Review on BRD4 as a protein with pleiotropic functions: scaffold for chromatin and transcription factors, chromatin remodeler through HAT activity, and regulator of transcription through kinase activity.
Keywords: BRD4, kinase, Pol II, acetyltransferase, nucleosome eviction
Abstract
Bromodomain protein 4 (BRD4) is a transcriptional and epigenetic regulator that plays a pivotal role in cancer and inflammatory diseases. BRD4 binds and stays associated with chromatin during mitosis, bookmarking early G1 genes and reactivating transcription after mitotic silencing. BRD4 plays an important role in transcription, both as a passive scaffold via its recruitment of vital transcription factors and as an active kinase that phosphorylates RNA polymerase II, directly and indirectly regulating transcription. Through its HAT activity, BRD4 contributes to the maintenance of chromatin structure and nucleosome clearance. This review summarizes the known functions of BRD4 and proposes a model in which BRD4 actively coordinates chromatin structure and transcription.
Introduction
BRD4, a chromatin-binding protein, was first identified as a mitotic bookmark and implicated in cell-cycle control [1]. Since its original description, BRD4 has become the subject of considerable interest as a possible therapeutic target in both cancer and autoimmune disease. A number of hematopoietic malignancies, including AML [2], depend on the continued expression of BRD4; a BRD4 translocation fusion product, BRD4-NUT, is responsible for aggressive midline carcinomas [3]. BRD4 also plays a role in autoimmunity and inflammatory diseases via its interactions with NF-κB [4]. Recent studies have also shown a role for BRD4 in the regulation of repression of HIV-1 latency, kidney disease, cardiac hypertrophy, and drug addiction [5–9]. Despite the clear involvement of BRD4 in mediating disease, understanding of its actual biologic functions is only now emerging. In this review, we summarize the current status of our understanding of BRD4 structure and function, emphasizing the roles of its HAT and kinase activities in the regulation of chromatin structure, transcription, and development.
BRD4 STRUCTURE
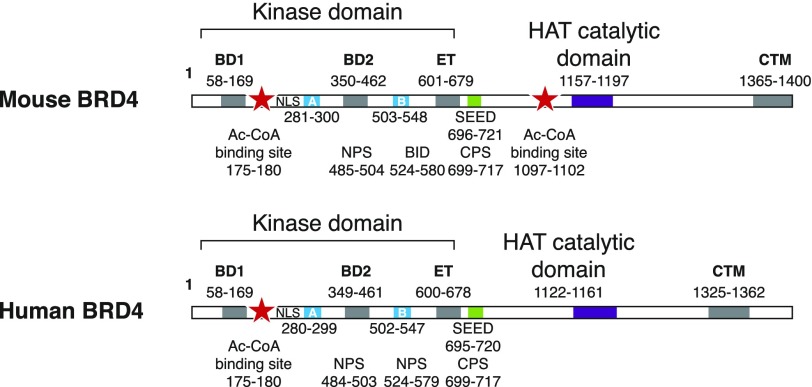
BRD4 is a member of the BET family of proteins that is comprised of BRD2, BRD3, BRD4, and BRDt [10]. The 1362-aa human BRD4 protein contains 2 bromodomains (BD1 and BD2), 2 conserved motifs termed A and B, an N terminal cluster of phosphorylation sites, a basic residue–enriched interaction domain, an ET domain, a SEED domain enriched in serine residues mixed with glutamic and aspartic acid residues, as well as a CTM (Fig. 1) [11].
Figure 1. Functional domains on mouse and human BRD4.
Numbers on cartoon indicate amino acid boundaries of known BRD4 domains. BID, basic residue–enriched interaction domain, CPS, C terminal cluster of phosphorylation sites, NPS, N terminal cluster of phosphorylation sites.
Alternative splicing of the BRD4 gene gives rise to a short 722-aa isoform C that retains the 2 bromodomains but lacks all regions C terminal to the SEED domain. The relative abundance of the long and short forms of BRD4 vary among different cell types [11]. A third isoform, B, has been identified that is truncated at 722 aa, similar to isoform C, but contains an additional C terminal peptide of 76 aa [12]. Isoform B has only been described in U2OS cells.
BRD4 N TERMINAL BROMODOMAINS
Bromodomains occur in a variety of nuclear proteins that function as scaffolds for transcriptional regulators, chromatin modulators, and chromatin-modifying enzymes. Bromodomains are commonly found in chromatin-binding proteins, such as HATs TAF1 and CBP/p300, as well as remodeling factors, such as SWI/SNF family members; 46 bromodomain-containing proteins have been identified that fall into 8 distinct families [13]. The BET family of bromodomain proteins, which contains BRD4, is one of those families and is distinguished by the presence of 2 bromodomains and an ET domain.
The 2 bromodomains of BRD4 are located in tandem within the N terminus and mediate the binding of BRD4 to chromatin via their interactions with acetylated lysines on histones H3 and H4. Whereas the bromodomains largely bind to specific acetylated lysines on histones (H3 and H4) [14], the BD2 domain of BRD4 also interacts with acetylated lysines on other protein partners, including the relA subunit of NF-κB [15] and the cyclin T1 subunit of PTEFb [16]. The structures of BRD4 BD1 (aa 58–169) and BD2 (aa 349–461) have been solved and consist of the archetypal bromodomain fold comprised of 2 interconnected loops (ZA and BC) from their N and C terminal regions and 4 α-helices (Z, A, B, C) [17]. The 2 loops form a pocket that encompasses a recognition site for acetylated lysines. A left-handed helical, hydrophobic core in each bromodomain is created by the 4 helices. The extended loop ZA that connects helices Z and A is comprised of 3 short helices [17]. Of interest, the BRD2 BD1 bromodomain has been shown to form an intact homodimer and BRD4 is known to dimerize in vivo through BD1 [17, 18]. The 2 BRD4 bromodomains have relatively low homology with bromodomains from other bromodomain families; however, they are highly homologous (>75%) with the bromodomains of other BET proteins [11]. Yeast Bdf1 protein, an ortholog of the mammalian TAF1 bromodomain protein, is one of these other BET proteins, which suggests that it might be an evolutionary progenitor for both TAF1 and BRD4 [11].
BRD4 C TERMINAL DOMAINS
Whereas the N terminal domain of BRD4 largely governs its interactions with acetylated lysine residues, the C terminal domains mediate its interactions with the spectrum of proteins for which BRD4 is a scaffold. The ET domain, which defines the BET family, is located between aa 600–678 and consists of 3 α-helices [19]. Although its function remains to be fully characterized, evidence suggests that it is a site for protein–protein interaction with some of the BRD4 partners. Among these are the histone methyl transferase NSD3, the histone demethylase JMJD6, and a component of the nuclear remodeling and deacetylase repressor complex, CHD4, all 3 of which regulate transcription, presumably via their interaction with the BRD4 ET domain [20].
The 2 conserved A (aa 280–299) and B (aa 502–547) domains are believed to be regions through which BRD4 contacts nucleosomal DNA [21]. The basic residue–enriched interaction domain (aa 524–580) and N terminal cluster of phosphorylation sites (aa 485–504) domains have been reported to be part of a phosphoswitch mechanism that regulates BRD4 binding to chromatin as well as its binding to and recruitment of p53 to transcription sites [22]. The region that encompasses the SEED domain (aa 695–720), part of which is called the C terminal cluster of phosphorylation sites, has been reported to be the site of phosphorylation by CK2, which triggers conformational changes in BRD4, affecting its function [22]. Finally, the CTM region of human BRD4 (aa 1325–1362) spans its interaction site with the transcription elongation factor, PTEFb [6].
BRD4 ENZYMATIC ACTIVITIES
In addition to functioning as a scaffold for a variety of transcription factors, BRD4 has 2 intrinsic enzymatic activities. Work from our lab has demonstrated that BRD4 is an atypical kinase whose activity maps to its N terminal domain; deletion of segments N terminal to the ET domain abrogates the kinase activity [23]. One of the BRD4 substrates is the heptad repeat in RNA Pol II CTD, which BRD4 phosphorylates specifically on Ser2 residues [23]. Surprisingly, as we have recently demonstrated, BRD4 also has intrinsic HAT activity [24]. The HAT activity of human BRD4 maps to a consensus acetyl-CoA binding site between aa 175–180 and a catalytic domain located distal to the ET domain, in the segment between aa 1122 and aa 1161. Whereas both human and mouse BRD4 have a homologous acetyl-CoA binding site and a catalytic domain, mouse BRD4 has an additional acetyl-CoA binding site in the C terminus (aa 1097–1102) [24].
BRD4 IN TRANSCRIPTION
BRD4 plays multiple roles in transcription: as a scaffold for transcription factors, a nucleator of superenhancers, and a kinase that is involved in transcription elongation and pause release. BRD4 plays a major role as a scaffold in the life cycle of the papilloma virus family via its interaction with the immediate early proteins, E2. E2 proteins are required to initiate and maintain viral DNA replication and transcription. BRD4 binds to E2, which prevents its degradation, modulates E2-mediated transcription, and anchors it and the HPV episome to the host chromosome during mitosis to prevent loss of viral genomes [25]. Recruitment of HPV E2 to the HPV promoter by BRD4 prevents recruitment of transcription factor II D (TFIID) and Pol II, thereby repressing transcription [25]. BRD4 is also a cellular cofactor for the murine leukemia virus integrase, mediating murine leukemia virus integration at transcription start sites [26].
Evidence for the role of BRD4 in normal cellular transcription has come from studies that show that BRD4 functions as a mitotic bookmark, remaining associated with the transcription start sites of M/G1 genes. During telophase, BRD4 recruits PTEFb to promoters, which thus enables postmitotic transcription elongation of early G1 genes for cell cycle progression [27]. BRD4 also bookmarks genes that are expressed during the previous cell cycle, which leads to their rapid re-expression in G1 [28]. BRD4 is also known to play a critical role in immediate early gene transcription in neuronal cells, controlling the expression of many critical immediate early genes as well as synaptic receptor proteins, thus controlling memory formation [29]
BRD4 regulation of transcription is both passive and active, affecting only a subset of genes in a cell type–specific fashion. As a passive scaffold, BRD4 recruits a variety of transcription factors to transcription start sites, and among the best characterized is the transcription elongation factor, PTEFb [30]. BRD4 also binds to a variety of chromatin-modifying enzymes, as noted above, as well as specific subunits of the Mediator complex [11, 31], a critical component of the preinitiation complex that links transcription factors to Pol II. Of interest, BRD4 plays a role in thymic differentiation by bridging the transcriptional regulator, Aire, and the transcription elongation factor, PTEFb, to promote elongation of peripheral tissue antigen transcripts in thymic stromal cells [32].
Our discovery that BRD4 has intrinsic kinase activity through which it directly regulates RNA pol II identified its active role in transcription, beyond its passive role as a scaffold. BRD4 phosphorylates RNA Pol II CTD, a unique structure with repeating heptads of amino acids that is essential for Pol II function. Of the 3 serines residues on which Pol II CTD can be phosphorylated, BRD4 phosphorylates Ser2, which is essential for the transition of Pol II from initiation to productive transcription elongation and for the recruitment of RNA splicing factors [23]. Of importance, phosphorylation of Pol II at Ser2, specifically by BRD4, results in the activation of Top I [33]. Top I controls transcription by unwinding the DNA supercoils that create a mechanical barrier to Pol II progression. Whereas Top I is basally active when bound to Pol II, CTD phosphorylation specifically by BRD4 superactivates it, which results in transcriptional pause release [33].
BRD4 is also a central player in the regulatory network of crosstalk among the 3 RNA Pol II CTD kinases that governs transcription initiation and early elongation [34]. Thus, the CDK7 component of transcription factor II H (TFIIH) phosphorylates BRD4 to regulate its kinase activity. In turn, BRD4 phosphorylation of the CDK9 component of PTEFb modulates its kinase activity [34]. Initial biochemical analyses demonstrating such crosstalk have been extended by kinetic studies [35]. In addition, BRD4 directly interacts with and phosphorylates the TFIID component TAF7 to modulate its activity as a checkpoint regulator of transcription. TAF7, in turn, inhibits the kinase activity of BRD4 as well as the other 2 major CTD kinases, CDK7 and CDK9 [34]. In aggregate, these activities of BRD4 define it as a direct and active regulator of transcription.
Whereas early studies of BRD4 mostly focused on its function at promoters, recent work has demonstrated that BRD4 is also enriched at enhancers and plays a large role in controlling enhancer activity and transcription of enhancer RNA at these sites [36, 37]. Besides being found at thousands of typical enhancer elements, BRD4 nucleates superenhancers, which are clusters of enhancer elements that are highly enriched for transcription factors and coactivators, such as Mediator and BRD4, that often extend across many kilobases [38]. Most superenhancers are cell type specific, associated with cell type–specific master transcription factors, or enriched at oncogenes [38]. The cell-type specificity of BRD4 in the regulation of transcription, in large part, is likely a reflection of its association with superenhancers. For example, BRD4 is associated with superenhancers within the MYC locus in various cancers, including AML and multiple myeloma [38, 39].
BRD4 IN CHROMATIN STRUCTURE
In addition to its role in regulating transcription both directly and indirectly, BRD4 also plays a major role in regulating chromatin structure. It is critical in the maintenance of higher-order chromatin structure [18]. Full-length BRD4 (1–1362), via its intrinsic HAT activity, mediates large-scale chromatin decondensation [24]. Accordingly, dissociation of BRD4 from chromatin causes severely fragmented chromatin morphology [18]. Mechanistic studies with BRD4 truncation mutants showed that the C terminal segment of BRD4, which contains the HAT domains, was necessary for maintaining normal chromatin structure [24]. Consistent with the BRD4 HAT activity leading to chromatin remodeling, it has been shown that a dominant negative inhibitor of BRD4 consisting of the 2 N terminal BRD4 bromodomains—deleted of the HAT domains—led to fragmented chromatin [18].
As noted in the section on BRD4 structure, BRD4 also gives rise to an alternatively spliced short isoform of BRD4 (1–794) that does not encompass HAT domains. This short isoform has been shown to recruit the condensin II chromatin remodeling complex to chromatin, which leads to compacted chromatin and insulation from DNA damage [12]. Thus, the various isoforms seem to alternatively regulate chromatin structure, although the mechanisms by which this is achieved remain unknown. In this regard, it has also been established that BRD4 molecules interact intermolecularly on chromatin via the bromodomains, which raises the possibility that these interactions contribute to the regulation of local chromatin structure and further document the complexity of BRD4 function [18]. Although most cells contain both long and short BRD4 isoforms, the mechanisms that regulate the alternative splicing of BRD4 are still unknown. Other evidence that points toward an important role for BRD4 in chromatin remodeling was the demonstration that BRD4-associated mitotic loci are larger/decompacted relative to non-BRD4–associated loci [28]. Treatment of the cells with JQ1, a BRD4 inhibitor, returned these loci to their normal size.
The role of BRD4 in maintaining chromatin structure and histone acetylation has been clarified by our recent finding that BRD4 has intrinsic HAT activity, which plays a critical role in regulating chromatin structure [24]. BRD4 HAT activity is distinct from that of other HATs, as it acetylates most of the H3 tail lysines, except H3K14, a site acetylated by all other known H3 HATs. Crucially, BRD4 also acetylates the globular region of H3 at the K122 residue, which is located at the point on the dyad axis of the nucleosome where DNA-histone interactions are strongest. Acetylation at H3K122 breaks a salt bridge that leads to nucleosome destabilization and, ultimately, nucleosome clearance and chromatin decompaction [24, 40]. BRD4 preferentially remodels chromatin and reduces nucleosome occupancy around the promoters and genes that it is known to target and regulate, such as Myc, Fos and Aurora B kinase. Furthermore, overexpression of BRD4 results in global remodeling around promoters, accompanied by increased transcription [24].
BRD4 IN CELL CYCLE REGULATION
BRD4 plays a role throughout the cell cycle, and levels of BRD4 are critical to its regulation of cell cycle and proliferation. It is necessary for mitosis to proceed normally [41]. Depletion of BRD4 leads to aberrant mitosis, with a high incidence of lagging chromosomes, leading to micronuclei, and bridging chromosomes, which results in cytokinesis failure and multilobulated nuclei [42]. Levels of Aurora B, a kinase that is required for proper mitosis, are directly regulated by BRD4 [42]. Of importance, BRD4 remains associated with mitotic chromosomes, functioning as a mitotic bookmark for early G1 genes, such as myc and fos [1, 27, 43]. The transition from G1 to M likely depends on both BRD4’s HAT activity, which is necessary for chromatin decompaction, and its kinase activity, which mediates transcription and pause release [23, 24]. Thus, the association of BRD4 with M/G1 genes correlates with the maintenance of high levels of chromatin acetylation during mitosis [27]. Conversely, overexpression of the BRD4 short isoform B, which is devoid of HAT activity, leads to an alteration of chromatin structure and chromothrypsis [12]. By binding to the transcription start sites of M/G1 genes, BRD4 directs their rapid postmitotic transcription. Accordingly, depletion of BRD4 is associated with less production of newly synthesized M/G1 gene RNAs [43]. Indeed, BRD4 provides transcriptional memory of genes: its association via mitosis results in the more rapid re-expression of genes that were expressed during the previous cell cycle [28].
In addition, BRD4 has been reported to regulate the transition from G2 to M via its interaction with the GAP protein, SPA-1 [44]. SPA-1 is induced in lymphocytes in response to mitogen activation [45]; ectopic SPA-1 also blocks the G2 to M transition in HeLa cells. BRD4 negatively regulates SPA-1, which relieves the block to cell-cycle progression [44]. Deletion of BRD4 in either NIH3T3 or HeLa cells arrests cells in G1, whereas ectopic expression of BRD4 is paradoxically similarly inhibitory [27, 43, 46]. Depletion of BRD4 alternatively triggers apoptosis or senescence, although it is not clear what determines which will occur [47] (unpublished observations). BRD4 has been reported to play roles in both the DNA damage response and oxidative stress, where depletion of BRD4 results in aberrant stress responses [12, 48].
BRD4 IN CELL DIFFERENTIATION AND DEVELOPMENT
Consistent with its pleiotropic activities in regulating the cell cycle, BRD4 also plays a critical role in regulating differentiation and development; however, BRD4 is not a general transcription factor. It is only associated with approximately 10% of gene regulatory elements in any given cell type, both superenhancer and traditional enhancers as well as promoters [49]. Genes that are regulated by BRD4 are those that define the differentiation status of the cell type as well as cell cycle genes [38, 49, 50]. For example, BRD4 is essential for the maintenance of human and mouse ESC identity [51]. Silencing of BRD4 either by short hairpin RNA or treatment with BET inhibitors causes ESC cells to accumulate in G1 phase of the cell cycle and acquire the morphology of differentiating cells. Induction of differentiation reflects the down-regulation of genes that are important for the ESC identity, such as Oct4, Nanog, and Prdm14, and the up-regulation of genes that are involved in EMT and neuroectodermal differentiation [51]. Of interest, BRD4 not only regulates Oct4 gene expression but is recruited by OCT4 protein to specific regulatory regions in ESC, including those of the long non-coding RNAs that regulate X chromosome inactivation [52].
BRD4 is also required for the re-expression of stem cell genes during reprogramming of MEFs to induced pluripotent stem cells, where it is required for the release of poised RNA Pol II; depletion of BRD4 prevents the completion of reprogramming [53]. Reprogramming of C/EBP activated somatic B cells to induced pluripotent stem cells also depends on the binding of BRD4 to the superenhancers of pluripotency genes, presumably mediating chromatin remodeling and transcription [54]. In contrast to its role in maintaining stem cell identity, BRD4 is also required for the maintenance of multiple differentiation programs. Generation of hematopoietic cells depends on the presence of BRD4. In the absence of BRD4, bone marrow stem cells are unable to give rise to lymphoid stem cells, which results in a failure in the differentiation of B and T cells [55]. BRD4-depleted human ESC on OP9 culture do not generate hematopoietic progenitors or mature blood cells [56]. Taken together, the available evidence indicates that BRD4 plays a critical role in maintaining cell identity, whether of stem cells or differentiated cells, consistent with its role in regulating chromatin structure and transcription.
Reflecting its importance in maintaining the cell differentiation state, BRD4 is critical to normal development and organogenesis. Germline deletion of BRD4 is embryonically lethal, with arrest occurring by 6 d postcoitus, possibly as a result of the failure of ESCs to undergo differentiation [57]. Sustained BRD4 silencing in mice induced multiple developmental defects. Among them were skin hyperplasia, which is characterized by follicular dysplasia and abnormal hair growth, and loss of secretory cells populations (lysozyme-positive paneth cells, mucin and goblet cells) as well as stem cells at the base of crypts in the intestine [55]. BRD4 seems to contribute to spermatogenesis, where there is a correlation between the hyperacetylation of chromatin and the binding of BRD4 to activated genes during spermatogenesis. During spermiogenesis, BRD4 relocalizes to spermatid acrosomes, concomitant with the condensation of chromatin and loss of hyperacetylated histones [58]. Finally, recent studies from our laboratory demonstrate that the absence of BRD4 during early thymic development leads to a marked loss of peripheral T cells (unpublished observations).
The absolute level of expression of BRD4 is critical for embryonic development. BRD4+/− heterozygous embryos can develop to day 17 postcoitus, but only one half survive. Heterozygous BRD4 neonates and pups are smaller than wild type. Adult mice display various morphologic abnormalities in the skin, liver, brain, testis, and eye [57]. Thus, 1 allele deletion of Brd4 is sufficient to allow the ESC to commit to differentiation but not sufficient for complete mouse development.
BRD4 IN CANCER
BRD4 is currently receiving considerable attention as a possible therapeutic target in cancer because of its regulation of cell cycle genes, especially Myc. A number of hematopoietic malignancies, notably AML, depend on BRD4 activation of Myc transcription. Inhibition of BRD4 binding to chromatin by small-molecule inhibitors, such as JQ1 or iBET, blocks Myc expression and suppresses the activity of hematopoietic transcription factors, inducing arrest of AML cell proliferation accompanied by myeloid differentiation [2, 59]. The loss of cell proliferation upon global BRD4 inhibition is accompanied by a global loss of histone acetylation, which suggests that BRD4 HAT activity serves to maintain the Myc locus—and other cell cycle genes—in an open chromatin configuration [24]. In vivo, transplantation of BRD4-depleted AML cells into recipient mice markedly delays leukemia progression [2]. Similar findings have been reported for other hematopoietic malignancies, including mixed-lineage leukemia fusion–driven leukemia, DLBCL and Burkitt lymphomas [60–63]. However, not all Myc-driven cancer cells are susceptible to BRD4 inhibition [63], and not all hematopoietic tumors are BRD4 dependent [61].
In addition to its regulation of Myc expression, BRD4 also plays a critical role in regulating Myc-independent pathways. For example, although inhibition of BRD4 inhibits the growth of DLBCL cells, ectopic expression of Myc does not restore cell growth, which suggests that other pathways are regulated by BRD4. Indeed, both the BCR signaling pathway and E2F target genes are affected by BRD4 inhibition. In DLBCL, BRD4 is associated with superenhancers of the POU2AF1 locus (encoding OCA-B) and the BCL6 gene, which is frequently mutated in DLBCL. OCA-B is a regulatory factor involved in B cell development, maturation, and germinal center formation. Inhibition of BRD4 decreases OCA-B expression, which leads to decreased proliferation of DLBCL cells [63]. Similar results were observed in mixed-lineage leukemia fusion leukemia, where BCL2 and CDK6 expression were affected by BRD4 inhibition [60].
Solid tumors are also regulated by BRD4, independent of Myc. In estrogen receptor–positive breast cancer, estrogen-induced transcriptional activity is enhanced by BRD4. Depletion of BRD4 in estrogen receptor–positive breast cancer cells leads to a global decrease of estrogen-induced gene expression [64]. Accordingly, BRD4 is found to be associated with estrogen-responsive genes at the transcription start site and within the transcribed regions. Association of BRD4 with estrogen response elements correlates with both Pol II occupancy at promoters and enhancers as well as increased mRNA and enhancer RNA synthesis at induced genes. In vitro, BRD4 depletion or inhibition affects the proliferation of breast cancer cell lines MCF7 and Ishikawa cells in either the absence or presence of estrogen [65]. BRD4 also regulates genetically diverse glioblastoma tumors: inhibition of BRD4 by the BET inhibitor JQ1 represses tumor growth [66].
Whereas BRD4 HAT activity is likely to contribute to its protumorigenic effects in most hematopoietic and solid tumors, it does not contribute to the aggressive phenotype of the NUT midline carcinoma. These tumors are characterized by the presence of a fusion between the N-terminal domain of BRD4 and the NUT gene [3]. This fusion truncates the BRD4 HAT domain but leaves the bromodomains of BRD4 intact, which allows the fusion protein to bind to chromatin. Of interest, the BRD4-NUT fusion results in the hyperacetylation of chromatin megadomains as a result of the recruitment by NUT of the HAT, p300, which aberrantly acetylates chromatin over stretches of 100 kb to 2 Mb, nucleated at enhancers or promoters where BRD4 binds, which results in increased transcription of the genes in and nearby the domain [67].
The role of BRD4 in metastasis, as opposed to primary tumors, is less clear. It has been described as a metastasis susceptibility gene as ectopic expression of BRD4 led to a repression of metastasis and primary tumor growth in human breast cancer [64]. Conversely, expression of the truncated isoform C of BRD4 restores metastatic capacity [68]. Of interest, deletion of the proline-rich region between the ET domain and the CTM on BRD4 led to the promotion of EMT, a central feature of metastasis [68]. BRD4 is also known to interact with the EMT transcription factor Twist when it is diacetylated [69]. Twist recruitment of BRD4 directs WNT5A expression in BLBC, which suggests that the Twist-BRD4–activated EMT program confers growth advantages to BLBC. Accordingly, BET inhibition disrupted the BRD4-Twist interaction, reduced Wnt5A expression, and reduced invasion and tumorigenicity of BLBC [69]. Thus, BRD4 plays an important role in EMT and metastasis, although the molecular mechanisms involved are yet to be elucidated in detail.
CONCLUSIONS
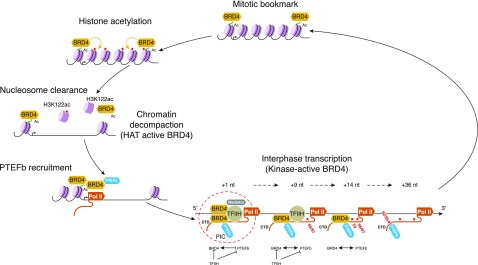
BRD4 is a protein with pleiotropic functions: it is a scaffold for various chromatin and transcription factors, a chromatin remodeler via its HAT activity, and a regulator of transcription via its kinase activity. It is essential for normal cell cycle, differentiation, and development. Taken together, these findings lead to a model in which BRD4 actively coordinates these processes (Fig. 2). We hypothesize that the pleiotropic activities of BRD4 are integrated to achieve transcriptional activation: it functions as a mitotic bookmark of early G1 genes, mediates chromatin decompaction—either in response to activation signals or entry into G1—at its target enhancers and promoters, recruits relevant transcription factors, including PTEFb, and contributes to transcriptional pause release through its phosphorylation of Pol II CTD Ser2. The mechanisms by which BRD4’s various activities are themselves regulated to ensure an orderly progression through cell cycle and transcription remain to be determined.
Figure 2. Model incorporating BRD4 molecular functions and the proposed sequence in which its enzymatic activities function.
BRD4 binds to mitotic chromatin, bookmarking early G1 genes. Toward the end of mitosis, BRD4 acetylates histones, initially on the tail lysines and eventually on H3K122 in the globular region of histone H3, which leads to nucleosome clearance and enables the recruitment of transcription machinery. BRD4 then regulates transcriptional pause release and elongation via its phosphorylation of RNA Pol II CTD and its recruitment of PTEFb to the preinitiation complex containing transcription factor II H (TFIIH).
AUTHORSHIP
B.N.D., A.G. and D.S.S. performed the literature review and prepared the manuscript together. B.N.D. and D.S.S. planned and prepared figures and models.
ACKNOWLEDGMENTS
The authors are supported by the Intramural Research Program of the U.S. National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Glossary
- AML
acute myeloid leukemia
- BET
bromodomain and extraterminal domain
- BLBC
basal-like breast cancer
- BRD4
bromodomain 4
- CTM
C terminal interaction motif
- DLBCL
diffuse large B cell lymphoma
- EMT
epithelial-to-mesenchymal transition
- ESC
embryonic stem cell
- ET
extraterminal
- HAT
histone acetyltransferase
- HPV
human papillomavirus
- OCA-B
Oct co-activator from B cells
- PTEFb
positive transcription elongation factor b
- RNA Pol II CTD
RNA polymerase II C terminal domain Ser2
- SEED motif
Ser (S)/Glu (E)/ Asp (D) motif
- SPA-1
signal-induced proliferation-associated protein 1
- TAF1
TFIID associated factor 1
- Top I
topoisomerase I
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Dey A., Ellenberg J., Farina A., Coleman A. E., Maruyama T., Sciortino S., Lippincott-Schwartz J., Ozato K. (2000) A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G(2)-to-M transition. Mol. Cell. Biol. 20, 6537–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuber J., Shi J., Wang E., Rappaport A. R., Herrmann H., Sison E. A., Magoon D., Qi J., Blatt K., Wunderlich M., Taylor M. J., Johns C., Chicas A., Mulloy J. C., Kogan S. C., Brown P., Valent P., Bradner J. E., Lowe S. W., Vakoc C. R. (2011) RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 478, 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.French C. A., Miyoshi I., Kubonishi I., Grier H. E., Perez-Atayde A. R., Fletcher J. A. (2003) BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 63, 304–307. [PubMed] [Google Scholar]
- 4.Nicodeme E., Jeffrey K. L., Schaefer U., Beinke S., Dewell S., Chung C. W., Chandwani R., Marazzi I., Wilson P., Coste H., White J., Kirilovsky J., Rice C. M., Lora J. M., Prinjha R. K., Lee K., Tarakhovsky A. (2010) Suppression of inflammation by a synthetic histone mimic. Nature 468, 1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu J., Gaiha G. D., John S. P., Pertel T., Chin C. R., Gao G., Qu H., Walker B. D., Elledge S. J., Brass A. L. (2012) Reactivation of latent HIV-1 by inhibition of BRD4. Cell Reports 2, 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisgrove D. A., Mahmoudi T., Henklein P., Verdin E. (2007) Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proc. Natl. Acad. Sci. USA 104, 13690–13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G., Liu R., Zhong Y., Plotnikov A. N., Zhang W., Zeng L., Rusinova E., Gerona-Nevarro G., Moshkina N., Joshua J., Chuang P. Y., Ohlmeyer M., He J. C., Zhou M. M. (2012) Down-regulation of NF-κB transcriptional activity in HIV-associated kidney disease by BRD4 inhibition. J. Biol. Chem. 287, 28840–28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiltoir J. I., Stratton M. S., Cavasin M. A., Demos-Davies K., Reid B. G., Qi J., Bradner J. E., McKinsey T. A. (2013) BET acetyl-lysine binding proteins control pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 63, 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sartor G. C., Powell S. K., Brothers S. P., Wahlestedt C. (2015) Epigenetic readers of lysine acetylation regulate cocaine-induced plasticity. J. Neurosci. 35, 15062–15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florence B., Faller D. V. (2001) You bet-cha: a novel family of transcriptional regulators. Front. Biosci. 6, D1008–D1018. [DOI] [PubMed] [Google Scholar]
- 11.Wu S. Y., Chiang C. M. (2007) The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J. Biol. Chem. 282, 13141–13145. [DOI] [PubMed] [Google Scholar]
- 12.Floyd S. R., Pacold M. E., Huang Q., Clarke S. M., Lam F. C., Cannell I. G., Bryson B. D., Rameseder J., Lee M. J., Blake E. J., Fydrych A., Ho R., Greenberger B. A., Chen G. C., Maffa A., Del Rosario A. M., Root D. E., Carpenter A. E., Hahn W. C., Sabatini D. M., Chen C. C., White F. M., Bradner J. E., Yaffe M. B. (2013) The bromodomain protein Brd4 insulates chromatin from DNA damage signalling. Nature 498, 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferri E., Petosa C., McKenna C. E. (2016) Bromodomains: structure, function and pharmacology of inhibition. Biochem. Pharmacol. 106, 1–18. [DOI] [PubMed] [Google Scholar]
- 14.Dey A., Chitsaz F., Abbasi A., Misteli T., Ozato K. (2003) The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 100, 8758–8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang B., Yang X. D., Zhou M. M., Ozato K., Chen L. F. (2009) Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol. Cell. Biol. 29, 1375–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang M. K., Mochizuki K., Zhou M., Jeong H. S., Brady J. N., Ozato K. (2005) The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19, 523–534. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura Y., Umehara T., Nakano K., Jang M. K., Shirouzu M., Morita S., Uda-Tochio H., Hamana H., Terada T., Adachi N., Matsumoto T., Tanaka A., Horikoshi M., Ozato K., Padmanabhan B., Yokoyama S. (2007) Crystal structure of the human BRD2 bromodomain: insights into dimerization and recognition of acetylated histone H4. J. Biol. Chem. 282, 4193–4201. [DOI] [PubMed] [Google Scholar]
- 18.Wang R., Li Q., Helfer C. M., Jiao J., You J. (2012) Bromodomain protein Brd4 associated with acetylated chromatin is important for maintenance of higher-order chromatin structure. J. Biol. Chem. 287, 10738–10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowe B. L., Larue R. C., Yuan C., Hess S., Kvaratskhelia M., Foster M. P. (2016) Structure of the Brd4 ET domain bound to a C-terminal motif from γ-retroviral integrases reveals a conserved mechanism of interaction. Proc. Natl. Acad. Sci. USA 113, 2086–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman S., Sowa M. E., Ottinger M., Smith J. A., Shi Y., Harper J. W., Howley P. M. (2011) The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol. Cell. Biol. 31, 2641–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larue R. C., Plumb M. R., Crowe B. L., Shkriabai N., Sharma A., DiFiore J., Malani N., Aiyer S. S., Roth M. J., Bushman F. D., Foster M. P., Kvaratskhelia M. (2014) Bimodal high-affinity association of Brd4 with murine leukemia virus integrase and mononucleosomes. Nucleic Acids Res. 42, 4868–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S. Y., Lee A. Y., Lai H. T., Zhang H., Chiang C. M. (2013) Phospho switch triggers Brd4 chromatin binding and activator recruitment for gene-specific targeting. Mol. Cell 49, 843–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devaiah B. N., Lewis B. A., Cherman N., Hewitt M. C., Albrecht B. K., Robey P. G., Ozato K., Sims R. J. III, Singer D. S. (2012) BRD4 is an atypical kinase that phosphorylates serine2 of the RNA polymerase II carboxy-terminal domain. Proc. Natl. Acad. Sci. USA 109, 6927–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devaiah B. N., Case-Borden C., Gegonne A., Hsu C. H., Chen Q., Meerzaman D., Dey A., Ozato K., Singer D. S. (2016) BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat. Struct. Mol. Biol. 23, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu S. Y., Lee A. Y., Hou S. Y., Kemper J. K., Erdjument-Bromage H., Tempst P., Chiang C. M. (2006) Brd4 links chromatin targeting to HPV transcriptional silencing. Genes Dev. 20, 2383–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A., Larue R. C., Plumb M. R., Malani N., Male F., Slaughter A., Kessl J. J., Shkriabai N., Coward E., Aiyer S. S., Green P. L., Wu L., Roth M. J., Bushman F. D., Kvaratskhelia M. (2013) BET proteins promote efficient murine leukemia virus integration at transcription start sites. Proc. Natl. Acad. Sci. USA 110, 12036–12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dey A., Nishiyama A., Karpova T., McNally J., Ozato K. (2009) Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol. Biol. Cell 20, 4899–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao R., Nakamura T., Fu Y., Lazar Z., Spector D. L. (2011) Gene bookmarking accelerates the kinetics of post-mitotic transcriptional re-activation. Nat. Cell Biol. 13, 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korb E., Herre M., Zucker-Scharff I., Darnell R. B., Allis C. D. (2015) BET protein Brd4 activates transcription in neurons and BET inhibitor Jq1 blocks memory in mice. Nat. Neurosci. 18, 1464–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z., Yik J. H., Chen R., He N., Jang M. K., Ozato K., Zhou Q. (2005) Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19, 535–545. [DOI] [PubMed] [Google Scholar]
- 31.Bhagwat A. S., Roe J. S., Mok B. Y., Hohmann A. F., Shi J., Vakoc C. R. (2016) BET bromodomain inhibition releases the Mediator complex from select cis-regulatory elements. Cell Reports 15, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida H., Bansal K., Schaefer U., Chapman T., Rioja I., Proekt I., Anderson M. S., Prinjha R. K., Tarakhovsky A., Benoist C., Mathis D. (2015) Brd4 bridges the transcriptional regulators, Aire and P-TEFb, to promote elongation of peripheral-tissue antigen transcripts in thymic stromal cells. Proc. Natl. Acad. Sci. USA 112, E4448–E4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baranello L., Wojtowicz D., Cui K., Devaiah B. N., Chung H. J., Chan-Salis K. Y., Guha R., Wilson K., Zhang X., Zhang H., Piotrowski J., Thomas C. J., Singer D. S., Pugh B. F., Pommier Y., Przytycka T. M., Kouzine F., Lewis B. A., Zhao K., Levens D. (2016) RNA polymerase II regulates topoisomerase 1 activity to favor efficient transcription. Cell 165, 357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devaiah B. N., Singer D. S. (2012) Cross-talk among RNA polymerase II kinases modulates C-terminal domain phosphorylation. J. Biol. Chem. 287, 38755–38766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pradhan M. A., Blackford J. A. Jr., Devaiah B. N., Thompson P. S., Chow C. C., Singer D. S., Simons S. S. Jr (2016) Kinetically defined mechanisms and positions of action of two new modulators of glucocorticoid receptor-regulated gene induction. J. Biol. Chem. 291, 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W., Prakash C., Sum C., Gong Y., Li Y., Kwok J. J., Thiessen N., Pettersson S., Jones S. J., Knapp S., Yang H., Chin K. C. (2012) Bromodomain-containing protein 4 (BRD4) regulates RNA polymerase II serine 2 phosphorylation in human CD4+ T cells. J. Biol. Chem. 287, 43137–43155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanno T., Kanno Y., LeRoy G., Campos E., Sun H. W., Brooks S. R., Vahedi G., Heightman T. D., Garcia B. A., Reinberg D., Siebenlist U., O’Shea J. J., Ozato K. (2014) BRD4 assists elongation of both coding and enhancer RNAs by interacting with acetylated histones. Nat. Struct. Mol. Biol. 21, 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovén J., Hoke H. A., Lin C. Y., Lau A., Orlando D. A., Vakoc C. R., Bradner J. E., Lee T. I., Young R. A. (2013) Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelish H. E., Liau B. B., Nitulescu I. I., Tangpeerachaikul A., Poss Z. C., Da Silva D. H., Caruso B. T., Arefolov A., Fadeyi O., Christie A. L., Du K., Banka D., Schneider E. V., Jestel A., Zou G., Si C., Ebmeier C. C., Bronson R. T., Krivtsov A. V., Myers A. G., Kohl N. E., Kung A. L., Armstrong S. A., Lemieux M. E., Taatjes D. J., Shair M. D. (2015) Mediator kinase inhibition further activates super-enhancer-associated genes in AML. Nature 526, 273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tropberger P., Pott S., Keller C., Kamieniarz-Gdula K., Caron M., Richter F., Li G., Mittler G., Liu E. T., Bühler M., Margueron R., Schneider R. (2013) Regulation of transcription through acetylation of H3K122 on the lateral surface of the histone octamer. Cell 152, 859–872. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z., He N., Zhou Q. (2008) Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression. Mol. Cell. Biol. 28, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You J., Li Q., Wu C., Kim J., Ottinger M., Howley P. M. (2009) Regulation of aurora B expression by the bromodomain protein Brd4. Mol. Cell. Biol. 29, 5094–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mochizuki K., Nishiyama A., Jang M. K., Dey A., Ghosh A., Tamura T., Natsume H., Yao H., Ozato K. (2008) The bromodomain protein Brd4 stimulates G1 gene transcription and promotes progression to S phase. J. Biol. Chem. 283, 9040–9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farina A., Hattori M., Qin J., Nakatani Y., Minato N., Ozato K. (2004) Bromodomain protein Brd4 binds to GTPase-activating SPA-1, modulating its activity and subcellular localization. Mol. Cell. Biol. 24, 9059–9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurachi H., Wada Y., Tsukamoto N., Maeda M., Kubota H., Hattori M., Iwai K., Minato N. (1997) Human SPA-1 gene product selectively expressed in lymphoid tissues is a specific GTPase-activating protein for Rap1 and Rap2. Segregate expression profiles from a rap1GAP gene product. J. Biol. Chem. 272, 28081–28088. [DOI] [PubMed] [Google Scholar]
- 46.Maruyama T., Farina A., Dey A., Cheong J., Bermudez V. P., Tamura T., Sciortino S., Shuman J., Hurwitz J., Ozato K. (2002) A Mammalian bromodomain protein, brd4, interacts with replication factor C and inhibits progression to S phase. Mol. Cell. Biol. 22, 6509–6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tasdemir N., Banito A., Roe J. S., Alonso-Curbelo D., Camiolo M., Tschaharganeh D. F., Huang C. H., Aksoy O., Bolden J. E., Chen C. C., Fennell M., Thapar V., Chicas A., Vakoc C. R., Lowe S. W. (2016) BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 6, 612–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hussong M., Börno S. T., Kerick M., Wunderlich A., Franz A., Sültmann H., Timmermann B., Lehrach H., Hirsch-Kauffmann M., Schweiger M. R. (2014) The bromodomain protein BRD4 regulates the KEAP1/NRF2-dependent oxidative stress response. Cell Death Dis. 5, e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whyte W. A., Orlando D. A., Hnisz D., Abraham B. J., Lin C. Y., Kagey M. H., Rahl P. B., Lee T. I., Young R. A. (2013) Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown J. D., Lin C. Y., Duan Q., Griffin G., Federation A. J., Paranal R. M., Bair S., Newton G., Lichtman A. H., Kung A. L., Yang T., Wang H., Luscinskas F. W., Croce K. J., Bradner J. E., Plutzky J. (2014) NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol. Cell 56, 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Micco R., Fontanals-Cirera B., Low V., Ntziachristos P., Yuen S. K., Lovell C. D., Dolgalev I., Yonekubo Y., Zhang G., Rusinova E., Gerona-Navarro G., Cañamero M., Ohlmeyer M., Aifantis I., Zhou M. M., Tsirigos A., Hernando E. (2014) Control of embryonic stem cell identity by BRD4-dependent transcriptional elongation of super-enhancer-associated pluripotency genes. Cell Reports 9, 234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu T., Pinto H. B., Kamikawa Y. F., Donohoe M. E. (2015) The BET family member BRD4 interacts with OCT4 and regulates pluripotency gene expression. Stem Cell Rep. 4, 390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L., Xu Y., He M., Zhang M., Cui F., Lu L., Yao M., Tian W., Benda C., Zhuang Q., Huang Z., Li W., Li X., Zhao P., Fan W., Luo Z., Li Y., Wu Y., Hutchins A. P., Wang D., Tse H. F., Schambach A., Frampton J., Qin B., Bao X., Yao H., Zhang B., Sun H., Pei D., Wang H., Wang J., Esteban M. A. (2014) Transcriptional pause release is a rate-limiting step for somatic cell reprogramming. Cell Stem Cell 15, 574–588. [DOI] [PubMed] [Google Scholar]
- 54.Di Stefano B., Collombet S., Jakobsen J. S., Wierer M., Sardina J. L., Lackner A., Stadhouders R., Segura-Morales C., Francesconi M., Limone F., Mann M., Porse B., Thieffry D., Graf T. (2016) C/EBPα creates elite cells for iPSC reprogramming by upregulating Klf4 and increasing the levels of Lsd1 and Brd4. Nat. Cell Biol. 18, 371–381. [DOI] [PubMed] [Google Scholar]
- 55.Bolden J. E., Tasdemir N., Dow L. E., van Es J. H., Wilkinson J. E., Zhao Z., Clevers H., Lowe S. W. (2014) Inducible in vivo silencing of Brd4 identifies potential toxicities of sustained BET protein inhibition. Cell Reports 8, 1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez R. M., Suarez-Alvarez B., Salvanes R., Huidobro C., Torano E. G., Garcia-Perez J. L., Lopez-Larrea C., Fernandez A. F., Bueno C., Menendez P., Fraga M. F. (2014) Role of BRD4 in hematopoietic differentiation of embryonic stem cells. Epigenetics 9, 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houzelstein D., Bullock S. L., Lynch D. E., Grigorieva E. F., Wilson V. A., Beddington R. S. (2002) Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol. Cell. Biol. 22, 3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryant J. M., Berger S. L. (2012) Low-hanging fruit: targeting Brdt in the testes. EMBO J. 31, 3788–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roe J. S., Mercan F., Rivera K., Pappin D. J., Vakoc C. R. (2015) BET bromodomain inhibition suppresses the function of hematopoietic transcription factors in acute myeloid leukemia. Mol. Cell 58, 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dawson M. A., Prinjha R. K., Dittmann A., Giotopoulos G., Bantscheff M., Chan W. I., Robson S. C., Chung C. W., Hopf C., Savitski M. M., Huthmacher C., Gudgin E., Lugo D., Beinke S., Chapman T. D., Roberts E. J., Soden P. E., Auger K. R., Mirguet O., Doehner K., Delwel R., Burnett A. K., Jeffrey P., Drewes G., Lee K., Huntly B. J., Kouzarides T. (2011) Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 478, 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mertz J. A., Conery A. R., Bryant B. M., Sandy P., Balasubramanian S., Mele D. A., Bergeron L., Sims R. J. III (2011) Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl. Acad. Sci. USA 108, 16669–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delmore J. E., Issa G. C., Lemieux M. E., Rahl P. B., Shi J., Jacobs H. M., Kastritis E., Gilpatrick T., Paranal R. M., Qi J., Chesi M., Schinzel A. C., McKeown M. R., Heffernan T. P., Vakoc C. R., Bergsagel P. L., Ghobrial I. M., Richardson P. G., Young R. A., Hahn W. C., Anderson K. C., Kung A. L., Bradner J. E., Mitsiades C. S. (2011) BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chapuy B., McKeown M. R., Lin C. Y., Monti S., Roemer M. G., Qi J., Rahl P. B., Sun H. H., Yeda K. T., Doench J. G., Reichert E., Kung A. L., Rodig S. J., Young R. A., Shipp M. A., Bradner J. E. (2013) Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. Cancer Cell 24, 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crawford N. P., Alsarraj J., Lukes L., Walker R. C., Officewala J. S., Yang H. H., Lee M. P., Ozato K., Hunter K. W. (2008) Bromodomain 4 activation predicts breast cancer survival. Proc. Natl. Acad. Sci. USA 105, 6380–6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagarajan S., Hossan T., Alawi M., Najafova Z., Indenbirken D., Bedi U., Taipaleenmäki H., Ben-Batalla I., Scheller M., Loges S., Knapp S., Hesse E., Chiang C. M., Grundhoff A., Johnsen S. A. (2014) Bromodomain protein BRD4 is required for estrogen receptor-dependent enhancer activation and gene transcription. Cell Reports 8, 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng Z., Gong Y., Ma Y., Lu K., Lu X., Pierce L. A., Thompson R. C., Muller S., Knapp S., Wang J. (2013) Inhibition of BET bromodomain targets genetically diverse glioblastoma. Clin. Cancer Res. 19, 1748–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang R., You J. (2015) Mechanistic analysis of the role of bromodomain-containing protein 4 (BRD4) in BRD4-NUT oncoprotein-induced transcriptional activation. J. Biol. Chem. 290, 2744–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alsarraj J., Walker R. C., Webster J. D., Geiger T. R., Crawford N. P., Simpson R. M., Ozato K., Hunter K. W. (2011) Deletion of the proline-rich region of the murine metastasis susceptibility gene Brd4 promotes epithelial-to-mesenchymal transition- and stem cell-like conversion. Cancer Res. 71, 3121–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi J., Wang Y., Zeng L., Wu Y., Deng J., Zhang Q., Lin Y., Li J., Kang T., Tao M., Rusinova E., Zhang G., Wang C., Zhu H., Yao J., Zeng Y. X., Evers B. M., Zhou M. M., Zhou B. P. (2014) Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell 25, 210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]