Abstract
Purpose
Antepartum depression and anxiety are risk factors for postpartum depression (PPD). Postpartum abnormalities in hypothalamic-pituitary-adrenal (HPA) reactivity are associated with PPD. It is not known if antepartum HPA abnormalities exist in women at risk for PPD (AR-PPD).
Methods
We measured salivary cortisol response to the Trier Social Stress Test (TSST) in 44 (24 AR-PPD, 20 healthy comparison) pregnant women. Depression and anxiety was measured using the Edinburgh Postnatal Depression Scale (EPDS) and Spielberger State-Trait Anxiety Inventory-State (STAI-S). We analyzed longitudinal changes in cortisol using generalized estimating equation methods to control for the correlation within subjects at the six TSST time points. Group differences in area under the curve (AUC) were examined.
Results
A majority (70.8%) of the AR-PPD had prior depression. EPDS total score was higher in ARPPD vs. comparison women (mean EPDS=9.8±4.9 vs. mean EPDS=2.4±2.0 respectively; p<0.001). Mean STAI-S total score was higher in AR-PPD vs. comparison women at all TSST time points and over time (z=2.71, df=1, p=0.007). There was no significant difference in cortisol concentration over time between groups.
Conclusion
We observed no detectable difference in cortisol response to psychosocial stress induced by the TSST despite clinically significant between-group differences in current/past depression and current symptomatology.
Keywords: postpartum, cortisol, depression, anxiety
Introduction
Postpartum depression (PPD) affects 1 in 8 women (Cox et al. 1993) and negatively impacts infant attachment, cognitive development and behavior. The presence of antepartum anxiety or depressive symptoms during pregnancy increases the risk for the development of PPD (Beck 2001), and may represent an early manifestation of the disorder. Recently, DSM-5 revised the “postpartum onset” specifier for major depression to “peripartum onset” in recognition that 50% of “postpartum” major depressive episodes begin antepartum (Diagnostic and Statistical Manual of Mental Disorders, DSM-5 American Psychiatric Association, 2013). Abnormalities in the hypothalamic-pituitary-adrenal (HPA) axis during the peripartum period have been suggested as a possible vulnerability factor for developing peripartum depressive and anxiety disorders (O'Keane et al. 2011; Smith et al. 1990).
In healthy women, pregnancy is associated with physiological changes in the HPA axis including suppressed hypothalamic corticotropin releasing hormone (CRH) and increased basal plasma CRH, adenocorticotropin (ACTH) and cortisol concentrations(Mastorakos and Ilias 2003; Nolten et al. 1980). Some studies of maternal HPA response to pharmacological and physical stressors in healthy pregnant women suggest that there is a dampened response to these stressors across gestation (Buss et al. 2009; Entringer et al. 2010; Schulte et al. 1990); other studies suggest comparable antepartum HPA responsivity to stress to non-pregnant women (De Weerth et al. 2007; Giesbrecht et al. 2013; Nierop et al. 2006a).
Preliminary studies which examined healthy euthymic pregnant women who developed postpartum depressive symptoms report abnormalities in antepartum HPA responsivity to pharmacologic and psychosocial stressors. Nierop (2006) reported higher cortisol reactivity to psychosocial stress induced by the Trier Social Stress Test (TSST) in healthy pregnant women who later developed mild depressive symptoms within two weeks of delivery(Nierop et al. 2006b). In an experimental model of pregnancy, women with a history of PPD demonstrated a greater cortisol response to ovine CRH stimulation in the presence of elevated levels of gonadal steroids compared with healthy comparison subjects (Bloch et al. 2005) suggesting that euthymic women with a history of PPD have an enhanced sensitivity of the HPA axis to gonadal steroids. However, it is not known if the antepartum salivary cortisol response to the TSST differs in women with a history of PPD or women with antepartum depressive and anxiety symptoms compared to healthy comparison women at low risk for developing PPD. Since up to a quarter of pregnant women have depressive or anxiety symptoms, it is important to determine if there are changes in salivary cortisol response in these women as it may represent a marker of those women who go on to develop PPD.
Given the above evidence of an abnormal antepartum cortisol response to the TSST in euthymic women who later developed postpartum depressive symptoms (Nierop et al. 2006b) we examined basal and dynamic salivary cortisol response to the TSST (Kirschbaum et al. 1993a) in pregnant women at-risk for PPD (AR-PPD) compared to healthy comparison pregnant women. Our aim was to examine potential biological underpinnings of the aforementioned antepartum risk factors for PPD. Since antepartum depressive and anxiety symptoms may be an early manifestation of PPD (Diagnostic and Statistical Manual of Mental Disorders, DSM-5 American Psychiatric Association, 2013; Righetti-Veltema et al. 1998; Stowe and Nemeroff 1995), we hypothesized that AR-PPD women would have exaggerated salivary cortisol stress reactivity as compared to the healthy comparison women.
Materials and Methods
Participant selection
English-speaking pregnant subjects were consented and screened for eligibility with the Edinburgh Postnatal Depression Scale (EPDS) (Cox et al. 1987) at 24-34 weeks gestational age as determined by first trimester ultrasound. A total of 44 eligible women between 18-40 years old enrolled in the study. Two groups were enrolled: (1) 20 healthy low-risk healthy comparison women (2) 24 AR-PPD women. The EPDS was used to assess peripartum depressive and anxiety symptoms (Cox et al. 1987; Lydsdottir et al. 2014) and a cut-off score of 10 was chosen based on validation of the EPDS during pregnancy (Berrettini et al. 1982). The healthy comparison group included women with an EPDS ≤5 and no current or past psychiatric diagnosis or family history of psychiatric illness, as ascertained by clinical and research interviews (First et al. 2001) conducted by a board-certified psychiatrist. The AR-PPD group included women at-risk for developing PPD. As the EPDS is not sufficiently accurate in predicting risk of postpartum depressive symptoms alone (Meijer et al. 2014), the AR-PPD group included women who had an elevated EPDS score (indicating current depressive and/or anxiety symptomatology) or a history of PPD or non-puerperal depression (Austin et al. 2010) as determined by the SCID-IV. Since anxiety and depression symptoms during pregnancy is associated with postpartum depressive symptomatology (Milgrom et al. 2008; O'Hara and Swain 1996), women who met criteria for an anxiety disorder or depressive disorder not otherwise specified were included in the AR-PPD group; however subjects who met SCID-IV criteria for a current MDE were excluded since the study was designed to identify a potential unifying biomarker in those women at risk for developing a major depressive episode.
Subjects were excluded for multiple gestation pregnancy, surrogate pregnancy, current major depressive episode (MDE) or lifetime history of manic episode or any psychotic disorder as determined by the Structured Clinical Interview for DSM-IV TR Disorders (SCID-IV), Patient Edition(First et al. 2001); elevated suicidal risk; and alcohol, tobacco or substance abuse/dependence in the 6 months prior to study entry. Based on medical and laboratory record review, subjects were excluded if they had active or a history of serious medical, neurological or endocrine disorder (e.g., adrenal, pituitary, thyroid, glucose or calcium homeostasis, polycystic ovarian syndrome). Prenatal vitamins and as-needed docusate was allowed during the study; other medication use was excluded. The Institutional Review Board (IRB) granted a waiver to review the medical records of obstetrics patients at our affiliated tertiary medical center during the study period between January 2010 and March 2013 and approved the study protocol. All subjects provided written informed consent and each received monetary compensation.
Study procedures
We conducted a cross-sectional study. The study visit was conducted between 28-33 weeks gestation and included the TSST (Kirschbaum et al. 1993a), serial saliva collection and measurement of depression and anxiety. EPDS was administered on the day of the TSST to confirm continued eligibility. The TSST has been used in stress research to induce a moderate psychological stress in children (Buske-Kirschbaum et al. 2003), adults and in pregnant women (Nierop et al. 2006b) and is protocol-based(Kirschbaum et al. 1993b). The TSST consists of an unprepared speech and mental arithmetic task performed in front of research team members (Kirschbaum et al. 1993a) and elicits robust salivary cortisol responses (Meinlschmidt et al. 2010). Subjects were instructed to avoid food intake for 2 hours and to abstain from caffeine, alcohol and running or other strenuous activity during the 24 hours prior the TSST. The TSST was performed between 1100 and 1700 hours. Subjects rested seated during a ten minute acclimation phase. Using prepared scripts, subjects were then told that they would complete two tasks in the experiment. First they were to assume the role of a job applicant who was invited for a personal interview with the company's job selection committee. Subjects were told to introduce themselves to the selection committee and convince them that they were the perfect applicant for their “ideal” job and to speak for the entire five minutes. Subjects were informed that the selection committee was trained in assessing nonverbal behavior and may ask additional questions during the interview. Subjects were informed that during the second task they would perform a serial subtraction test for five minutes. After subjects were informed of the two tasks, they were confronted with the public speaking and mental arithmetic tasks performed in front of a panel of three standing evaluators in white lab coats and carrying conspicuous hand-held voice recorders. Evaluators were unknown to the subject, took notes on clip boards and critiqued the subjects’ performance during the stress tasks using prepared scripts. A debriefing occurred after final research assessments were completed.
Outcome measures
Research assessments included: SCID-IV, past medical and obstetric history, demographics, Edinburgh Postnatal Depression Scale (EPDS) (Cox et al. 1987) and Childhood Trauma Questionnaire (CTQ)(Bernstein and Fink 1997). As a history of childhood maltreatment can dampen the cortisol response to the TSST, the CTQ was administered to control for possible confounding effects(Carpenter et al. 2011) and was administered after subjects completed the TSST. The Spielberger State-Trait Anxiety Inventory (STAI-S) (state anxiety) was completed at time 0 (T0=prior to TSST), T15 (immediately post-TSST), T25, T35, T45 and T75 min. Saliva samples were collected at the same time points as the STAI-S. As in other studies(Buss et al. 2009; Hellgren et al. 2013), we measured salivary cortisol to noninvasively and repeatedly examine bioavailable cortisol (Hampson et al. 2013) as there are known peripartum changes in serum cortisol binding protein which effect serum cortisol concentration.
Salivary cortisol quantification
Saliva was collected by Salivette® using a synthetic swab specifically designed for cortisol determination. Salivettes were centrifuged for 2 minutes at 1,000 x g, yielding a clear saliva sample. Cortisol concentration was analyzed by ARUP® Laboratories (Salt Lake City, Utah) within one day of collection. Salivary free cortisol concentration was analyzed using enzyme-linked immunosorbent assay (ELISA) with an analytical measurement range = 0.31 nmol/L – 77.91 nmol/L and lower limit of sensitivity of 0.19 nmol/L. Intra-assay coefficient of variation (CV) was 3-5% and inter-assay CV was 3-9% for mean cortisol concentrations of 4.41-11.59 nmol/L and 4.97-11.86 nmol/L respectively.
Statistical analysis
Baseline characteristics were compared between our two primary comparison groups (AR-PPD vs. healthy comparison) using Fisher's exact test for categorical variables and Student's independent samples t-test for continuous variables (with Satterthwaite adjustment for unequal variances, when appropriate). Pearson correlation coefficients were computed for comparisons between continuous variables of interest.
We analyzed cortisol changes during the visit longitudinally using generalized estimating equation (GEE) methods to control for the correlation within the subjects at the six time points during the TSST. Models were run examining potential associations between cortisol and AR-PPD/healthy comparison group categories, EPDS score, STAI-S score and CTQ score. Main effects for risk group and variables of interest were modeled separately and then in combined models with risk group and one additional variable at a time, to examine any potential differences in scales or moods by risk group. We also examined STAI-S total score as the dependent variable to examine possible effects of risk group on STAI-S score. We used an autoregressive covariance structure in all of our GEE models. For main effects models, P values are reported from a z test that a single regression coefficient was equal to 0; for multivariable models, P values are reported from the overall Type 3 test of any difference among levels of a factor (such as risk group) adjusting for other variables in the model.
In addition, we calculated area under the curve with respect to increase (AUCi) using a trapezoidal formula for salivary cortisol at all six time points. Following the methods of Fisher (2012) (Fisher et al. 2012) and based on recommendations from Pruessner 2003, (Pruessner et al. 2003), we retained negative AUCi values to avoid omitting information and examined the difference between positive and negative AUCi classifications. We dichotomized patients into those with positive AUC values, corresponding to a stronger increase than decrease in cortisol levels over time, and those with negative AUC values, corresponding to a stronger decrease than increase in cortisol values over time(Fisher et al. 2012). AUCi at each time point was compared between the negative and positive classifications using Student's independent samples t-test for continuous variables. We also examined if there was a difference between AUCi classifications between AR-PPD and healthy comparison women using a Χ2 test.
All analyses were conducted in SAS Version 9.3 (SAS Institute, Inc., Cary, North Carolina). Study data was managed using Research Electronic Data Capture (REDCap)(O'Keane et al. 2011).
Results
Study population characteristics at time of TSST
A total of 44 women participated in the study. The average age of subjects was 32.9 years (± standard deviation [SD] 4.8). The majority of the subjects were white, non-Hispanic, employed and married. There were no between group differences in number of children in household or household income (Table 1). Overall, women were 31.3 weeks gestational age (±2.3 weeks) at the time of the TSST. AR-PPD women had a higher EPDS total score compared to HCS women. A majority (70.8%) of the AR-PPD women had a past depression diagnosis, including 16.7% with a past major depressive episode with postpartum onset, and 25% had a past anxiety diagnosis.
Table 1.
Study Population Characteristics at time of Trier Social Stress Test (TSST)
| Variablea | AR-PPD (n=24) | HCS (n=20) | P-value* |
|---|---|---|---|
| Age, mean (SD) | 34.02 (5.21) | 31.44 (3.97) | 0.08 |
| Race | |||
| White | 22 (91.67) | 17 (85.00) | 0.65 |
| Non-white | 2 (8.33) | 3 (15.00) | |
| Ethnicity | |||
| Hispanic | 4 (16.67) | 2 (10.00) | 0.67 |
| Not Hispanic or Latino | 20 (83.33) | 18 (90.00) | |
| Education level | |||
| High school diploma or less | 4 (16.67) | 2 (10.00) | 0.035 |
| Partial or completed college | 15 (62.50) | 6 (30.00) | |
| Graduate/professional degree | 5 (20.83) | 12 (60.00) | |
| Marital status | |||
| Never married | 7 (29.17) | 2 (10.00) | 0.15 |
| Married | 16 (66.67) | 18 (90.00) | |
| Divorced/separated/widowed | 1 (4.17) | 0 (0.00) | |
| Number of children in household | |||
| 0 | 9 (37.50) | 11 (55.00) | 0.56 |
| 1 | 10 (41.67) | 6 (30.00) | |
| 2+ | 4 (16.67) | 3 (15.00) | |
| Gestational age in weeks, mean (SD) | 31.55 (2.12) | 30.96 (2.61) | 0.42 |
| Weight at time of TSST (lbs), mean (SD) | 181.70 (32.78) | 174.50 (32.74) | 0.42 |
| EPDS total score, mean (SD) AUCi. | 9.83 (4.86) | 2.40 (1.98) | <.001 |
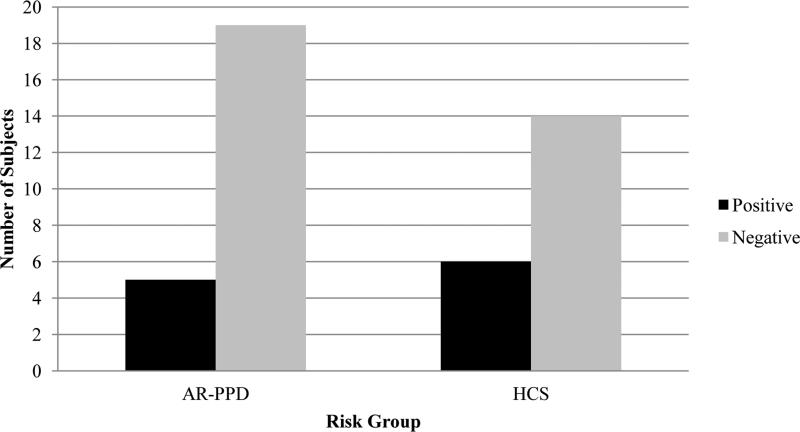
| Positive (>0) | 5 (20.83) | 6 (30.00) | 0.51 |
| Negative (<0) | 19 (79.17) | 14 (70.00) |
Unless otherwise indicated, data are expressed as number (percentage) of subjects. Percentages have been rounded and may not total 100.
P-values from Fisher's Exact Test for categorical variables and Student's independent samples t-test for continuous variables (with Satterthwaite adjustment for unequal variances, when appropriate)
SD: Standard Deviation; AR-PPD: At-risk postpartum depression; HCS: Healthy comparison subject; EPDS: Edinburgh Postnatal Depression Scale; AUCi: area under the curve with respect to the increase, where positive AUC values correspond to a stronger increase than decrease in cortisol levels over time and negative AUC values correspond to a stronger decrease than increase in cortisol values over time.
Psychological rating scales and salivary cortisol response to Trier Social Stress Test
Mean STAI total score significantly differed between groups during the TSST (T0-T75; z=2.71, df=1, p=0.007) with AR-PPD subjects reporting higher anxiety compared to HCS (Table 2). However, a test of group-by-time interaction did not show a significant difference in the rate change in STAI-S by risk group over time (p=0.23). AR-PPD subjects had a higher average CTQ score (13.41 ± 12.75) compared to HCS (3.95 ± 5.59). A comparison of these mean values using Student's t-test showed a significant difference between groups (p=0.003).
Table 2.
Salivary Cortisol (nmol/L) and STAI over time in minutes (T0-T75) during TSST
| Time | T0 | T15 | T25 | T35 | T45 | T75 | |
|---|---|---|---|---|---|---|---|
| Mean (SD) | P-value* | ||||||
| CORTISOL | |||||||
| AR-PPD (n=24) | 6.61 (2.46) | 6.79 (2.99) | 6.42 (2.20) | 6.23 (2.23) | 5.70 (2.11) | 5.09 (2.13) | 0.876 |
| Healthy comparison (n=20) | 6.58 (2.19) | 6.59 (2.68) | 6.78 (2.80) | 6.70 (3.03) | 6.11 (2.54) | 5.27 (2.06) | |
| STAI-S | |||||||
| AR-PPD (n=24) | 31.75 (7.83) | 45.08 (12.44) | 35.00 (12.03) | 32.54 (10.44) | 30.45 (9.48) | 28.67 (7.58) | 0.007 |
| Healthy comparison (n=20) | 26.55 (5.19) | 36.40 (8.20) | 28.45 (6.46) | 25.75 (5.66) | 25.65 (6.60) | 24.90 (4.98) | |
P-values from z test using generalized estimating equations (GEE)
SD: Standard Deviation; AR-PPD: At-risk postpartum depression; STAI-S: Spielberger State-Trait Anxiety Inventory
Overall women had a cortisol level of 6.60 nmol/L (±2.32) at baseline (T0). There was no correlation between EPDS score and baseline cortisol (r=0.1407, p=NS). The average cortisol values at each of the six TSST time points did not differ between AR-PPD and healthy comparison groups (Table 2). Average cortisol values decreased in both groups over time (test for trend p=<.001), but did not differ by group (p=NS), suggesting that cortisol recovery was not prolonged in the AR-PPD group as compared to the HCS group, as hypothesized. GEE models examining longitudinal changes in cortisol to control for the inherent correlation between subjects did not differ significantly by risk group (z=−0.16; df=1; p=NS), EPDS score (z=0.65; df=1; p=NS) or CTQ score (z=0.58; df=1; p=NS). STAI total score also did not affect cortisol concentration over time (z=1.24, df=1, p=NS) and did not differ by group (Χ2=0.05, df=1, p=NS).
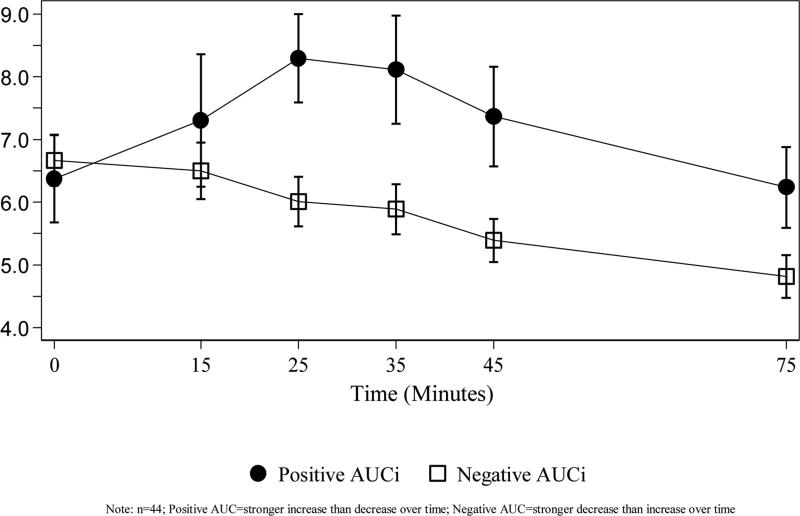
Figure 1 depicts the change in mean cortisol (nmol/L) over time by AUCi group. There was a statistically significant difference in mean cortisol between the positive and negative AUCi groups at T25, T35, T45, and T75 (p=0.006, 0.012, 0.012, 0.048, respectively). The majority (75%) of subjects had a negative AUCi. No significant differences between the proportion of positive and negative AUCi classifications existed between AR-PPD and healthy comparison women (Figure 2). CTQ score did not differ by AUCi group, with a mean score of 7.18 (±8.95) in the positive AUCi subjects and a mean score of 9.94 (±11.90) in the negative AUCi subjects (t-test p-value=0.49).
Figure 1.
Mean (±SE) Salivary Cortisol for AR-PPD and Healthy Comparison Subjects
AUCi: area under the curve with respect to the increase, where positive AUC values correspond to a stronger increase than decrease in cortisol levels over time and negative AUC values correspond to a stronger decrease than increase in cortisol values over time.
AR-PPD: At-risk for postpartum depression; HCS: healthy comparison subject
Figure 2.
Number of Subjects with Positive and Negative AUCi* by Risk Group
* AUCi: area under the curve with respect to the increase, where positive AUC values correspond to a stronger increase than decrease in cortisol levels over time and negative AUC values correspond to a stronger decrease than increase in cortisol values over time.
AR-PPD: At-risk for postpartum depression; HCS: healthy comparison subject
Discussion
This study, which examined salivary cortisol response to the TSST in late gestation in healthy women and those at-risk for PPD, yielded two main findings. First, in this study, healthy pregnant women and those at-risk of PPD had no detectable difference in salivary cortisol response to psychosocial stress induced by the TSST despite clinically significant between-group differences in current/past depression diagnoses and current symptomatology. 75% of women in late gestation showed little elevation in salivary cortisol concentration during the TSST despite increases in state anxiety as measured by the STAI. Our finding is similar to what is reported with the cortisol awakening response across gestation (Entringer et al. 2010) and is comparable to findings by de Weerth (2007) who reported that approximately half of the healthy pregnant women lacked a cortisol response to the TSST (i.e. <2.5nmol/L change over time) (De Weerth et al. 2007).
Our results are in contrast to a prior study which reported cortisol reactivity in early second trimester (i.e. 13-18 weeks) and early third trimester (i.e. 26-31 weeks) healthy euthymic women(Nierop et al. 2006a). For a similar sample size (N=30) among comparison groups, the absolute increase in cortisol reported in that study was greater than we report in our study. Potential explanations for the difference in findings may include; differences in the controlled time frames for collection of cortisol samples including a shorter baseline period compared to other studies and measurement of baseline cortisol at one time point before TSST rather than averaging across the resting period, and study of all pregnant women (i.e. healthy and at-risk) at a later gestational age compared to other studies.
Childhood abuse is associated with increased HPA-axis sensitivity to stress in non-pregnant women with current symptoms of depression and anxiety(Heim et al. 2000) and in healthy non-pregnant subjects it is associated with hypo-responsiveness of the HPA-axis to psychosocial stress(Carpenter et al. 2007; Carpenter et al. 2011). Although at-risk pregnant subjects reported greater childhood abuse than healthy subjects, there was no association between CTQ score and salivary cortisol measurements in late gestation. It is likely that we did not find an association between CTQ and cortisol responsivity since there was only one AR-PPD subject whose CTQ physical abuse subscale score reached the moderate to severe threshold, a threshold which has been shown to be associated with cortisol hyporesponsivity(Carpenter et al. 2011). Future studies should examine the role of maternal early life stress exposure as a risk for PPD.
We hypothesized that women at-risk for PPD would demonstrate an exaggerated salivary cortisol response to the TSST based on findings of higher cortisol awakening response in patients with current and/or a past history of depression(Vreeburg et al. 2009) compared to those without depression. However, consistent with the results of several other studies(Harville et al. 2009; Hellgren et al. 2013; Meinlschmidt et al. 2010; Rothenberger et al. 2011; Shea et al. 2007; Voegtline et al. 2013), we did not find an association between cortisol concentration and anxiety rating scores induced by the psychosocial stressor, the findings suggest that the psychological and the endocrine response to acute psychosocial stress may represent two separate aspects of the stress response in late gestation when both the HPA(Buss et al. 2009; Entringer et al. 2010) and psychological responses to stress are attenuated(Glynn et al. 2001).
Strengths of this study include stringent inclusion/exclusion criteria; subjects underwent thorough research diagnostic and symptomatic evaluations, including the CTQ. Although childhood abuse has profound effects on the HPA-axis, measurement of its moderating effect on cortisol reactivity is not widespread among studies, making comparisons between studies challenging. Another strength of this study is that salivary cortisol was determined by enzyme immunoassay which is preferable to serum total cortisol in the assessment of dynamic HPA activity (Gozansky et al. 2005) and reflects unbound concentrations of cortisol in serum. To maximize salivary cortisol recovery, we used a polyester tampon rather than a cotton tampon within the Salivette®(Hansen et al. 2008). To reduce the variance of salivary cortisol between subjects, the majority (80%) of subjects completed the TSST between 1200 and 1700. We chose this time frame to maximize our ability to elicit a cortisol response by controlling for the difference in circadian variation to the TSST which has been reported in pregnancy, with smaller or absent cortisol reactivity earlier in the day (De Weerth et al. 2007) however 20% women completed the TSST between 1100-1200 due to the challenges in scheduling. No salivary stimulants were utilized. Also, medication use in our study was restricted to prenatal vitamins and stool softeners and all subjects were nonsmokers and not using alcohol, so we were able to limit the impact of these on cortisol concentrations. The sample size among comparison groups, while not large, is comparable to published reports(Hellgren et al. 2013; Meinlschmidt et al. 2010). The differences we observed were not as great as those we used to plan the study; however, a post-experiment power analysis would not be informative in this situation(Hoenig and Heisey 2001; Simon 1986).
Limitations of this study include our sample size and investigation of psychological and endocrine responses but not autonomic responses to the TSST. Additionally, another limitation is that we could not include a healthy non-pregnant comparative population due to limits of the funding mechanism which supported this study. Inclusion of this comparison group would clarify if our results were due to a gestation-dependent diminution of the stress response or if the TSST did not achieve an adequate stress response in all subjects, an alternative explanation for our results. We designed the study with an approach consistent with the Research Domain Criteria (RDoC) (Ostergaard et al. 2014) and included a heterogeneous at-risk population. However, since the AR-PPD group included a variety of clinical phenotypes, the underlying neuroendocrine mechanisms for each clinical phenotype may differ(Schiller et al. 2014) and inclusion as a group may have obscured the ability to see between-group differences in cortisol reactivity. Antepartum anxiety is a risk factor for PPD: in our study sixty-two percent of AR-PPD subjects had an anxiety disorder. There is an emerging literature describing the effects of anxiety on cortisol during pregnancy (Kane et al. 2014; King et al. 2010; O'Connor et al. 2014) and in the postpartum (Labad et al. 2011; Lord et al. 2011) but substantial methodological variation among studies makes it difficult to identify clear associations. The study sample size did not allow us to examine potential differences in cortisol reactivity among the clinical phenotypes.
The majority of previous studies examining the relationship between HPA functioning and peripartum mood and anxiety have focused on the postpartum period. HPA axis reactivity to stress is reduced during the postpartum period(Brunton et al. 2008; Tu et al. 2005) and suppression of HPA reactivity in the postpartum is associated with PPD(Cox et al. 2015; Magiakou et al. 1996; Taylor et al. 2009). Preliminary data suggest antepartum HPA axis abnormalities may contribute to the development of PPD (Bloch et al. 2005; Nierop et al. 2006b). Preclinical and clinical studies indicate that hormonal adaptations during late pregnancy affect endocrine mechanisms associated with maternal postpartum physiological functioning (Brunton et al. 2008; Meinlschmidt et al. 2010) but few studies have investigated the relationship between antepartum HPA functioning with maternal peripartum mood. Furthermore, more studies are needed to examine antepartum HPA functioning in relationship to infant neurodevelopmental outcomes, especially in antepartum women AR-PPD and antepartum depressed women. Understanding how stress impacts maternal HPA functioning during pregnancy is critical to understanding potential neuroendocrine mechanisms of risk for perinatal depressive and anxiety disorders and the impact on the developing fetus(Bolten et al. 2013). This study builds upon prior research on HPA reactivity in the peripartum period(Entringer et al. 2010; Hellgren et al. 2013; Meinlschmidt et al. 2010) and includes a novel and understudied population, women at-risk for developing PPD.
Acknowledgements
This study was supported by National Center for Advancing Translational Sciences, National Institutes of Health Grant (UL1 TR000161) and National Institutes of Health Grant (5K23MH097794) awarded to Dr. Deligiannidis. The sponsoring agency had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this article are those of the authors and do not necessarily reflect the position of the NIH.
We thank Dr. Peter J. Schmidt for his thoughtful comments during preparation of the manuscript and gratefully thank the research subjects for their participation.
Footnotes
Declaration of interest
The authors of this manuscript do not have conflicts of interest relevant to the subject of this manuscript.
The work described in this paper was presented as a poster presentation at the American College of Neuropsychopharmacology (ACNP) 52nd Annual Meeting, Hollywood, FL on December 10, 2013.
References
- Austin MP, Hadzi-Pavlovic D, Priest SR, Reilly N, Wilhelm K, Saint K, Parker G. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? Arch Womens Ment Health. 2010;13:395–401. doi: 10.1007/s00737-010-0153-7. doi:10.1007/s00737-010-0153-7. [DOI] [PubMed] [Google Scholar]
- Beck CT. Predictors of postpartum depression: an update. Nursing research. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire. 1997 doi: 10.1097/00004583-199703000-00012. www.pearsonassessments.com. [DOI] [PubMed]
- Berrettini WH, Nurnberger JI, Jr., Worthington EK, Simmons-Alling S, Gershon ES. Platelet vasopressin receptors in bipolar affective illness. Psychiatry Res. 1982;7:83–86. doi: 10.1016/0165-1781(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Bloch M, Rubinow DR, Schmidt PJ, Lotsikas A, Chrousos GP, Cizza G. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocrinol Metab. 2005;90:695–699. doi: 10.1210/jc.2004-1388. doi:jc.2004-1388 [pii]10.1210/jc.2004-1388. [DOI] [PubMed] [Google Scholar]
- Bolten M, Nast I, Skrundz M, Stadler C, Hellhammer DH, Meinlschmidt G. Prenatal programming of emotion regulation: neonatal reactivity as a differential susceptibility factor moderating the outcome of prenatal cortisol levels. Journal of psychosomatic research. 2013;75:351–357. doi: 10.1016/j.jpsychores.2013.04.014. doi:10.1016/j.jpsychores.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA, Douglas AJ. Adaptive responses of the maternal hypothalamic-pituitary- adrenal axis during pregnancy and lactation. Journal of neuroendocrinology. 2008;20:764–776. doi: 10.1111/j.1365-2826.2008.01735.x. doi:10.1111/j.1365-2826.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease? Psychosom Med. 2003;65:806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- Buss C, Entringer S, Reyes JF, Chicz-DeMet A, Sandman CA, Waffarn F, Wadhwa PD. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. American journal of obstetrics and gynecology. 2009;201:398, e391–398. doi: 10.1016/j.ajog.2009.06.063. doi:10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. doi:10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2011;214:367–375. doi: 10.1007/s00213-010-2007-4. doi:10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EQ, Stuebe A, Pearson B, Grewen K, Rubinow D, Meltzer-Brody S. Oxytocin and HPA Stress Axis Reactivity in Postpartum Women. Psychoneuroendocrinology. 2015 doi: 10.1016/j.psyneuen.2015.02.009. doi: http://dx.doi.org/doi:10.1016/j.psyneuen.2015.02.009. [DOI] [PMC free article] [PubMed]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. Br J Psychiatry. 1993;163:27–31. doi: 10.1192/bjp.163.1.27. [DOI] [PubMed] [Google Scholar]
- De Weerth C, Wied GD, Jansen LM, Buitelaar JK. Cardiovascular and cortisol responses to a psychological stressor during pregnancy Acta obstetricia et gynecologica. Scandinavica. 2007;86:1181–1192. doi: 10.1080/00016340701547442. doi:10.1080/00016340701547442. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders, DSM-5 (American Psychiatric Association, 2013) Fifth Edition edn. American Psychiatric Publishing; Washington, D.C.: [Google Scholar]
- Entringer S, et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010;13:258–268. doi: 10.3109/10253890903349501. doi:10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 2001. [Google Scholar]
- Fisher PA, Kim HK, Bruce J, Pears KC. Cumulative Effects of Prenatal Substance Exposure and Early Adversity on Foster Children's HPA Axis Reactivity During a Psychosocial Stressor. International journal of behavioral development. 2012;36:29–35. doi: 10.1177/0165025411406863. doi:10.1177/0165025411406863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht GF, Campbell T, Letourneau N, Kaplan BJ. Advancing gestation does not attenuate biobehavioural coherence between psychological distress and cortisol. Biological psychology. 2013;93:45–51. doi: 10.1016/j.biopsycho.2013.01.019. doi:10.1016/j.biopsycho.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. American journal of obstetrics and gynecology. 2001;184:637–642. doi: 10.1067/mob.2001.111066. doi:10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Gozansky WS, Lynn JS, Laudenslager ML, Kohrt WM. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic--pituitary--adrenal axis activity. Clinical endocrinology. 2005;63:336–341. doi: 10.1111/j.1365-2265.2005.02349.x. doi:10.1111/j.1365- 2265.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- Hampson E, Phillips SD, Soares CN, Steiner M. Steroid concentrations in antepartum and postpartum saliva: normative values in women and correlations with serum. Biology of sex differences. 2013;4:7. doi: 10.1186/2042-6410-4-7. doi:10.1186/2042-6410-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AM, Garde AH, Persson R. Measurement of salivary cortisol--effects of replacing polyester with cotton and switching antibody. Scandinavian journal of clinical and laboratory investigation. 2008;68:826–829. doi: 10.1080/00365510802056207. doi:10.1080/00365510802056207. [DOI] [PubMed] [Google Scholar]
- Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM. Stress questionnaires and stress biomarkers during pregnancy. J Womens Health (Larchmt) 2009;18:1425–1433. doi: 10.1089/jwh.2008.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Jama. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hellgren C, Akerud H, Skalkidou A, Sundstrom-Poromaa I. Cortisol awakening response in late pregnancy in women with previous or ongoing depression. Psychoneuroendocrinology. 2013;38:3150–3154. doi: 10.1016/j.psyneuen.2013.08.007. doi:10.1016/j.psyneuen.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Hoenig JM, Heisey DM. The Abuse of Power: The pervasive fallacy of power calculations for data analysis. The American Statistician. 2001;55:19–24. [Google Scholar]
- Kane HS, Dunkel Schetter C, Glynn LM, Hobel CJ, Sandman CA. Pregnancy anxiety and prenatal cortisol trajectories. Biological psychology. 2014;100:13–19. doi: 10.1016/j.biopsycho.2014.04.003. doi:10.1016/j.biopsycho.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King NM, Chambers J, O'Donnell K, Jayaweera SR, Williamson C, Glover VA. Anxiety, depression and saliva cortisol in women with a medical disorder during pregnancy. Arch Womens Ment Health. 2010;13:339–345. doi: 10.1007/s00737-009-0139-5. doi:10.1007/s00737-009-0139-5. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer D. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993a;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kuirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993b;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Labad J, et al. Increased morning adrenocorticotrophin hormone (ACTH) levels in women with postpartum thoughts of harming the infant. Psychoneuroendocrinology. 2011;36:924–928. doi: 10.1016/j.psyneuen.2010.11.006. doi:10.1016/j.psyneuen.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Lord C, Hall G, Soares CN, Steiner M. Physiological stress response in postpartum women with obsessive-compulsive disorder: A pilot study. Psychoneuroendocrinology. 2011;36:133–138. doi: 10.1016/j.psyneuen.2010.04.014. doi:10.1016/j.psyneuen.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Lydsdottir LB, Howard LM, Olafsdottir H, Thome M, Tyrfingsson P, Sigurdsson JF. The mental health characteristics of pregnant women with depressive symptoms identified by the Edinburgh Postnatal Depression Scale. J Clin Psychiatry. 2014;75:393–398. doi: 10.4088/JCP.13m08646. doi:10.4088/JCP.13m08646. [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab. 1996;81:1912–1917. doi: 10.1210/jcem.81.5.8626857. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- Meijer JL, et al. Predictive accuracy of Edinburgh postnatal depression scale assessment during pregnancy for the risk of developing postpartum depressive symptoms: a prospective cohort study. BJOG : an international journal of obstetrics and gynaecology. 2014;121:1604–1610. doi: 10.1111/1471-0528.12759. doi:10.1111/1471-0528.12759. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Martin C, Neumann ID, Heinrichs M. Maternal cortisol in late pregnancy and hypothalamic-pituitary-adrenal reactivity to psychosocial stress postpartum in women. Stress. 2010;13:163–171. doi: 10.3109/10253890903128632. doi:10.3109/10253890903128632. [DOI] [PubMed] [Google Scholar]
- Milgrom J, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108:147–157. doi: 10.1016/j.jad.2007.10.014. doi:10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, Klinkenberg A, Nater UM, Zimmermann R, Ehlert U. Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary alpha-amylase responses to psychosocial stress in human pregnancy. J Clin Endocrinol Metab. 2006a;91:1329–1335. doi: 10.1210/jc.2005-1816. doi:jc.2005-1816 [pii]10.1210/jc.2005-1816. [DOI] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med. 2006b;68:931–937. doi: 10.1097/01.psy.0000244385.93141.3b. doi:68/6/931 [pii]10.1097/01.psy.0000244385.93141.3b. [DOI] [PubMed] [Google Scholar]
- Nolten WE, Lindheimer MD, Rueckert PA, Oparil S, Ehrlich EN. Diurnal patterns and regulation of cortisol secretion in pregnancy. J Clin Endocrinol Metab. 1980;51:466–472. doi: 10.1210/jcem-51-3-466. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Tang W, Gilchrist MA, Moynihan JA, Pressman EK, Blackmore ER. Diurnal cortisol patterns and psychiatric symptoms in pregnancy: short-term longitudinal study. Biological psychology. 2014;96:35–41. doi: 10.1016/j.biopsycho.2013.11.002. doi:10.1016/j.biopsycho.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara MW, Swain A. Rates and risk of postpartum depression- a meta-analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- O'Keane V, et al. Increased pituitary-adrenal activation and shortened gestation in a sample of depressed pregnant women: a pilot study. J Affect Disord. 2011;130:300–305. doi: 10.1016/j.jad.2010.10.004. doi:10.1016/j.jad.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Ostergaard SD, Fava M, Rothschild AJ, Deligiannidis KM. The implications of the National Institute of Mental Health Research Domain Criteria for researchers and clinicians. Acta Psychiatr Scand. 2014;130:409–414. doi: 10.1111/acps.12331. doi:10.1111/acps.12331. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Righetti-Veltema M, Conne-Perreard E, Bousquet A, Manzano J. Risk factors and predictive signs of postpartum depression. J Affect Disord. 1998;49:167–180. doi: 10.1016/s0165-0327(97)00110-9. [DOI] [PubMed] [Google Scholar]
- Rothenberger SE, Moehler E, Reck C, Resch F. Prenatal stress: course and interrelation of emotional and physiological stress measures. Psychopathology. 2011;44:60–67. doi: 10.1159/000319309. doi:10.1159/000319309. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, Rubinow DR. The role of reproductive hormones in postpartum depression. CNS Spectr. 2014:1–12. doi: 10.1017/S1092852914000480. doi:10.1017/s1092852914000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte HM, Weisner D, Allolio B. The corticotrophin releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clinical endocrinology. 1990;33:99–106. doi: 10.1111/j.1365-2265.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. doi:10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Simon R. Confidence intervals for reporting results of clinical trials. Annals of internal medicine. 1986;105:429–435. doi: 10.7326/0003-4819-105-3-429. [DOI] [PubMed] [Google Scholar]
- Smith R, et al. Mood changes, obstetric experience and alterations in plasma cortisol, beta- endorphin and corticotrophin releasing hormone during pregnancy and the puerperium. Journal of psychosomatic research. 1990;34:53–69. doi: 10.1016/0022-3999(90)90008-r. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Nemeroff CB. Women at risk for postpartum-onset major depression. American journal of obstetrics and gynecology. 1995;173:639–645. doi: 10.1016/0002-9378(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Taylor A, Glover V, Marks M, Kammerer M. Diurnal pattern of cortisol output in postnatal depression. Psychoneuroendocrinology. 2009;34:1184–1188. doi: 10.1016/j.psyneuen.2009.03.004. doi:10.1016/j.psyneuen.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Tu MT, Lupien SJ, Walker CD. Measuring stress responses in postpartum mothers: perspectives from studies in human and animal populations. Stress. 2005;8:19–34. doi: 10.1080/10253890500103806. doi:10.1080/10253890500103806. [DOI] [PubMed] [Google Scholar]
- Voegtline KM, Costigan KA, Kivlighan KT, Laudenslager ML, Henderson JL, DiPietro JA. Concurrent levels of maternal salivary cortisol are unrelated to self-reported psychological measures in low-risk pregnant women. Arch Womens Ment Health. 2013;16:101–108. doi: 10.1007/s00737-012-0321-z. doi:10.1007/s00737-012-0321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg SA, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. doi:10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]