186. [PubMed] [Google Scholar]
- 11.Jiang X, Dong M, Cheng J, Huang S, He Y, Ma K, Tang B, Guo Y. 2013. Decreased leukocyte telomere length (LTL) is associated with stroke but unlikely to be causative. PLoS ONE 8, e68254 ( 10.1371/journal.pone.0068254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. 2007. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 369, 107–114. ( 10.1016/S0140-6736(07)60071-3) [DOI] [PubMed] [Google Scholar]
- 13.Tamura Y, et al. 2014. beta-cell telomere attrition in diabetes: inverse correlation between HbA1c and telomere length. J. Clin. Endocrinol. Metab. 99, 2771–2777. ( 10.1210/jc.2014-1222) [DOI] [PubMed] [Google Scholar]
- 14.Ilmonen P, Kotrschal A, Penn DJ. 2008. Telomere attrition due to infection. PLoS ONE 3, e2143 ( 10.1371/journal.pone.0002143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahin E, et al. 2011. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365. ( 10.1038/nature09787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin E, DePinho RA. 2010. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528. ( 10.1038/nature08982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaskelioff M, et al. 2011. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–U1700. ( 10.1038/nature09603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pathai S, et al. 2013. Accelerated biological ageing in HIV-infected individuals in South Africa: a case–control study. AIDS 27, 2375–2384. ( 10.1097/QAD.0b013e328363bf7f) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson MW, McGuinness D, Swann R, Barclay S, Mills PR, Patel AH, McLauchlan J, Shiels PG. 2013. Non cell autonomous upregulation of CDKN2 transcription linked to progression of chronic hepatitis C disease. Aging Cell 12, 1141–1143. ( 10.1111/acel.12125) [DOI] [PubMed] [Google Scholar]
- 20.Reichert S, Criscuolo F, Verinaud E, Zahn S, Massemin S. 2013. Telomere length correlations among somatic tissues in adult zebra finches. PLoS ONE 8, e81496 ( 10.1371/journal.pone.0081496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasselquist D, Nilsson J-A. 2012. Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds? Anim. Behav. 83, 1303–1312. ( 10.1016/j.anbehav.2012.03.025) [DOI] [Google Scholar]
- 22.Palinauskas V, Valkiunas G, Bolshakov CV, Bensch S. 2011. Plasmodium relictum (lineage SGS1) and Plasmodium ashfordi (lineage GRW2): the effects of the co-infection on experimentally infected passerine birds. Exp. Parasitol. 127, 527–533. ( 10.1016/j.exppara.2010.10.007) [DOI] [PubMed] [Google Scholar]
- 23.LaPointe DA, Atkinson CT, Samuel MD. 2012. Ecology and conservation biology of avian malaria. Year Ecol. Conserv. Biol. 1249, 211–226. ( 10.1111/j.1749-6632.2011.06431.x) [DOI] [PubMed] [Google Scholar]
- 24.Marzal A, Asghar M, Rodriguez L, Reviriego M, Hermosell IG, Balbontin J, Garcia-Longoria L, de Lope F, Bensch S et al. . 2013. Co-infections by malaria parasites decrease feather growth but not feather quality in house martin. J. Avian Biol. 44, 437–444. ( 10.1111/j.1600-048x.2013.00178.x) [DOI] [Google Scholar]
- 25.Marzal A, de Lope F, Navarro C, Moller AP. 2005. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142, 541–545. ( 10.1007/s00442-004-1757-2) [DOI] [PubMed] [Google Scholar]
- 26.Atkinson CT, Dusek RJ, Woods KL, Iko WM. 2000. Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. J. Wildl. Dis. 36, 197–204. ( 10.7589/0090-3558-36.2.197) [DOI] [PubMed] [Google Scholar]
- 27.Valkiūnas G. 2005. Avian malaria parasites and other haemosporidia. Boca Raton, FL: CRC Press. [Google Scholar]
- 28.Asghar M, Westerdahl H, Zehtindjiev P, Ilieva M, Hasselquist D, Bensch S. 2012. Primary peak and chronic malaria infection levels are correlated in experimentally infected great reed warblers. Parasitology 139, 1246–1252. ( 10.1017/S0031182012000510) [DOI] [PubMed] [Google Scholar]
- 29.Hellgren O, Waldenstrom J, Bensch S. 2004. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 90, 797–802. ( 10.1645/GE-184R1) [DOI] [PubMed] [Google Scholar]
- 30.Palinauskas V, Žiegytė R, Ilgūnas M, Iezhova TA, Bernotienė R, Bolshakov C, Valkiūnas G. 2015. Description of the first cryptic avian malaria parasite, Plasmodium homocircumflexum n. sp., with experimental data on its virulence and development in avian hosts and mosquitoes. Int. J. Parasitol. 45, 51–62. ( 10.1016/j.ijpara.2014.08.012) [DOI] [PubMed] [Google Scholar]
- 31.Asghar M, Hasselquist D, Bensch S. 2011. Are chronic avian haemosporidian infections costly in wild birds? J. Avian Biol. 42, 530–537. ( 10.1111/j.1600-048X.2011.05281.x) [DOI] [Google Scholar]
- 32.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning, a labratory manuel. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 33.Asghar M, Bensch S, Tarka M, Hansson B, Hasselquist D. 2015. Maternal and genetic factors determine early life telomere length. Proc. R. Soc. B 282, 20142263 ( 10.1098/rspb.2014.2263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG, Griffiths K, Gault EA, Monaghan P. 2009. Real-time quantitative PCR assay for measurement of avian telomeres. J. Avian Biol. 40, 342–347. ( 10.1111/j.1600-048X.2008.04623.x) [DOI] [Google Scholar]
- 35.Westerdahl H, Asghar M, Hasselquist D, Bensch S. 2012. Quantitative disease resistance: to better understand parasite-mediated selection on major histocompatibility complex. Proc. R. Soc. B 279, 577–584. ( 10.1098/rspb.2011.0917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bejerano G, Pheasant M, Makunin I, Stephen S, Kent WJ, Mattick JS, Haussler D. 2004. Ultraconserved elements in the human genome. Science 304, 1321–1325. ( 10.1126/science.1098119) [DOI] [PubMed] [Google Scholar]
- 37.von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344. ( 10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 38.Videvall E, Cornwallis CK, Palinauskas V, Valkiunas G, Hellgren O. 2015. The avian transcriptome response to malaria infection. Mol. Biol. Evol. 32, 1255–1267. ( 10.1093/molbev/msv016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilgunas M, Bukauskaitė D, Palinauskas V, Iezhova TA, Dinhopl N, Nedorost N, Weissenbacher-Lang C, Weissenböck H, Valkiūnas G. 2016. Mortality and pathology in birds due to Plasmodium (Giovannolaia) homocircumflexum infection, with emphasis on the exoerythrocytic development of avian malaria parasites. Malar. J. 15, 256 ( 10.1186/s12936-016-1310-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from Dryad: http://dx.doi.org/10.5061/dryad.3477v.