Abstract
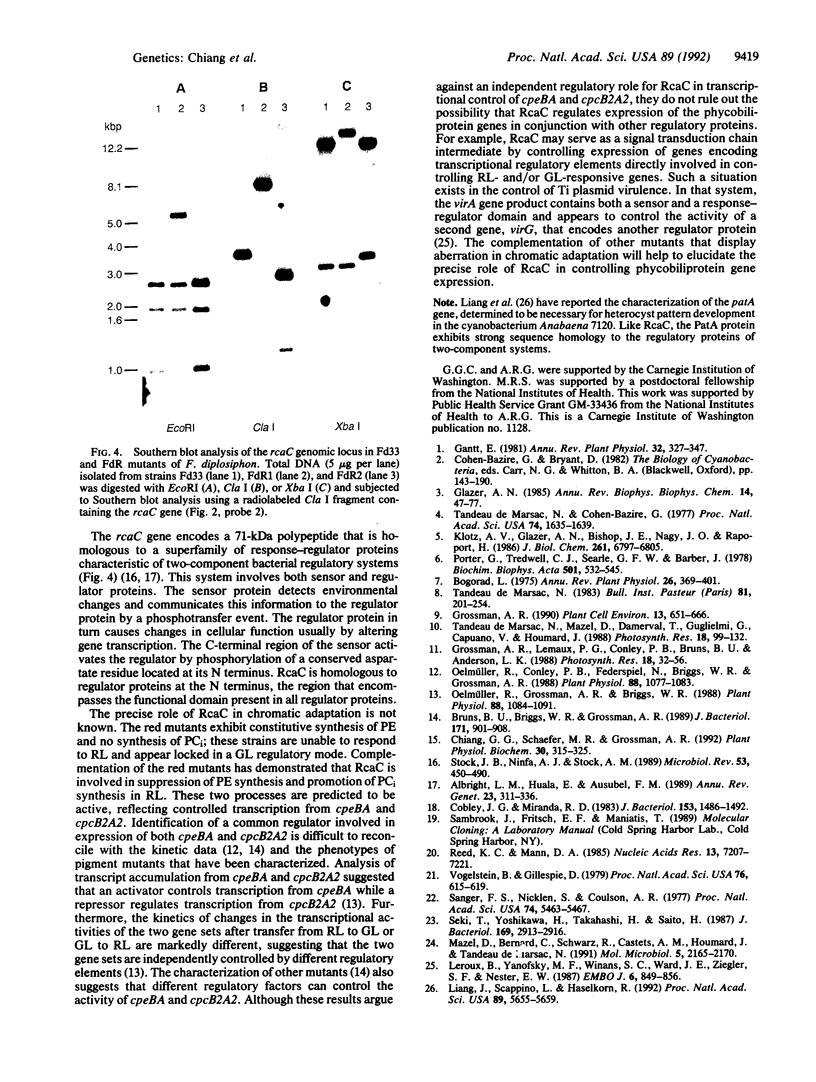
Many cyanobacteria alter their phycobilisome composition in response to changes in light wavelength in a process termed complementary chromatic adaptation. Mutant strains FdR1 and FdR2 of the filamentous cyanobacterium Fremyella diplosiphon are characterized by aberrant chromatic adaptation. Instead of adjusting to different wavelengths of light, FdR1 and FdR2 behave as if they are always in green light; they do not respond to red light. We have previously reported complementation of FdR1 by conjugal transfer of a wild-type genomic library. The complementing DNA has now been localized by genetic analysis to a region on the rescued genomic subclone that contains a gene designated rcaC. This region of DNA is also able to complement FdR2. Southern blot analysis of genomic DNA from FdR1 and FdR2 indicates that these strains harbor DNA insertions within the rcaC sequence that may have resulted from the activity of transposable genetic elements. The predicted amino acid sequence of RcaC shares strong identity to response regulators of bacterial two-component regulatory systems. This relationship is discussed in the context of the signal-transduction pathway mediating regulation of genes encoding phycobilisome polypeptides during chromatic adaptation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright L. M., Huala E., Ausubel F. M. Prokaryotic signal transduction mediated by sensor and regulator protein pairs. Annu Rev Genet. 1989;23:311–336. doi: 10.1146/annurev.ge.23.120189.001523. [DOI] [PubMed] [Google Scholar]
- Bruns B. U., Briggs W. R., Grossman A. R. Molecular characterization of phycobilisome regulatory mutants of Fremyella diplosiphon. J Bacteriol. 1989 Feb;171(2):901–908. doi: 10.1128/jb.171.2.901-908.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley J. G., Miranda R. D. Mutations affecting chromatic adaptation in the cyanobacterium Fremyella diplosiphon. J Bacteriol. 1983 Mar;153(3):1486–1492. doi: 10.1128/jb.153.3.1486-1492.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Light harvesting by phycobilisomes. Annu Rev Biophys Biophys Chem. 1985;14:47–77. doi: 10.1146/annurev.bb.14.060185.000403. [DOI] [PubMed] [Google Scholar]
- Klotz A. V., Glazer A. N., Bishop J. E., Nagy J. O., Rapoport H. Phycobiliprotein-bilin linkage diversity. II. Structural studies on A- and D-ring-linked phycoerythrobilins. J Biol Chem. 1986 May 25;261(15):6797–6805. [PubMed] [Google Scholar]
- Leroux B., Yanofsky M. F., Winans S. C., Ward J. E., Ziegler S. F., Nester E. W. Characterization of the virA locus of Agrobacterium tumefaciens: a transcriptional regulator and host range determinant. EMBO J. 1987 Apr;6(4):849–856. doi: 10.1002/j.1460-2075.1987.tb04830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Scappino L., Haselkorn R. The patA gene product, which contains a region similar to CheY of Escherichia coli, controls heterocyst pattern formation in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5655–5659. doi: 10.1073/pnas.89.12.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel D., Bernard C., Schwarz R., Castets A. M., Houmard J., Tandeau de Marsac N. Characterization of two insertion sequences, IS701 and IS702, from the cyanobacterium Calothrix species PCC 7601. Mol Microbiol. 1991 Sep;5(9):2165–2170. doi: 10.1111/j.1365-2958.1991.tb02146.x. [DOI] [PubMed] [Google Scholar]
- Oelmüller R., Conley P. B., Federspiel N., Briggs W. R., Grossman A. R. Changes in Accumulation and Synthesis of Transcripts Encoding Phycobilisome Components during Acclimation of Fremyella diplosiphon to Different Light Qualities. Plant Physiol. 1988 Dec;88(4):1077–1083. doi: 10.1104/pp.88.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelmüller R., Grossman A. R., Briggs W. R. Photoreversibility of the Effect of Red and Green Light Pulses on the Accumulation in Darkness of mRNAs Coding for Phycocyanin and Phycoerythrin in Fremyella diplosiphon. Plant Physiol. 1988 Dec;88(4):1084–1091. doi: 10.1104/pp.88.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T., Yoshikawa H., Takahashi H., Saito H. Cloning and nucleotide sequence of phoP, the regulatory gene for alkaline phosphatase and phosphodiesterase in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):2913–2916. doi: 10.1128/jb.169.7.2913-2916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marsac N. T., Cohen-bazire G. Molecular composition of cyanobacterial phycobilisomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1635–1639. doi: 10.1073/pnas.74.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]