Abstract
Stress hormones might represent a key link between individual-level infection outcome, population-level parasite transmission, and zoonotic disease risk. Although the effects of stress on immunity are well known, stress hormones could also affect host–vector interactions via modification of host behaviours or vector-feeding patterns and subsequent reproductive success. Here, we experimentally manipulated songbird stress hormones and examined subsequent feeding preferences, feeding success, and productivity of mosquito vectors in addition to defensive behaviours of hosts. Despite being more defensive, birds with elevated stress hormone concentrations were approximately twice as likely to be fed on by mosquitoes compared to control birds. Moreover, stress hormones altered the relationship between the timing of laying and clutch size in blood-fed mosquitoes. Our results suggest that host stress could affect the transmission dynamics of vector-borne parasites via multiple pathways.
Keywords: mosquito, epizootic, corticosterone, glucocorticoid, transmission
1. Introduction
Stress can have negative effects on animals including reduced growth [1,2], diminished reproductive success [3,5], impaired cognitive ability [4,6,7], altered social behaviour [8], immunosuppression, and increased susceptibility to infection [4,9,10]. Stressors—aversive environmental conditions that challenge the body's ability to maintain homeostasis—often activate neuroendocrine pathways that result in the release of molecules that help to ameliorate adversity. For example, the hypothalamus-pituitary-adrenal (HPA) axis of vertebrates mediates responses to stressors via glucocorticoid hormones [4]. Corticosterone, the major glucocorticoid stress hormone in birds and many vertebrates, coordinates emergency life-history responses to help individuals endure or recover from stressors [10,11]. Although many of the molecular and cellular mechanisms of glucocorticoid actions are understood [4], few studies have considered how stress hormone effects on individuals might influence populations [12,13].
For vector-borne parasites, corticosterone could influence epidemiological dynamics directly, by influencing host susceptibility, parasite burden and shedding rate or duration, and morbidity or mortality of hosts [14–16]. Corticosterone could also influence disease spread indirectly via effects on host contact rates, defensive behaviours, or even attractiveness to or responses towards vectors. Surprisingly though, whereas corticosterone is well known to affect many behaviours [17,18], its role in anti-vector defence has been little investigated. Moreover, the effects of corticosterone on vector-feeding choices are also, to our knowledge, unknown. Mosquitoes and other vectors use certain host traits conveyed by olfactory and visual signals to locate hosts [19], and many such traits (e.g. carbon dioxide (CO2) output, body odour, body temperature, etc.) can be affected by corticosterone. Subsequently, vectors might be able to discern a vulnerable and/or favourable host by attending to corticosterone-mediated traits. Furthermore, although glucocorticoids are not known to affect insects directly, corticosterone could impact mosquito longevity and/or reproductive output via effects relayed through host blood meals.
Our goals in this study were to investigate the effects of corticosterone on (i) vector preferences and feeding success on songbird hosts, (ii) songbird anti-vector behaviours, and (iii) vector survival and reproductive success post-choice of host. We experimentally manipulated corticosterone in adult zebra finches (Taeniopygia guttata) and used the southern house mosquito (Culex quinquefasciatus) to query vector preference, host defence, and subsequent vector productivity. We chose C. quinquefasciatus because it is a geographically widespread, often abundant, and an epidemiologically relevant vector of multiple parasites (e.g. West Nile and St Louis encephalitis virus, and multiple filarial worms and protozoa) [20].
2. Material and methods
We used laboratory-reared adult male and female zebra finches (T. guttata) and wild-caught laboratory-reared southern house mosquitoes (C. quinquefasciatus) for all our experiments. See the electronic supplementary material, Methods, for detailed information about finch and vector husbandry.
(a). Corticosterone implant surgery
Corticosterone levels were altered via implantation of either empty (controls) or one or two corticosterone-filled (Sigma Aldrich, St. Louis, MO, USA, product #27840) silastic tubules (7 mm long; inner diameter 1.5 mm, Dow Corning, Midland, MI, USA, product #508-006) according to Ouyang et al. [21]. Birds receiving either one or two implants are hereafter referred to as belonging to ‘CORT+’ and ‘CORT++’ treatments. All implants were sealed with multi-purpose silicone sealant (Dow Corning, product #732), but just before implantation, a 0.5 mm hole was made through both sides of the implant to facilitate release of the hormone [21]. Implants were administered subcutaneously on the flank of each bird while birds were under light isoflurane anaesthesia. After implantation, the less than 3 mm wound was sealed with a surgical adhesive (Vetbond, 3M, St Paul, MN, USA, product #1469). Finches were allowed to recover from surgery for 2 days before initiation of any additional procedures. See the electronic supplementary material, Methods, for assessment of implant effects on circulating CORT (i.e. validation protocol) and electronic supplementary material, figure S1 for individual concentration values.
(b). Experiment 1: mosquito preferences for avian hosts
This study occurred in November, 2014. Two days after surgery, male (n = 27) and female (n = 27) zebra finches were transferred in same-sex groups of three into 30.48 cm3 mesh cages (BioQuip, Rancho Dominguez, CA, USA, product # 1450 BSV). A clear plastic panel on one side of each cage enabled visualization of birds. All other sides of the cage were composed of fine mesh to prevent the escape of vectors. Within each cage, we housed one bird of each treatment: control, CORT+, and CORT++. We initiated the mosquito challenge 1 h before lights went off (dusk) by adding 50 blood-seeking female mosquitoes to multi-bird cages. Between the hours of 17.00 and 18.00, we dimmed lights with a single 60 W bulb to mimic lighting conditions for ideal mosquito feeding (i.e. dusk). After this time, lights went off completely, as usual. We allowed mosquitoes to feed on birds until the following dawn (06.00), at which point all birds were bled for corticosterone and DNA (for later vector–host matching procedures, see the electronic supplementary material, Methods). Mosquitoes were aspirated from each cage and pooled into a single plastic vial per cage and maintained at −80°C until processing. We matched vector blood meals with host identity by microsatellite genotyping. See the electronic supplementary material, Methods, for the genotyping protocol.
(c). Experiment 2: host behaviour and vector-feeding success
In May 2015, 3 days after surgery, male (n = 20) and female (n = 20) zebra finches were transferred singly to the screen cages described earlier. Finches were then exposed to 25 host-seeking female mosquitoes at 17.00, 1 h prior to lights off. No ‘dusk simulation’ took place for this study to allow clear video recording and behavioural scoring. This study involved two cohorts of 20 birds each. For 1 h prior to and until 1 h after mosquitoes were initially introduced into cages, all individual behaviours were recorded using a Foscam FI9821 W V2 1.0 Megapixel Wireless IP Camera (Foscam, Shenzhen, China). For each individual bird, we obtained counts of the number of times we observed a set of pre-defined behaviours, which we predicted would affect vector-feeding success, including (i) the number of hops within cages (‘hopping activity’) and (ii) the number of vector-directed behaviours, which we specifically defined as including the number of head, tail, and body shakes, feather fluffs, pecks at mosquitoes, and preening-like attempts to remove mosquitoes from the feathers or body observed in videos. After the 12 h vector challenge, all mosquitoes were aspirated from cages, and the number of total blood-fed and total unfed mosquitoes counted. See the electronic supplementary material, Methods, for further description of behavioural assessments in Experiment 2.
(d). Experiment 3: host blood-meal characteristics and vector productivity
In September 2015, 20 additional zebra finches were implanted as in previous experiments to investigate the impact of corticosterone on host physiological traits and mosquito fitness. Experimental procedures were similar to those above. Blood was sampled immediately after the vector challenge and tested for blood glucose levels (milligrams per decilitre), haematocrit (proportion of red blood cells in whole blood), body temperature (nearest 0.1°C), and body mass (nearest 0.1 g). See the electronic supplementary material, Methods, for further details of blood metrics for Experiment 3.
The first six fully blood-fed mosquitoes collected from each cage at the end of the vector challenge were individually aspirated into plastic containers (Glad, 32 oz Tupperware containers with 1.5 oz plastic cups in the bottom) that served as mosquito domiciles for the next 20 days. We monitored mosquito survival over the 20 day trial and also quantified the timing of egg laying and number of eggs laid for each individual mosquito. See the electronic supplementary material, Methods, for details about the Experiment 3 vector survival and productivity trial.
(e). Data analyses
We performed statistical analyses using R v. 3.2.3 [22]. We examined vector-feeding preferences, vector-feeding success, and host behaviours with generalized linear mixed models (lmer function, lme4 package) available in the R statistical package (v. 3.2.1; [23]) and summarized the results of models using Wald χ2 tests (‘Anova’ function in the R package ‘car’ [24]). We visualized the relationship between levels within model predictors using the R package ‘effects’ [25]. We used univariate analysis of variance to explore treatment-level differences in host physiological parameters (body mass, body temperature, blood glucose levels, and haematocrit score) and a Tukey post hoc test for multiple comparisons. We used Cox Mixed Effect Proportional Hazards Models in R [26] to examine the differences in (i) rate of survival and (ii) rate of egg laying in female mosquitoes. We used a linear mixed effect model to examine the relationship between clutch size and time to egg laying as a function of treatment with host ID as a random effect. We limited our analyses of reproductive success of mosquitoes (clutch size and day of egg laying) to one week after blood feeding, twice the length of the typical gonotrophic cycle of C. quinquefasciatus [27]; 70% of vectors collected from bird cages laid eggs within this period of time, 18% laid eggs after day 6, and 12% did not lay clutches at all or died before laying eggs. Effects of corticosterone on mosquito survival were assessed over the full 20 day trial. See the electronic supplementary material, Methods, for detailed description of data analysis for each experiment.
3. Results
(a). Vector preferences and feeding success
Corticosterone treatment affected vector choice of hosts in Experiment 1 (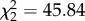 , p < 0.001); figure 1): birds with experimentally elevated corticosterone levels were almost twice as likely to be fed on by vectors compared with controls (control versus CORT+ z = 6.69, p < 0.001; control versus CORT++ z = 5.23, p < 0.001). There was no difference in choice between CORT+ and CORT++ birds (z = 1.59, p = 0.112). Though most vectors fed on a single host, some took blood meals from multiple hosts (electronic supplementary material, figure S2a,b). Similar results were observed in Experiment 2, where birds were housed alone (figure 2a): a greater number of blood-fed mosquitoes were recovered from cages of CORT-treated birds than from control bird cages (
, p < 0.001); figure 1): birds with experimentally elevated corticosterone levels were almost twice as likely to be fed on by vectors compared with controls (control versus CORT+ z = 6.69, p < 0.001; control versus CORT++ z = 5.23, p < 0.001). There was no difference in choice between CORT+ and CORT++ birds (z = 1.59, p = 0.112). Though most vectors fed on a single host, some took blood meals from multiple hosts (electronic supplementary material, figure S2a,b). Similar results were observed in Experiment 2, where birds were housed alone (figure 2a): a greater number of blood-fed mosquitoes were recovered from cages of CORT-treated birds than from control bird cages (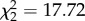 , p < 0.001; control versus CORT+ z = 4.02, p < 0.001; control versus CORT++ z = 3.50, p < 0.001; CORT+ versus CORT++ z = 0.494, p = 0.621).
, p < 0.001; control versus CORT+ z = 4.02, p < 0.001; control versus CORT++ z = 3.50, p < 0.001; CORT+ versus CORT++ z = 0.494, p = 0.621).
Figure 1.
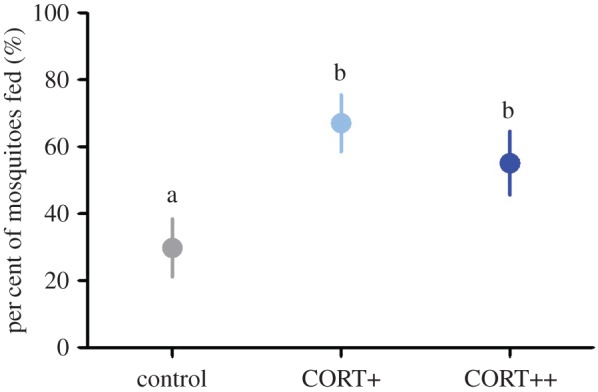
Corticosterone manipulation increased vector-feeding preference in co-housed zebra finches (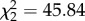 , p < 0.001). Raw average percentages displayed, statistical pairwise comparisons based on generalized linear mixed model and indicated with letters at p < 0.05 level, bars ± standard error (s.e.). (Online version in colour.)
, p < 0.001). Raw average percentages displayed, statistical pairwise comparisons based on generalized linear mixed model and indicated with letters at p < 0.05 level, bars ± standard error (s.e.). (Online version in colour.)
Figure 2.
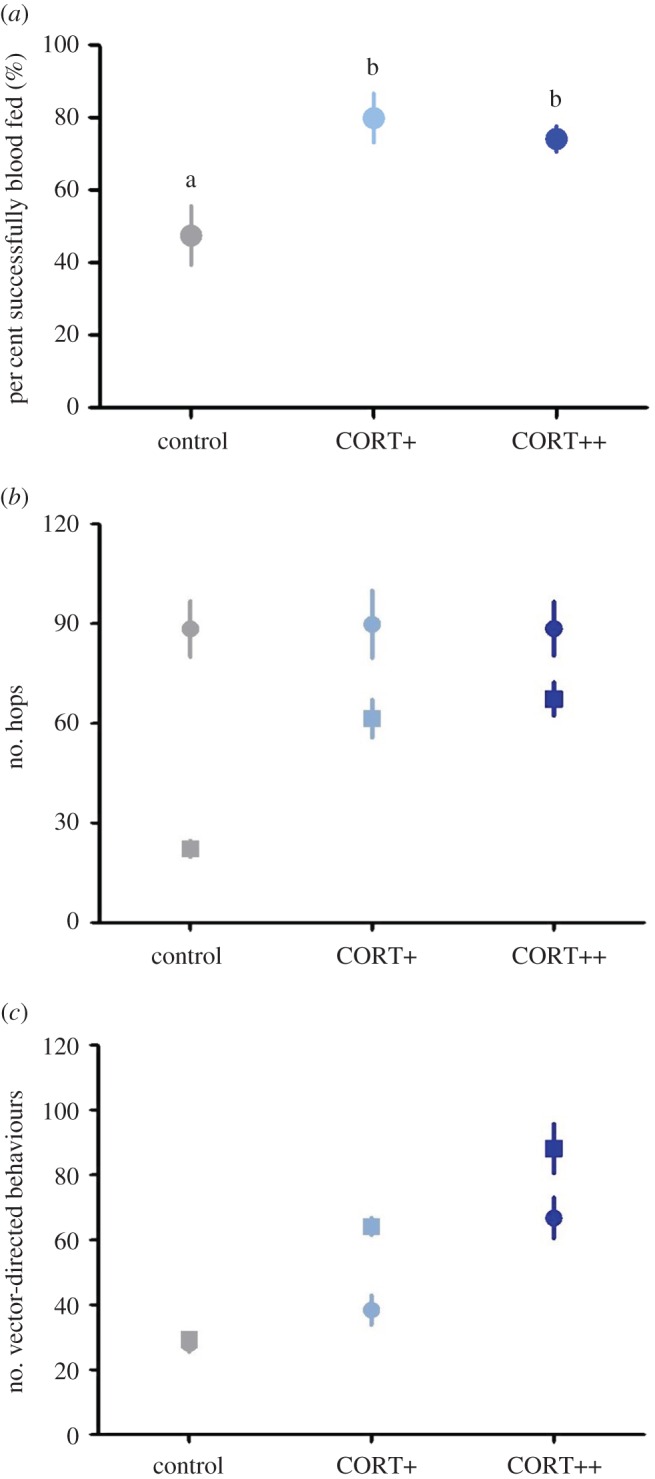
(a) Corticosterone increased vector-feeding success in singly housed zebra finches (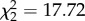 , p < 0.001). Raw average percentages displayed, statistical pairwise comparisons based on a generalized linear mixed model and indicated with letters at p < 0.05 level, bars ± s.e. (b,c) Corticosterone and time (before versus after the addition of vectors to bird cages) affected the number of hops (b) and vector-directed behaviours (c) of singly housed zebra finches (linear mixed model with Poisson distribution, treatment×time interaction for both hops and vector-directed behaviours; p < 0.0001). There was an overall treatment effect of corticosterone on the number of vector-directed behaviours (p = 0.012). Circles represent pre-mosquito-challenge behaviours of finches and squares represent behaviours observed during the mosquito challenge, when vectors were present in bird cages. Raw average values displayed (symbols), bars ± s.e. (Online version in colour.)
, p < 0.001). Raw average percentages displayed, statistical pairwise comparisons based on a generalized linear mixed model and indicated with letters at p < 0.05 level, bars ± s.e. (b,c) Corticosterone and time (before versus after the addition of vectors to bird cages) affected the number of hops (b) and vector-directed behaviours (c) of singly housed zebra finches (linear mixed model with Poisson distribution, treatment×time interaction for both hops and vector-directed behaviours; p < 0.0001). There was an overall treatment effect of corticosterone on the number of vector-directed behaviours (p = 0.012). Circles represent pre-mosquito-challenge behaviours of finches and squares represent behaviours observed during the mosquito challenge, when vectors were present in bird cages. Raw average values displayed (symbols), bars ± s.e. (Online version in colour.)
(b). Host behaviour and influence on vector-feeding success
The number of hops a bird made in the cage (i.e. hopping activity) was predicted by time (before versus after the addition of mosquitoes into cages) and the interaction between time and CORT treatment (time 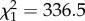 , p < 0.0001; time × treatment
, p < 0.0001; time × treatment 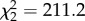 , p < 0.0001, figure 2b). But, there was no main effect of CORT on the number of hops (
, p < 0.0001, figure 2b). But, there was no main effect of CORT on the number of hops (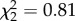 , p = 0.67). Birds tended to display the same number of hops regardless of treatment before mosquitoes were present but decreased hopping activity after vectors were added to cages (figure 2b). CORT treatment, time, and the interaction between time and CORT treatment predicted the number of vector-directed behaviours exhibited by finches (treatment
, p = 0.67). Birds tended to display the same number of hops regardless of treatment before mosquitoes were present but decreased hopping activity after vectors were added to cages (figure 2b). CORT treatment, time, and the interaction between time and CORT treatment predicted the number of vector-directed behaviours exhibited by finches (treatment 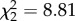 , p = 0.012, time
, p = 0.012, time 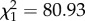 , p < 0.001, time × treatment
, p < 0.001, time × treatment 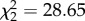 , p < 0.0001; figure 2c). Vector-directed behaviours varied across treatments before and during the vector challenge (figure 2c), and control birds displayed the same number of vector-directed behaviours before and after mosquitoes were added to cages, whereas CORT+ and CORT++ birds tended to increase the number of vector-directed behaviours they displayed during the mosquito challenge.
, p < 0.0001; figure 2c). Vector-directed behaviours varied across treatments before and during the vector challenge (figure 2c), and control birds displayed the same number of vector-directed behaviours before and after mosquitoes were added to cages, whereas CORT+ and CORT++ birds tended to increase the number of vector-directed behaviours they displayed during the mosquito challenge.
The effect of hopping activity and vector-directed behaviours on the blood-feeding success of vectors (e.g. number of blood-fed vectors recovered, Experiment 2) depended on both CORT treatment and an interaction between CORT and the behavioural metric measured. That is, there was a significant effect of the number of hops (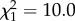 , p = 0.001), CORT treatment (
, p = 0.001), CORT treatment (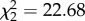 , p < 0.001), and a significant interactive effect of hops and CORT treatment (
, p < 0.001), and a significant interactive effect of hops and CORT treatment (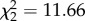 , p = 0.002) on vector-feeding success. Similarly, the number of vector-directed behaviours (
, p = 0.002) on vector-feeding success. Similarly, the number of vector-directed behaviours (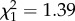 , p = 0.238), as well as CORT treatment (
, p = 0.238), as well as CORT treatment (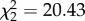 , p < 0.001) and the interaction of vector-directed behaviours and CORT (
, p < 0.001) and the interaction of vector-directed behaviours and CORT (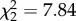 , p = 0.019) significantly predicted vector-feeding success. In both cases, the CORT+ treatment was associated with a reduction in the number of blood-fed vectors as the number of hops or vector-directed behaviours increased (electronic supplementary material, figure S3a,b). However, a reduction in blood-fed vectors with increased host activity level (measured specifically as the number of hops or vector-directed behaviours) was not observed in the CORT++ treatment (electronic supplementary material, figure S3a,b).
, p = 0.019) significantly predicted vector-feeding success. In both cases, the CORT+ treatment was associated with a reduction in the number of blood-fed vectors as the number of hops or vector-directed behaviours increased (electronic supplementary material, figure S3a,b). However, a reduction in blood-fed vectors with increased host activity level (measured specifically as the number of hops or vector-directed behaviours) was not observed in the CORT++ treatment (electronic supplementary material, figure S3a,b).
(c). Blood-meal traits and vector productivity
CORT treatment increased host blood glucose levels (F2,11 = 4.71, p = 0.033; control versus CORT+ p = 0.028; control versus CORT++ p = 0.019; electronic supplementary material, figure S4a), decreased host haematocrit (F2,15 = 3.829; p = 0.045; control versus CORT++ p = 0.014; electronic supplementary material, figure S4b), and had no effect on host body mass or host body temperature (F2,15 = 0.182; p = 0.835 and F2,15 = 0.262; p = 0.773), respectively. There was no significant effect of CORT on the rate of mosquito reproduction (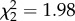 , p = 0.372; figure 3a). Mosquito clutch size was predicted by an interaction between CORT treatment and days to egg laying (
, p = 0.372; figure 3a). Mosquito clutch size was predicted by an interaction between CORT treatment and days to egg laying ( , p = 0.026; the main effects of CORT and days to egg laying were not significant,
, p = 0.026; the main effects of CORT and days to egg laying were not significant, 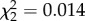 , p = 0.992 and
, p = 0.992 and 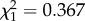 , p = 0.545, respectively). In this model, days to egg laying was treated as a continuous variable. We then repeated this analysis but treated days to egg laying as a fixed factor with three levels. Here, the interaction term was non-significant (
, p = 0.545, respectively). In this model, days to egg laying was treated as a continuous variable. We then repeated this analysis but treated days to egg laying as a fixed factor with three levels. Here, the interaction term was non-significant ( , p = 0.058) but the effect size estimates and errors around estimates of both models were similar (η2 = 0.10, CI = 0–0.24 continuous and η2 = 0.13, CI = 0–0.22 fixed [28,29]); see the electronic supplementary material, table S7a,b for further information. For our clutch size analyses, we grouped clutches on days 3 and 4 as the small number of mosquitoes laying eggs in that window might unduly have affected the analysis (via leverage); however, we present all days separately for reference (figure 3b). CORT did not affect the rate of vector survival (
, p = 0.058) but the effect size estimates and errors around estimates of both models were similar (η2 = 0.10, CI = 0–0.24 continuous and η2 = 0.13, CI = 0–0.22 fixed [28,29]); see the electronic supplementary material, table S7a,b for further information. For our clutch size analyses, we grouped clutches on days 3 and 4 as the small number of mosquitoes laying eggs in that window might unduly have affected the analysis (via leverage); however, we present all days separately for reference (figure 3b). CORT did not affect the rate of vector survival (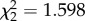 , p = 0.449; figure 3c).
, p = 0.449; figure 3c).
Figure 3.
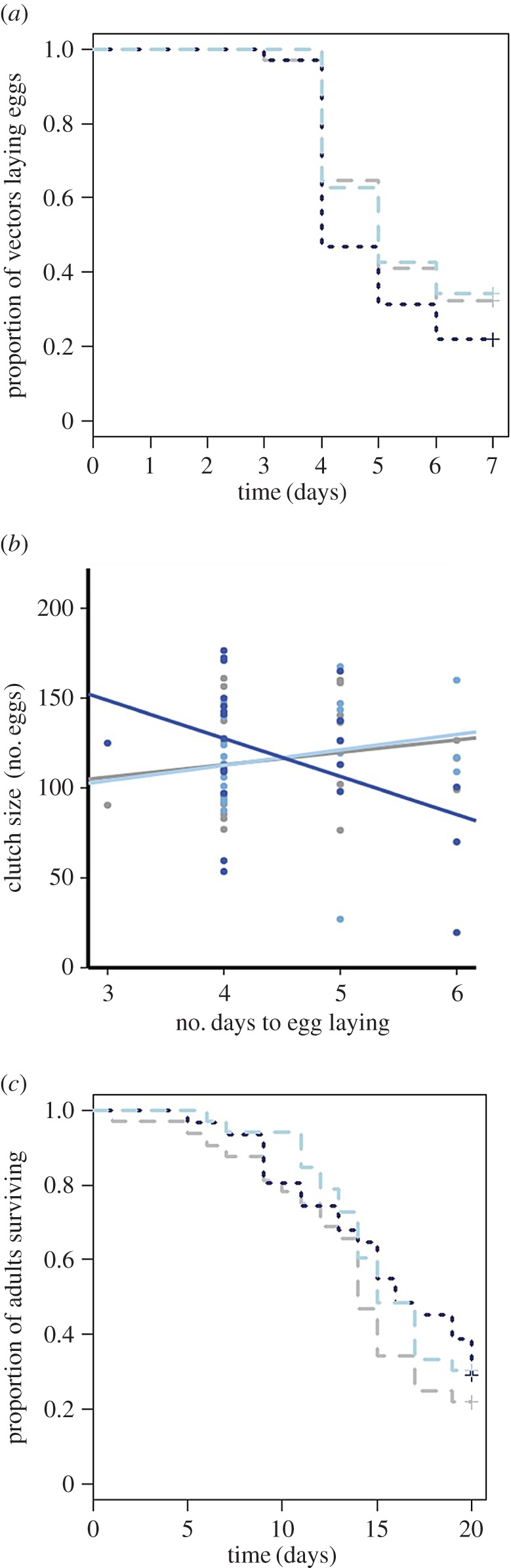
(a) Corticosterone treatment of fed-upon hosts did not affect vector egg-laying rate (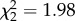 , p = 0.372). Kaplan–Meier curves for rate of reproduction displayed: grey, large-dashed line = control; light blue, large-dashed line = CORT+; dark blue, small-dashed line = CORT++ treatment. (b) Corticosterone treatment was associated with variation in the pattern of mosquito reproductive output (i.e. clutch size) over time (
, p = 0.372). Kaplan–Meier curves for rate of reproduction displayed: grey, large-dashed line = control; light blue, large-dashed line = CORT+; dark blue, small-dashed line = CORT++ treatment. (b) Corticosterone treatment was associated with variation in the pattern of mosquito reproductive output (i.e. clutch size) over time (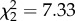 , p = 0.026, for the effect of the interaction of treatment and time on clutch size in a model with time treated as continuous). Grey line and points = control; light blue line and points = CORT+; dark blue line and points = CORT++ treatment. (c) Vector mortality rate was not affected by the corticosterone treatment of hosts on which vectors fed (
, p = 0.026, for the effect of the interaction of treatment and time on clutch size in a model with time treated as continuous). Grey line and points = control; light blue line and points = CORT+; dark blue line and points = CORT++ treatment. (c) Vector mortality rate was not affected by the corticosterone treatment of hosts on which vectors fed (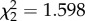 , p = 0.449). Kaplan–Meier survival curves are plotted: grey, large-dashed line and points = control; light blue, large-dashed line and points = CORT+; dark blue, small-dashed line = CORT++ treatment. (Online version in colour.)
, p = 0.449). Kaplan–Meier survival curves are plotted: grey, large-dashed line and points = control; light blue, large-dashed line and points = CORT+; dark blue, small-dashed line = CORT++ treatment. (Online version in colour.)
4. Discussion
Our data suggest that the negative impacts of glucocorticoids on individuals might heighten disease risk for other members of a population or community. Zebra finches with experimentally elevated CORT were preferred approximately 2 : 1 for feeding by southern house mosquitoes, a geographically widespread and abundant parasite vector (figure 1). These results were not simply due to reduced movement (hopping activity) or lack of attendance to biting vectors in CORT-treated birds [30]; indeed, birds in the CORT+ group displayed more vector-directed behaviours during a challenge with host-seeking mosquitoes (figure 2a–c). Combined with the effects of corticosterone on vector reproduction, stress hormones might also impact disease risk in a community through positive or negative effects on vector abundance.
(a). Stress hormones and host traits
Vectors use a variety of cues to locate hosts [19]. Among many traits, CO2 output, body size, and body temperature are used to determine host presence and navigate towards hosts [19]. However, mosquitoes are well known to exploit hosts based on additional features, including age [31,32], sex [19,33], and species [34–36]. Also, vector preferences for the same individual can change over a trajectory of infection [37–39], and can be dependent on the composition of microbiota living on the skin of hosts [40]. Selectivity means that some groups of hosts may be disproportionately responsible for transmission dynamics of vector-borne parasites [34–36]. Understanding mediators of vector preference may allow the identification of key hosts (individuals or cohorts) that support transmission within the larger host community and may lead to targeted disease management approaches. We hypothesize that corticosterone affects many proximate mediators of vector feeding. For example, changes in volatile metabolites are associated with parasitic infection [41] and could alter host attractiveness [42]. Stress can also affect body odour [43–44] and may act on vector behaviours. Relatedly, feedbacks between neuroendocrine hormones and the microbiome exist [45] and could influence how vectors choose hosts. Additionally, the prime physiological effect of corticosterone is gluconeogenesis, and associated signals such as CO2 output, metabolic rate, and changes in the composition of blood or expired breath could attract vectors to hosts. Regardless of the mechanism, our study is the first to demonstrate that host corticosterone directly affects the frequency of contact with vectors.
We hypothesized that hopping as well as head, tail, and body shakes, preening, and feather fluffing would increase in the presence of vectors and that these types of behaviours would also be most effective at discouraging or dislodging biting vectors. A comparison of pre and post-mosquito-challenge behaviours revealed that the number of hops around the cage decreased in all treatment groups during the vector challenge, though more so in the control birds than in CORT+ or CORT++ birds (figure 2b,c). We did observe finches standing still and visually following vectors immediately after they were added into the cage. Some birds actively consumed vectors in mid-air or pecked at vectors in the cage, suggesting that an additional host defence strategy might involve increased vector predation by avian hosts. While comparatively less active in terms of hopping behaviour, many finches increased the number of vector-directed behaviours after mosquitoes were placed in cages. This effect was seen in the CORT+ and CORT++ treatments.
In terms of efficacy of behaviours in preventing successful vector feeding (electronic supplementary material, figure S3a), greater hopping activity was associated with a decrease in vector-feeding success, but this pattern was only apparent in the CORT+ treatment. Thus, what we defined as defensive might have instead had context-specific effects on vector-feeding success. For example, vectors strongly preferred to take meals from CORT++ finches, and CORT++ birds also hopped more than control birds during the mosquito challenge, though not more than CORT+ birds. More importantly, hopping in CORT++ birds did not prevent vectors from feeding. Thus, hopping was either completely ineffective or, beyond some functionally relevant threshold, increased hopping activity acted to strengthen proximate cues to vectors (e.g. increased body temperature and/or CO2 output [19]). By comparison, elevated hopping activity within CORT+ birds was associated with slightly reduced vector-feeding success (electronic supplementary material, figure S3a). With respect to vector-directed behaviours, again, only within the CORT+ treatment did these behaviours protect birds from successful mosquito bites (electronic supplementary material, figure S3). Perhaps, what we operationally defined as ‘defence’ peaks in utility at some point, beyond which hosts may become so attractive to vectors that their defences are overwhelmed. In essence, CORT effects on defensive behaviours appear nonlinear.
(b). Stress hormones and vector traits
CORT treatment affected the relationship between the timing of egg laying and clutch size, but we do not feel it appropriate yet to conclude whether such effects were positive or negative. Specifically, mosquitoes feeding on CORT++ birds showed a decrease in clutch size over time, a trend not observed in vectors feeding on control or CORT+ birds (electronic supplementary material, figure S6a,b). Although these results are compelling, we encourage future work on this topic with more statistical power. Such research will be demanding as it is natural for few mosquitoes to breed soon after blood feeding. Here, peak vector reproduction occurred, on average, 4 days after taking a host blood meal but some vectors reproduced earlier (3 days after feeding). This bias in the number of clutches laid at early time points presents a statistical challenge (low sample size and high leverage of early points in the analysis). With respect to the timing of reproduction in nature, even slight differences in the timing of egg laying could be biologically meaningful and epidemiologically influential [46]. The sooner a female lays her eggs, the sooner she herself can go on to bite another host and potentially transmit parasites [46].
(c). Ecological relevance
Average corticosterone levels in our study were within a range of observed values of other songbirds exposed to natural stressors [47,48] or experimental conditions where hormones are exogenously manipulated [49,50]. Exceptions include one outlier in Experiment 1 and multiple outliers in Experiment 2 (electronic supplementary material, figure S1). The latter outcome is perhaps due to our sampling method; design constraints prevented us from rapidly sampling blood within 3 min of capture in a separate room outside of the housing facility, as was performed in Experiment 1. It is challenging to conduct naturally representative, yet manipulative studies of CORT when CORT itself is but one aspect of a regulated, dynamic homeostatic system. These challenges are well summarized in several recent papers on the context and dose dependency of implant effects [51,52] and alternative implant methods [53]. We chose the zebra finch as our study species because many wild species respond adversely to captivity [54,55]. Unfortunately, even though the role of corticosterone in the zebra finch's life history and physiology has been studied [56,57], corticosterone has not been measured in wild zebra finches. Our use of two CORT doses here meant to emulate variation in CORT levels achieved under stressed conditions in the wild. Many stressors (i.e. road noise, pesticide and other toxicant exposures, predation risk in a habitat, infection, light pollution, etc.) span (at least) the time frame over which we examined hormone levels. Much more work is necessary regarding the interplay of host CORT dynamics, vector-feeding behaviour, host defences, and mosquito productivity.
5. Conclusion
Heterogeneity in vector interactions with hosts has been implicated as a key mediator of epizootic risk [34–36,58,59]. For example, the American Robin (Turdus migratorius) in some regions of North America and the common blackbird (T. merula) in a region of Italy are bitten more frequently than expected from their relative abundance [34,35]. Mechanistic investigations of why these hosts are preferred over others would be particularly useful in the light of our findings, especially in locations where vector-borne diseases are emerging and hosts are naive [39]. In general, understanding the ramifications of individual host stress biology for community disease risk is important, as host organisms are forced to endure or exploit increasingly modified areas [60]. As inter-individual variation in competence is likely to vary extensively among hosts, disease risk may be greater than predicted based on current models [61] because of the disproportionate effects of some individuals on vector feeding and subsequent productivity when hosts are stressed [62,63].
Supplementary Material
Supplementary Material
Acknowledgements
We thank I. Ford, J. Miller, and L. Hebert for assistance with mosquito fitness trials, T. Stenn for mosquito husbandry, J. Giarrizzo for technical assistance with video recording, and L. Brown and M. Betts for statistical advice. We thank the Martin and Unnasch labs for helpful comments.
Ethics
Procedures were carried out under permit of the Institutional Animal Care and Use Committee permit number 0396 from the University of South Florida, Department of Comparative Medicine.
Data accessibility
All data for statistical analyses are provided in the Dryad repository, doi: http://dx.doi.org/10.5061/dryad.s7m33. Additional information and data provided in the electronic supplementary material file.
Authors' contributions
S.S.G, L.B.M., T.R.U., and N.B.-C. designed experiments; S.S.G., L.B.M., S.C.B., and N.B.-C. performed experimental procedures; A.W.S. performed microsatellite genotyping for Experiment 1; H.K.H. provided technical expertise for experimental diagnostics; S.S.G. and L.B.M. performed statistical analysis and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NSF IOS grant #1257773 to L.B.M. and T.R.U.
References
- 1.Laugero KD, Moberg CP. 2000. Energetic response to repeated restraint stress in rapidly growing mice. Am. J. Physiol. Endocrinol. Meta. 279, E33–E43. [DOI] [PubMed] [Google Scholar]
- 2.Kitaysky AS, Wingfield JC, Piatt JF. 2003. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav. Ecol. 12, 619–625. ( 10.1093/beheco/12.5.619) [DOI] [Google Scholar]
- 3.Greenberg N, Wingfield JC. 1987. Stress and reproduction: reciprocal relationships. In Hormones and reproduction in fishes, amphibians, and reptiles (eds Norris DO, Jones RE), pp. 461–503. New York, NY: Springer. [Google Scholar]
- 4.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. ( 10.1210/edrv.21.1.0389) [DOI] [PubMed] [Google Scholar]
- 5.French SS, McLemore R, Vernon B, Johnston GIH, Moore MC. 2007. Corticosterone modulation of reproductive and immune system trade-offs in female tree lizards: long-term corticosterone manipulations via injectable gelling material. J. Exp Biol. 210, 2859–2865. ( 10.1242/jeb.005348) [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS, Wingfield JC. 2003. The concept of allostasis in biology and medicine. Horm. Behav. 43, 2–15. ( 10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- 7.Lupien SJ, McEwen BS, Gunnar MR, Heim C. 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature 10, 434–445. ( 10.1038/nrn2639) [DOI] [PubMed] [Google Scholar]
- 8.Blanchard RJ, McKittrick CR, Blanchard DC. 2001. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol. Behav. 73, 261–271. ( 10.1016/S0031-9384(01)00449-8) [DOI] [PubMed] [Google Scholar]
- 9.Dhabhar FS. 2009. A hassle a day may keep the pathogens away: the flight-or-fight stress response and the augmentation of immune function. Integr. Comp. Biol. 49, 215–236. ( 10.1093/icb/icp045) [DOI] [PubMed] [Google Scholar]
- 10.Martin LB. 2009. Stress and immunity in wild vertebrates: timing is everything. Gen. Comp. Endocrinol. 163, 70–76. ( 10.1016/j.ygcen.2009.03.008) [DOI] [PubMed] [Google Scholar]
- 11.Wingfield JC. 2003. Control of behavioural strategies for capricious environments. Anim. Behav. 66, 807–816. ( 10.1006/anbe.2003.2298) [DOI] [Google Scholar]
- 12.Love OP, McGowan PO, Sheriff MJ. 2013. Maternal adversity and ecological stressors in natural populations: the role of stress axis programming in individuals, with implications for populations and communities. Funct. Ecol. 27, 81–92. ( 10.1111/j.1365-2435.2012.02040.x) [DOI] [Google Scholar]
- 13.Sheriff MJ, McMahon EK, Krebs CJ, Boonstra R. 2015. Predator-induced maternal stress and population demography in snowshoe hares: the more severe the risk, the longer the generational effect. J. Zool. 296, 305–310. ( 10.1111/jzo.12249) [DOI] [Google Scholar]
- 14.Ben-Nathan D, Feuerstein G. 1990. The influence of cold or isolation stress on resistance of mice to West Nile virus encephalitis. Experientia 46, 285–290. ( 10.1007/BF01951768) [DOI] [PubMed] [Google Scholar]
- 15.Avitsur R, Hunzeker J, Sheridan JF. 2006. Role of early stress in the individual difference in host response to viral infection. Brain Behav. Immun. 20, 339–348. ( 10.1016/j.bbi.2005.09.006) [DOI] [PubMed] [Google Scholar]
- 16.Ben-Nathan D. 2013. Stress and virulence: West Nile virus encephalitis. Isr. J. Vet. Med. 68, 135–140. [Google Scholar]
- 17.DeNardo DF, Sinervo B. 1994. Effect of steroid hormone interaction on activity and home-range size of male lizards. Horm. Behav. 28, 273–287. ( 10.1006/hbeh.1994.1023) [DOI] [PubMed] [Google Scholar]
- 18.Breuner CW, Greenberg AL, Wingfield JC. 1998. Non-invasive corticosterone treatment rapidly increases activity in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii). Gen. Comp. Endocrinol. 111, 386–394. ( 10.1006/gcen.1998.7128) [DOI] [PubMed] [Google Scholar]
- 19.Takken W, Verhulst NO. 2013. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 58, 433–453. ( 10.1146/annurev-ento-120811-153618) [DOI] [PubMed] [Google Scholar]
- 20.Bartholomay LC, et al. 2010. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science 330, 88–90. ( 10.1126/science.1193162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouyang JQ, Muturi M, Quetting M, Hau M. 2013. Small increases in corticosterone before the breeding season increase parental investment but not fitness in a wild passerine bird. Horm. Behav. 63, 776–781. ( 10.1016/j.yhbeh.2013.03.002) [DOI] [PubMed] [Google Scholar]
- 22.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See; http:www.R-project.org/. [Google Scholar]
- 23.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 24.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn Thousand Oaks, CA: Sage Publications, Inc. [Google Scholar]
- 25.Fox J. 2003. Effects displays for R for generalised linear models. J. Stat. Softw. 8, 1–27. ( 10.18637/jss.v008.i15) [DOI] [Google Scholar]
- 26.Therneau T. 2015. Package ‘coxme’. R package version 2.2–5.
- 27.Elizondo-Quiroga A, et al. 2006. Gonotrophic cycle and survivorship of Culex quinquefasciatus (Diptera: Culicidae) using sticky ovitraps in Monterrey, northeastern Mexico. J. Am. Mosq. Control Assoc. 22, 10–14. ( 10.2987/8756-971X(2006)22%5B10:GCASOC%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 28.Navarro DJ. 2015. Learning statistics with R: a tutorial for psychology students and other beginners. (Version 0.5). Adelaide, Australia: University of Adelaide. [Google Scholar]
- 29.Kelley K. 2007. Confidence intervals for standardized effect sizes: theory, application, and implementation. J. Stat. Softw. 20, 1–24. ( 10.18637/jss.v020.i08) [DOI] [Google Scholar]
- 30.Day JF, Ebert KM, Edman JD. 1983. Feeding patterns of mosquitoes (Diptera: Culicidae) simultaneously exposed to malarious and healthy mice, including a method for separating blood meals from conspecific hosts. J. Med. Entomol 20, 120–127. ( 10.1093/jmedent/20.2.120) [DOI] [PubMed] [Google Scholar]
- 31.Ligon RA, Burkett-Cadena ND, Liu M, Hill GE, Hassan HK, Unnasch TR. 2009. Assessing mosquito feeding patterns on nestling and brooding adult birds using microsatellite markers. Am. J. Trop. Med. Hyg. 81, 534–537. [PubMed] [Google Scholar]
- 32.Burkett-Cadena ND, Ligon RA, Liu M, Hassan HK, Hill GE, Eubanks MD, Unnasch TR. 2010. Vector-host interactions in avian nests: do mosquitoes prefer nestlings over adults? Am. J. Trop. Med. Hyg. 83, 395–399. ( 10.4269/ajtmh.2010.10-0048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burkett-Cadena ND, Bingham AM, Unnasch TR. 2014. Sex-biased avian host use by arbovirus vectors. R. Soc. Open Sci. 1, 140262 ( 10.1098/rsos.140262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. 2006. Host heterogeneity dominates West Nile virus transmission. Proc. R. Soc. B 273, 2327–2333. ( 10.1098/rspb.2006.3575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzoli A, et al. 2015. Understanding West Nile virus ecology in Culex pipiens host feeding preference in a hotspot of virus emergence. Parasites Vectors 8, 213 ( 10.1186/s13071-015-0831-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamer GL, Chaves LF, Anderson TK, Kitron UD, Brawn JD, Ruiz MO, Loss SR, Walker ED, Goldberg TL. 2011. Fine-scale variation in vector host use and force of infection drive localized patterns of West Nile virus transmission. PLoS ONE 6, e23767 ( 10.1371/journal.pone.0023767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferguson HM, Read AF. 2004. Mosquito appetite for blood is stimulated by Plasmodium chabaudi infection in themselves and their vertebrate hosts. Malar. J. 3, 12 ( 10.1186/1475-2875-3-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornet S, Nicot A, Rivero A, Gandon S. 2013. Malaria infection increases bird attractiveness to uninfected vectors. Ecol. Lett. 16, 323–329. ( 10.1111/ele.12041) [DOI] [PubMed] [Google Scholar]
- 39.De Moras CM, Stanczyk NM, Betz HS, Pulido H, Sim DG, Read AF, Mescher MC. 2014. Malaria-induced changes in host odors enhance mosquito attraction. Proc. Natl Acad. Sci. USA 111, 11 079–11 084. ( 10.1073/pnas.1405617111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhulst NO, et al. 2011. Composition of human skin microbiotia affects attractiveness to malaria mosquitoes. PLoS ONE 6, e28991 ( 10.1371/journal.pone.0028991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penn D, Potts WK. 1998. Chemical signals and parasite-mediated sexual selection. Trends Ecol. Evol. 13, 391–396. ( 10.1016/S0169-5347(98)01473-6) [DOI] [PubMed] [Google Scholar]
- 42.Verhulst NO, Andriessen R, Groenhagen U, Bukovinszkiné Kiss G, Schulz S, Takken W, van Loon JJA, Schraa G, Smallegange RC. 2010. Differential attraction of malaria mosquitoes to volatile blends produced by human skin bacteria. PLoS ONE 5, e15829 ( 10.1371/journal.pone.0015829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaefer ML, et al. 2010. Mouse urinary biomarkers provide signatures of maturation, diet, stress level, and diurnal rhythm. Chem. Senses 35, 459–471. ( 10.1093/chemse/bjq032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zalaquett C, Thiessen D. 1991. The effect of odors from stressed mice on conspecific behavior. Physiol. Behav. 50, 221–227. ( 10.1016/0031-9384(91)90524-R) [DOI] [PubMed] [Google Scholar]
- 45.Neuman H, Debelius JW, Knight R, Koren O. 2015. Microbial endocrinology: the interplay between the microbiota and the endocrine system. Microbiol. Rev. 39, 509–521. ( 10.1093/femsre/fuu010) [DOI] [PubMed] [Google Scholar]
- 46.Vezilier J, Nicot A, Gandon S, Rivero A. 2015. Plasmodium infection brings forward mosquito oviposition. Biol. Lett. 11, 20140840 ( 10.1098/rsbl.2014.0840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newman AEM, Zanette LY, Clinchy M, Goodenough N, Soma KK. 2013. Stress in the wild: chronic predator pressure and acute restraint affect plasma DHEA and corticosterone levels in a songbird. Stress 16, 363–367. ( 10.3109/10253890.2012.723076) [DOI] [PubMed] [Google Scholar]
- 48.Vitousek M, Jenkins BR, Safran RJ. 2014. Stress and success: individual differences in the glucocorticoid stress response predict behavior and reproductive success under high predation risk. Horm. Behav. 66, 812–819. ( 10.1016/j.yhbeh.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 49.Breuner CW, Wingfield JC, Romero LM. 1999. Diel rhythms of basal and stress-induced corticosterone in a wild, seasonal vertebrate, Gambel's white-crowned sparrow. J. Exp. Zool. 284, 334–342. ( 10.1002/(SICI)1097-010X(19990801)284:3%3C334::AID-JEZ11%3E3.0.CO;2-%23) [DOI] [PubMed] [Google Scholar]
- 50.Perez EC, Elie JE, Soulage CO, Soula HA, Mathevon N, Vignal C. 2012. The acoustic expression of stress in a songbird: does corticosterone drive isolation-induced modifications of zebra finch calls? Horm. Behav. 61, 573–581. ( 10.1016/j.yhbeh.2012.02.004) [DOI] [PubMed] [Google Scholar]
- 51.Crossin GT, Love OP, Cooke SJ, Williams TD. 2016. Glucocorticoid manipulations in free-living animals: considerations of dose delivery, life-history context and reproductive state. Funct. Ecol. 30, 116–125. ( 10.1111/1365-2435.12482) [DOI] [Google Scholar]
- 52.Sopinka NM, Patterson LD, Redfern JC, Pleizier NK, Belanger CB, Midwood JD, Crossin GT, Cooke SJ. 2015. Manipulating glucocorticoids in wild animals: basic and applied perspectives. Conserv. Physiol. 3, cov031. ( 10.1093/conphys/cov031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quispe R, Trappschuh M, Gahr M, Goymann W. 2015. Towards more physiological manipulations of hormones in field studies: comparing the release dynamics of three kinds of testosterone implants: silastic tubing, time-release pellets and beeswax. Gen. Comp. Endocrinol. 212, 100–105. ( 10.1016/j.ygcen.2015.01.007) [DOI] [PubMed] [Google Scholar]
- 54.Martin LB, Kidd L, Liebl AL, Coon CAC. 2011. Captivity induces hyper-inflammation in the house sparrow (Passer domesticus). J. Exp. Biol. 214, 2579–2585. ( 10.1242/jeb.057216) [DOI] [PubMed] [Google Scholar]
- 55.Dickens MJ, Delehanty DJ, Romero LM. 2010. Stress: an inevitable component of animal translocation. Biol. Conserv. 143, 1329–1341. ( 10.1016/j.biocon.2010.02.032) [DOI] [Google Scholar]
- 56.Buchanan KL, Leitner S, Spencer KA, Goldsmith AR, Catchpole CK. 2004. Developmental stress selectively affects the song control nucleus HVC in the zebra finch. Proc. R. Soc. Lond. B 271, 2381–2386. ( 10.1098/rspb.2004.2874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crino OL, Prather CT, Driscoll SC, Good JM, Breuner CW. 2014. Developmental stress increases reproductive success in male zebra finches. Proc. R. Soc. B 281, 20141266 ( 10.1098/rspb.2014.1266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hassan HK, Cupp EW, Hill GE, Katholi CR, Klingler K, Unnasch TR. 2003. Avian host preference by vectors of eastern equine encephalomyelitis. Am. J. Trop. Med. Hyg. 69, 641–647. [PubMed] [Google Scholar]
- 59.Simpson JE, Hurtado PJ, Medlock J, Molaei G, Andreadis TG, Galvani AP, Diuk-Wasser MA. 2011. Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc. R. Soc. B 279, 925–933. ( 10.1098/rspb.2011.1282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin LB, Hopkins WA, Mydlarz LD, Rohr JR. 2010. The effects of anthropogenic global change on immune functions and disease resistance. Ann. N. Y. Acad. Sci. 1195, 129–148. ( 10.1111/j.1749-6632.2010.05454.x) [DOI] [PubMed] [Google Scholar]
- 61.Becker DJ, Streicker DG, Altizer S. 2015. Linking anthropogenic resources to wildlife-pathogen dynamics: a review and meta-analysis. Ecol. Lett. 18, 483–495. ( 10.1111/ele.12428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin LB, et al. 2016. Host competence: an organismal trait to integrate immunology and epidemiology. Integr. Comp. Biol. ( 10.1093/icb/icw064) [DOI] [PubMed] [Google Scholar]
- 63.Gervasi SG, Civitello DJ, Kilvitis HJ, Martin LB. 2015. The context of host competence: a role for plasticity in host-parasite dynamics. Trends Parasitol. 31, 419–425. ( 10.1016/j.pt.2015.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data for statistical analyses are provided in the Dryad repository, doi: http://dx.doi.org/10.5061/dryad.s7m33. Additional information and data provided in the electronic supplementary material file.


