OR-mediated increases in intracellular cAMP can modulate CD4+ T cell tissue trafficking.
Keywords: cell trafficking, extranasal, inflammation
Abstract
Retention of T cells within affected tissue is a critical component of adaptive immune inflammation. However, the mechanisms involved in T cell retention remain largely undefined. Previous studies revealed the capacity of cAMP signaling to regulate immune cell migration, as well as dynamic regulation of receptors that could induce cAMP production in immune cells. The potential for cAMP to act as a retention signal has been mostly unexplored, partially as a result of this second messenger’s well-characterized inhibition of effector function in immune cells. Here, we report that cAMP regulates the tissue retention of mouse T cells at concentrations well below those that inhibited proliferation or decreased acquisition of an effector phenotype. Stimulation of CD4+ T cells with odorants known to be cognate ligands for T cell-expressed olfactory receptors induced cAMP and inhibited chemokine-driven chemotaxis without decreasing T cell proliferation or effector functions. Similar effects were observed following treatment with relatively low concentrations of the cAMP analog Sp-5,6-dichloro-1-β-d-ribofuranosylbenzimidazole-3′,5′-monophosphorothioate. Furthermore, pretreatment with odorants or cAMP at concentrations that did not inhibit effector function induced T cell tissue retention in mice by inhibiting chemokine-dependent T cell egress from the footpad to the draining lymph node. Together, these results suggest that odorant receptor-mediated increases in intracellular cAMP can modulate T cell tissue trafficking and may offer new therapeutic targets for controlling T cell tissue accumulation.
Introduction
Immune cell chemotaxis is vital to the initiation, execution, and resolution of inflammation [1]. Interactions between chemokines and their cognate receptors are required for both immune cell extravasation to sites of inflammation and egress of cells from peripheral tissue through lymphatic vessels [2–4]. Thus, chemokine signaling regulates both T cell entry and egress, 2 functions that are critical for determining the numbers of T cells that can accumulate and participate in immune responses within nonlymphatic tissue. These vital roles for chemokine recognition in immune responses make modulation of chemokine/chemokine receptor interactions an attractive target for therapeutic manipulation.
As an example, T cell accumulation and lesion formation within the CNS during EAE, a model of CNS inflammation, involve modulation of T cell egress [5–7]. T cell retention within the CNS is thought to require induction of inflammation via on-site antigen recognition [5, 8, 9]. However, T cells can be retained within the CNS, following the initiation of inflammation, without regard to antigen specificity, suggesting that arrest within the CNS can be mediated by an antigen-independent mechanism [5, 8]. The nature of this mechanism remains unclear.
Previous studies demonstrated that increases in levels of cAMP can reduce lymphocyte activation and effector function, including chemokine-induced chemotaxis, in T cells [10, 11]. The studies that describe these negative effects on multiple T cell functions typically use high doses of cAMP (≥250 μM) or highly active cAMP-inducing agents. Thus, it remains unclear what roles lower cAMP concentrations might play in individual T cell activities.
The largest group of mammalian receptors that signal through cAMP is the family of G protein-coupled ORs [12, 13]. In the olfactory system, activation of ORs by volatile chemicals (odorants) initiates a G protein-coupled, cAMP-mediated signaling cascade that results in the depolarization of OSNs and the generation of action potentials. There is growing evidence that many ORs are also expressed outside of the nasal cavity and may regulate such diverse functions as sperm chemotaxis [14], myocyte migration and adhesion [15], and glomerular filtration in the kidney [16, 17]. Intriguingly, ORs have been detected in spleen [18, 19], but until recently, the cellular specificities of this expression and the functional roles of these extranasal ORs were unknown. Emerging data now suggest that functional ORs may be expressed in hematopoietic lineage cells [20, 21] and that the observed expression may be dynamic [22].
Here, we report that ORs are expressed in mature murine CD4+ T cells. Stimulation with cognate OR agonists induced cAMP production and prevented chemotaxis of T cells along a chemokine gradient in a cAMP-dependent manner without suppressing other aspects of T cell activation and function. In addition, pretreatment with this OR ligand decreased the rate of T cell egress from the footpad to the popliteal lymph node. Together, these data indicate that members of the OR superfamily are expressed in peripheral T cells and modulate chemokine/chemokine receptor-induced responses through production of cAMP.
MATERIALS AND METHODS
Mice
C57BL/6J and congenic CD45.1 (Ly5.2) mice were purchased from the National Cancer Institute (Bethesda, MD, USA) or The Jackson Laboratory (Bar Harbor, ME, USA) . All experiments were approved by the University of Maryland, Baltimore Animal Care and Use Committee, and the Uniformed Services University of the Health Sciences Animal Care and Use Committee, and all mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility.
T cell purification
Naïve mice were euthanized and spleens removed to ice-cold RPMI 1640 complete medium (10% FBS, Hepes, penicillin/streptomycin, l-glutamine, nonessential amino acids) before being ground to a single-cell suspension using sterile-frosted glass slides. Cells were washed and layered on Ficoll 1.077 and then centrifuged at 1900 rpm for 20 min with no deceleration brake. Cells were then washed twice with 1% FBS in HBSS and incubated in 1 ml per spleen of RPMI 1640 complete medium containing 1 μg/ml purified rat IgG anti-CD8α and anti-CD24 antibodies (BioLegend, San Diego, CA, USA). Cells were washed again and then treated with 0.5 ml/spleen prewashed anti-rat IgG, anti-mouse IgG, and anti-mouse IgM magnetic beads (Polysciences, Warrington, PA), per the manufacturer’s instructions. Beads and cells were placed on ice for 30 min and then separated using a flask magnet, while shaking at room temperature for 20 min. Purified CD4+ T cells were stimulated for 48 h with anti-CD3 (1 μg) and anti-CD28 (2 μg; BioLegend) at 37°C with 5% CO2 (for activated T cells) or used immediately following purification (for naïve cells).
Real-time PCR
An RNeasy Mini Kit (Qiagen, Germantown, MD, USA) was used for purification of mRNA, using the manufacturer's instructions, from naïve and activated T cells, as well as from the olfactory bulb recovered from the same animals. In addition, mRNA was generated from the mouse thymoma cell line EL4, grown according to the manufacturer’s instructions for 6 passages (American Type Culture Collection, Manassas, VA, USA). SuperScript II Reverse Transcriptase (Thermo Fisher Scientific, Waltham, MA, USA) was used to synthesize cDNA, according to the manufacturer’s instructions. SsoFast EvaGreen Supermix (Bio-Rad, Hercules, CA, USA) was used in a real-time PCR mix buffer, as recommended by the manufacturer. Reactions were run over 40 cycles using a 54°C annealing temperature. Primers used were: 18S forward: AGTCGGAGGTTCGAAGACGAT, 18S reverse: GCGGGTCATGGGAATAACG; Adcy3 forward: TGAGGAGAGCATCAACAACG, Adcy3 reverse: GCCATATAGGTGCTGCCAAT; Adrb2 forward: CTGGTGGTGATGGTCTTTGTC, Adrb2 reverse: GAGGTTTTGGGCGTGGAATCT; CCR7 forward: GGTTCCTGCCTCTCATGTATT, CCR7 reverse: GTAGGTATCCGTCATGGTCTTG; Cnga2 forward: TTGATTGGAGCTGTTGTGGA, Cnga2 reverse: GCTCTTCACACCGTTGGATT; CXCR4 forward: GCTGTTGCTGCATAATCTCTTC, CXCR4 reverse: GCA TAA CCA AAC AAT TTA AGA ATG GG; Gnal forward: TACACACCCACAGACCAGGA, Gnal reverse: CCACGTAAATGATCGCAGTG; Olfr16 forward: TTTCTGAGCATGTTGGCAAG, Olfr16 reverse: CAAGGATATGGGAAGGTT; Olfr544 forward: GGGACTCACTGTTCGCATCT, Olfr544 reverse: ATGAGGACATGGTGGAGGAG; Olfr642 forward: GGTCTTCCCACTTCCTTTCC, Olfr642 reverse: GCCCATACATGCTGTTGATG.
cAMP measurement
The CatchPoint cAMP Fluorescent Assay Kit (Molecular Devices, Sunnyvale, CA, USA) was used to measure cAMP production within T cells upon stimulation with the odorants AzA, NA, or 1-pentanol (Sigma-Aldrich, St. Louis, MO, USA). Purified CD4+ T cells (1 × 105) were treated with various concentrations of odorants in diluent or with an equal volume of diluent within a 96-well plate in a volume of 30 μl RPMI 1640 medium plus 1 mM 3-isobutyl-1-methylxanthine for 1 h in a 37°C incubator with 10% CO2. Cells were then lysed, and the cAMP content of the lysates was measured. Fluorescence intensity of samples, which is inversely proportional to cAMP concentration, was measured using a FlexStation 3 Benchtop Multi-Mode Microplate Reader (Molecular Devices). cAMP concentration was calculated using SoftMax Pro software (Molecular Devices) by calibrating florescence intensity of samples to an established 8-point standard curve, ranging from 0 to 400 pmol.
Flow cytometric staining
Staining was performed as described previously [23]. For detection of cell-surface markers, cells were stained on ice for 30 min using optimal antibody concentrations. Cells were also stained using the Live/Dead Fixable Near-IR Dead Cell Stain Kit for viability detection (Thermo Fisher Scientific), as recommended by the manufacturer for detection of viability. For detection of surface CCR7, protein cells were incubated with optimized concentrations of anti-CCR7 (clone 4B12; BioLegend) at 37°C for 15 min, as recommended by the manufacturer. For intracellular cytokine staining, cells were stimulated with anti-CD3 (1 μg) and anti-CD28 (2 μg) in the presence of the protein transport inhibitor BD GolgiStop (BD Biosciences, San Jose, CA, USA) and incubated at 37°C with 5% CO2 for 4 h. Cells were then resuspended in staining buffer (1× PBS, 1% FCS or BSA, 0.05% sodium azide), counted, and treated with an anti-CD4 (RM4-4) antibody conjugated to PE (BioLegend) for immunofluorescent staining, as well as a Live/Dead Fixable Near-IR Dead Cell Stain Kit for viability detection (Thermo Fisher Scientific). Cells were washed 2 times with staining buffer and then fixed, permeabilized, and incubated with anti-CCR7 or anti-IFN-γ antibody (BioLegend) or an isotype control antibody at 4°C for 30 min in the dark. Cells were then examined immediately by flow cytometric analysis. Populations were selected for analysis based on FSC-area compared with FSC-height to allow exclusion of conjoined cells, and initial gates were set, based on previously defined lymphocyte FSC and SSC profiles.
In vitro chemotaxis
T cell chemotaxis was tested in 24-well, 5 μm pore-size polycarbonate membrane Transwell plates (Sigma-Aldrich). Naïve T cells (5 × 105) were dispensed in the upper chamber, with or without AzA or cBiMPS (Sigma-Aldrich), whereas different chemokines (varying doses) or medium alone were added to the lower chamber. Plates were then incubated overnight at 37°C. After removal of the Transwell inserts, cells from the lower compartments were collected, and an aliquot was tested for viability by trypan blue staining and counted. The remaining cells were stained with anti-CD4 (RM4-4)-PE and anti-CD44 (IM7)-allophycocyanin antibodies, as well as the Live/Dead Fixable Near-IR Dead Cell Stain Kit for viability detection (Thermo Fisher Scientific) and then examined by flow cytometry. As equal volumes were used across all sample groups, we also used a set, 90-s window for flow cytometric examination of each sample, allowing us to compare relative numbers within each sample and to corroborate data recovered by counting. Each experiment was performed in duplicate, and results were means from duplicate wells.
Proliferation analysis
Purified CD4+ T cells from naïve mice were stimulated for 48 h with anti-CD3 (1 μg/ml) and anti-CD28 (2 μg/ml) in the presence of different concentrations of cBIMPS, AzA, or diluent. BrdU was then added at 10 μg per 3 ml and incubated overnight. Cells were then collected and examined by intracellular flow cytometry analyses with surface-stain for phenotypic markers and intracellular stain with anti-BrdU.
In vivo trafficking
Congenic, CD45.1+-purified spleen CD4+ T cells were incubated with 50 μM cBIMPS, 5 mM AzA, or diluent for 1 h in a 37°C incubator with 10% CO2 and then washed 3 times before being injected in a 20-μl vol of HBSS into the footpad of anesthetized wild-type CD45.2+ mice. After 16 h, mice were killed, and draining popliteal and nondraining axillary lymph nodes were removed, ground to a single-cell suspension using frosted glass slides, and examined for viability (Fixable Live Dye) and for CD4 and CD45.1 expression via flow cytometry. In parallel, some mice received the same cells via an intravenous injection as a test of transferred cell viability. The spleens were recovered and cells examined as above.
Statistics
All statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, Incorporated, La Jolla, CA, USA). All data were analyzed using a 1-way ANOVA, followed by Tukey’s multiple comparisons test.
RESULTS
To determine if the multifactorial, functional consequences of increased cAMP concentrations within T cells are separable, we examined the capacity of T cells to proliferate and up-regulate the activation marker CD69 when stimulated with anti-CD3 and anti-CD28 in the presence or absence of the pan adenylyl cyclase activator forskolin or the membrane-permeable phosphodiesterase-resistant cAMP analog cBiMPS. During stimulation with antibodies to CD3 and CD28, both forskolin (10 μM) and cBiMPS (500 μM) treatments significantly decreased cell viability, while also inhibiting proliferation, IFN-γ production, and expression of CD69 in surviving cells (Table 1). Treatment with a lower dose (250 μM) of cBiMPS resulted in statistically significant decreases in cell viability and CD69 expression, with no observable modulation of proliferation or cytokine production (Table 1). Finally, treatment with 50 μM cBiMPS had no observable effect on survival, proliferation, acquisition of CD69, or cytokine production (Table 1). Neither 10 μM forskolin nor any of the concentrations of cBiMPS tested had any significant effect on T cell viability in the absence of anti-CD3 and anti-CD28 (data not shown). These data suggest that the previously described inhibitory effects of cAMP on T cell activation and function [24–27] are highly concentration dependent and that a 50 μM dose of cBiMPS is not sufficient to inhibit many T cell effector functions.
TABLE 1.
cAMP analog effects on T cell function
| Concentration of cBiMPS | % viable | % CD69+a | % of cells proliferatinga | % of IFN-γ-producing cellsa |
|---|---|---|---|---|
| No drug added | 69 ± 11 | 61 ± 21 | 48 ± 14 | 8 ± 3 |
| 50 μM cBiMPS | 66 ± 9 | 59 ± 12 | 46 ± 13 | 9 ± 2 |
| 250 μM cBiMPS | 44 ± 10* | 29 ± 9* | 37 ± 9 | 8 ± 1 |
| 500 μM cBiMPS | 19 ± 12* | 13 ± 6* | 11 ± 4* | 2 ± 1* |
| 500 μM cBiMPS + 500 μM Rp-cAMPS | 52 ± 9 | 33 ± 12* | 38 ± 14 | 7 ± 1 |
| Forskolin | 8 ± 4* | 6 ± 3* | 7 ± 3* | 1 ± 1* |
| No CD3/CD28 stimulation | 71 ± 14 | 1 ± 0* | 5 ± 2* | 0 ± 0* |
Groups were compared by 1-way ANOVA using Tukey’s multiple comparison test.
Only viable cells were examined.
P < 0.05, group outcome was significantly different compared with the no-drug-added group.
Next, we asked if similar doses of cAMP are capable of decreasing T cell responsiveness to chemokine signals. We used an in vitro migration assay to determine if the various doses of cBiMPS could modulate naïve T cell trafficking to the chemokines CXCL12 and CCL21. All tested concentrations of cBiMPS, but not equivalent volumes of diluent controls, resulted in a statistically significant inhibition of naïve T cell migration down a CXCL12 gradient (100 ng/ml added to the bottom chamber; Fig. 1A).
Figure 1. A cAMP analog inhibits chemotaxis to multiple chemokines.
Purified CD4+ T cells from naïve mice were examined for their capacity to chemotax through a Transwell insert toward an optimal concentration (100 ng/ml) of CXCL12 (A) or an optimal (100 ng/ml; B) or suboptimal (25 ng/ml; C and D) and (E) concentration of CCL21 following pretreatment with various doses of the cAMP analog cBiMPS. (A and C) One group of cells was pretreated with the PKA inhibitor Rp-cAMPS to determine the role of PKA in the observed chemotaxis inhibition. (D) All cells were pretreated with the Epac inhibitor ESI-09 and then treated with 50 μM cBiMPS or left untreated. (E) One group of cells was pretreated with the Epac agonist 8-pCPT-2′-O-Me-cAMP. (A–C) Means ± sd represent 6 separate experiments, each using T cells pooled from ≥3 mice. (D and E) Means ± sd represent 4 separate experiments, each using T cells pooled from ≥4 mice. *P < 0.5.
In contrast to the results observed for CXCL12 (Fig. 1A), the 50, 100, and 200 μM doses of cBiMPS had no significant effect on naïve T cell migration to a near-optimal (>90% of the maximal response) dose of CCL21 (100 ng/ml; Fig. 1B). Given the large relative differences in naïve T cell responsiveness to near-optimal doses of CXL12 and CCL21 (∼2.5-fold/undirected and ∼10-fold/undirected, respectively), we further examined the effects of cBiMPS on T cell chemotaxis to a suboptimal (30–50% of the maximal response) dose (25 ng/ml) of CCL21. We found that cBiMPS significantly decreased T cell responsiveness to 25 ng/ml CCL21 at all doses tested (Fig. 1C). For both chemokines, the effects of cBiMPS were partially blocked by pretreatment with 1 mM of the cAMP-dependent kinase (PKA) antagonist Rp-cAMPS, indicating that the inhibition of cell migration at least partially requires PKA signaling. (Fig. 1A and C).
Whereas Rp-cAMPS-mediated inhibition of PKA signaling largely abolished the effects of cBiMPS on chemotaxis, it remained possible that an alternate cAMP signaling pathway, through the Epac, was also involved in cAMP-mediated inhibition of chemotaxis [28, 29]. To address the necessity of Epac for cBiMPS inhibition of chemotaxis, we treated cells with the Epac inhibitor ESI-09, as previously reported [30], and examined the capacity of cBiMPS to inhibit chemotaxis to CCL21. We found that inhibition of Epac had no effect on cBiMPS-mediated inhibition of chemotaxis (Fig. 1D). To determine if Epac stimulation alone was capable of modulating chemotaxis, we treated cells with the Epac agonist 8-pCPT-2′-O-Me-cAMP, as previously reported, and tested responses to CCL21 [31]. We found that Epac activation had no effect on chemotaxis to CCL21 (Fig. 1E).
Together, these data suggest that cAMP-mediated inhibition of chemokine induced chemotaxis through PKA and that inhibition is critically dependent on the specific chemokine to be inhibited, as well as chemokine concentration.
T cells express ORs
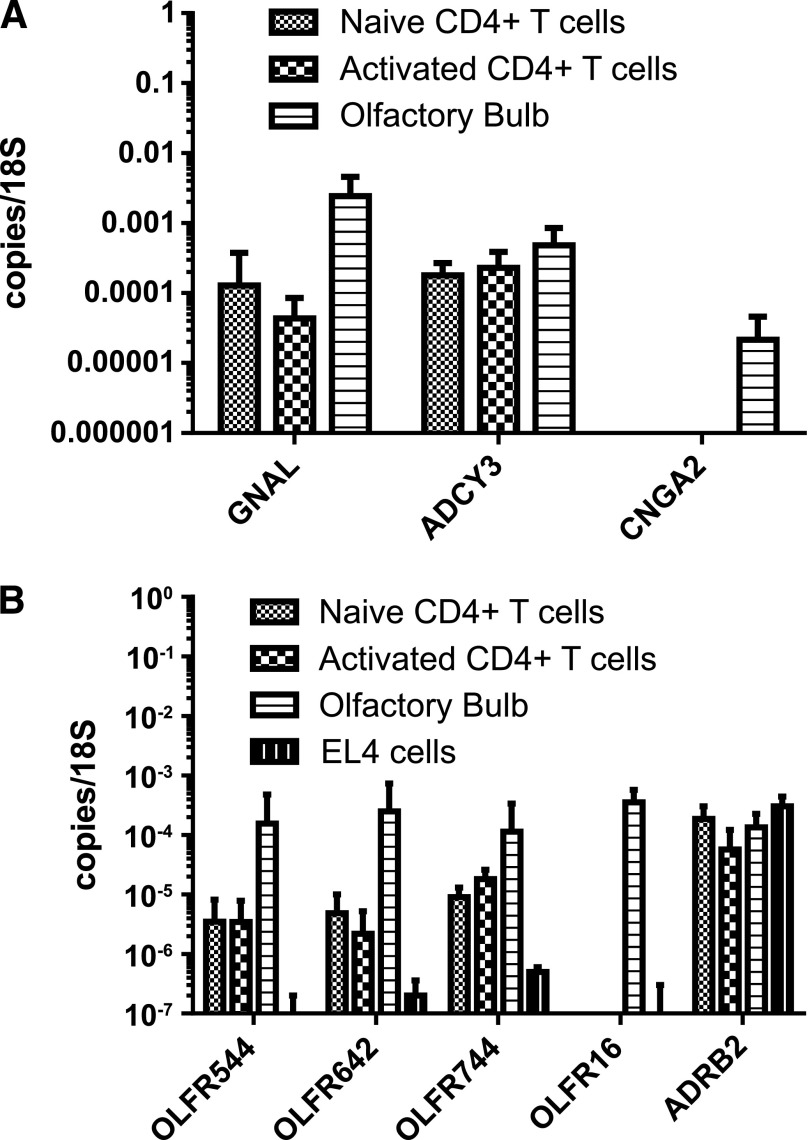
We next considered what receptor mechanisms might regulate cAMP in these cells. Our lab has an ongoing interest in tissue-retentive T cells, particularly within the CNS after induction of EAE. A recent microarray indicated that a number of ORs are expressed in T cells (unpublished results), suggesting that ORs may play a functional role in T cells. In the olfactory system, ORs signal via a cAMP-dependent transduction cascade that includes the Gαolf, the ADCY3, and a CNG ion channel composed of subunits CNGA2, CNGA4, and CNGB1b [32]. Given the ability of cAMP to modulate T cell trafficking, we hypothesized that ORs could regulate cAMP-mediated T cell retention and could couple retention to local chemical cues. Thus, we next asked if components of the canonical olfactory signaling pathway were expressed in CD4+ T cells. With the use of qPCR, we examined mRNA expression of Gαolf, ADCY3, and the obligatory CNG channel subunit CNGA2 (Fig. 2A). We found constitutive expression of both Gαolf and ADCY3; however, CNGA2 expression was not significantly above background. These results suggest that OR signaling in T cells could generate cAMP signals but may not lead to CNG channel activation. To determine if ORs are functional in T cells, we selected 3 ORs with known ligands for further examination: Olfr544 (a.k.a., MOR42-3) and Olfr642 (a.k.a., MOR13-6), known receptors for AzA [33, 34], and Olfr744 (a.k.a., MOR106-13P), a known receptor for pentanol [33]. To validate expression of these receptors, we examined naïve and anti-CD3/anti-CD28-stimulated T cell mRNA levels by qPCR for the expression of Olfr544, Olfr642, and Olfr744; all were constitutively expressed in naïve and activated CD4+ T cells (Fig. 2B). We also examined the mouse thymoma cell line EL4 and found reduced expression of Olfr544, Olfr642, and Olfr744 when compared with CD4+ T cells (Fig. 2B).
Figure 2. T cells express specific ORs and downstream signaling components.
Both naïve and CD3/CD28-activated, purified CD4+ T cells were examined for expression of OR signaling components, including the GNAL, ADCY3, and the CNG ion channel subunit CNGA2 by real-time PCR analysis of RNA expression (A). Additional experiments (B) examined the expression of the cAMP-producing receptors Olfr544, Olfr642, Olfr744, Olfr16, and the Adrb2. Means ± sd represent 3 separate experiments, each using T cells pooled from ≥3 mice.
Given the expression of OR signaling components observed, we predicted that T cells would respond to previously defined odorants for OLFR544 (responsive to octanedioic and AzA/nonanedioic acid), OLFR642 (responsive to a broad range of bromocarboxylic and dicarboxylic acids, including AzA), and OLFR744 (responsive to pentanol, hexanol, and heptanol) through production of cAMP. Incubation of CD4 T cells for 1 h with AzA or pentanol was sufficient to induce cAMP production in a dose-dependent manner (Fig. 3A and B). The production of cAMP following pentanol treatment supports a previous report that also demonstrated cAMP production in lymphocytes following treatment with short-chain alcohols, including pentanol [35]. The same study that defined ligand recognition for OLFR544, -642, and -744 also demonstrated that NA, the monocarboxylic equivalent of AzA, was broadly recognized by the majority of other ORs tested [33]. Given the large number of ORs potentially expressed in T cells, we wished to determine whether NA could also induce cAMP. Again, incubation of CD4 T cells for 1 h with NA was sufficient to induce cAMP production in a dose-dependent manner (Fig. 3C). Together, these data demonstrate that known odorants that activate T cell-expressed ORs could induce stimulation of cAMP production in CD4+ T cells, although we cannot rule out the possibility that T cells contain additional AzA- or pentanol-responsive receptors beyond OLFR544, -642, and -744.
Figure 3. Odorants induce cAMP in T cells.
Purified, naïve CD4+ T cells were examined for production of cAMP after 1 h treatments with indicated concentrations of AzA (A), 1-pentanol (B), or NA (C). Means ± sd represent 4 separate experiments, each using T cells pooled from ≥3 mice. *P < 0.5.
OR ligands inhibit T cell chemotaxis
Given the potential of cAMP to modulate T cell retention, we wished to determine whether odorant-evoked cAMP concentrations were sufficient to decrease T cell responsiveness to chemokine signals. We used an in vitro migration assay to determine if the presence of AzA was sufficient to modulate T cell trafficking to the chemokines CXCL12 and CCL21. In the absence of chemokines, a small number of CD44-high CD4+ T cells migrated through a Transwell membrane (data not shown). This T cell migration occurred to the same extent, with or without AzA (1–5 mM) added to either the bottom (Fig. 4A) or top (data not shown) Transwell chambers. Thus, AzA did not induce chemotaxis or chemofugetaxis in CD4+ T cells or inhibit nondirected T cell migration. By contrast, the increase of concentrations of AzA, but not equivalent volumes of diluent controls, resulted in a statistically significant inhibition of T cell migration down a 100 ng/ml CXCL12 gradient (Fig. 4B). Similar effects were observed following treatment with pentanol or NA (data not shown). The inhibition of chemotaxis could be blocked by pretreatment with 1 mM of the PKA inhibitor Rp-cAMPS, suggesting that the AzA-induced inhibition of cell migration is PKA dependent (Fig. 4B).
Figure 4. AzA inhibits chemotaxis to multiple chemokines in a PKA-dependent manner.
Purified CD4+ T cells from naïve mice were examined for their capacity to chemotax through a Transwell insert toward to an optimal concentration of CXCL12 (100 ng/ml; A) or a suboptimal (25 ng/ml; C) concentration of CCL21 following pretreatment with various doses of AzA (B). Means ± sd represent 6 separate experiments, each using T cells pooled from ≥3 mice. *P < 0.5.
As the chemokine receptor CCR7 is vital to T cell egress from multiple tissues [2, 3], we also examined the capacity of odorant-induced cAMP to block T cell responses to the CCR7 ligand CCL21. Again, 5 mM AzA was sufficient to block T cell migration toward a suboptimal (25 ng/ml) concentration of CCL21 via a PKA-dependent mechanism (Fig. 4C).
Whereas the experiments with cBiMPS demonstrate that chemotaxis inhibition could be observed without inhibition of other effector functions following treatment with low concentrations of exogenous cAMP (Table 1), it was not clear if the levels of cAMP generated by odorant treatment of T cells would be sufficient to impact other T cell effector functions. To answer this question, we pretreated cells with doses of AzA or NA, shown to achieve statistically significant chemotaxis inhibition, and examined the response of the cells to anti-CD3- and anti-CD28-mediated activation (Fig. 5). We found no significant changes in cell viability, proliferation, CD69 expression, or production of IFN-γ at the doses tested (combined flow cytometric data summarized in Table 2). Thus, the concentration of cAMP, produced upon stimulation with 5 mM AzA, has, at most, a minimal capacity to inhibit T cell activation.
Figure 5. Concentrations of odorants capable of inhibiting chemotaxis have no impact on CD69 expression and proliferation.
Purified CD4+ T cells from naïve mice were gated by FSC and SSC parameters (A) and then examined for their capacity to increase expression of the activation marker CD69 or incorporate the proliferation marker BrdU following pretreatment with a pan-adenylate cyclase activator (forskolin), a low (50 μM) concentration of cAMP analog (cBiMPS), or odorants recognized by T cell-expressed ORs (AzA and NA; B).
TABLE 2.
Effects of odorants on T cell functions other than chemotaxis
| Concentration of cBiMPS | % viable | % CD69+a | % of cells proliferatinga | % of IFN-γ-producing cellsa |
|---|---|---|---|---|
| No drug added | 69 ± 11 | 61 ± 21 | 48 ± 14 | 8 ± 3 |
| 5 mM AzA | 62 ± 13 | 66 ± 15 | 44 ± 9 | 10 ± 3 |
| 1 mM pentanol | 42 ± 12* | 56 ± 13 | 41 ± 15 | 9 ± 2 |
| 100 μM NA | 58 ± 12 | 60 ± 11 | 39 ± 13 | 7 ± 1 |
| Forskolin | 8 ± 4* | 6 ± 3* | 7 ± 3* | 1 ± 1* |
| No CD3/CD28 stimulation | 71 ± 14 | 1 ± 0* | 5 ± 2* | 0 ± 0* |
Groups were compared by 1-way ANOVA using Tukey’s multiple comparison test.
Only viable CD4+ cells were examined.
P < 0.05, group outcome was significantly different compared with the no-drug-added group.
Finally, we asked whether treatment with AzA would affect T cell egress in vivo. Freshly isolated, naïve T cells were pretreated with AzA (5 mM), cBiMPS (50 μM), or diluent for 1 h and injected into the footpad. After 16 h, we removed the draining popliteal lymph nodes and the nondraining axillary lymph nodes and examined them for the presence of injected cells by flow cytometry (Fig. 6A). Treatment with AzA or cBiMPS was sufficient to significantly block T cell egress to the draining lymph node (Fig. 6B). AzA-mediated inhibition of T cell egress was abrogated by pretreatment of the cells with a 50 μM concentration of the adenylyl cyclase inhibitor SQ22538 (Fig. 6C). Importantly, AzA-, cBiMPS-, and diluent-treated T cells were recovered in equal numbers from the spleen after parallel intravenous injections, indicating that the differences in cell recovery from the draining lymph node were not a result of in vivo toxicity associated with AzA treatment or cAMP signaling (Fig. 6D). Therefore, both exogenous and odorant-induced cAMP signals can act to retain T cells within nonlymphoid tissue in vivo.
Figure 6. T cell tissue egress is inhibited following treatment with odorants.
(A and B) Purified CD45.1+ congenic CD4+ T cells from naïve mice were examined for their capacity to exit the footpad over 16 h following pretreatment with 5 mM AzA, 50 μM cBiMPS, or diluent. Representative FACS plots are shown in A, with combined data shown in B. (B) Means ± sd represent 4 separate experiments, each using T cells pooled from ≥3 mice. In parallel, cells were treated with SQ22538 (SQ) or diluent before pretreatment with AzA or diluent (C) and examined for their capacity to exit the footpad over 16 h. (C) Means ± sd represent 4 separate experiments, each using T cells pooled from ≥3 mice. (D) Additionally, in parallel, cells were treated as for A and C and then injected intravenously. Spleen cells were examined for transferred cell number and viability after 16 h. *P < 0.05, **P < 0.01, ***P < 0.001. PLN, popliteal lymph node; ALN, axillary lymph node.
Given the significant inhibition of chemotaxis observed, we wished to determine whether cBiMPS modulated the expression of chemokine receptors in the CD4 T cells. We incubated naïve T cells with 100 μM cBiMPS over the same time period used in the chemotaxis experiments and examined mRNA levels of CCR7 and CXCR4 by qPCR (Fig. 7A). Treatment with cBiMPS had no observable effect on mRNA levels of CCR7 or CXCR4. Further examination of surface CCR7 protein expression levels in these cells also suggested no significant discernable effect of cBiMPS on chemokine receptor expression (Fig. 7B and C).
Figure 7. cBiMPS-mediated chemotaxis inhibition is not associated with changes in chemokine receptor expression levels.
Naïve, purified CD4+ T cells were incubated with cBiMPS, AzA, or the diluent complete RPMI 1640 medium for 16 h and then examined for expression of CCR7 and CXCR4 by real-time PCR analysis of RNA expression (A) or by flow cytometric intracellular (B) or surface (C) CCR7 protein detection. Means ± sd represent 4 separate experiments, each using T cells pooled from ≥2 mice.
DISCUSSION
Immune cell trafficking is a critical component of the cellular immune response. The complex interactions between the large numbers of chemokines and chemokine receptors are vital to establishing specific cues for trafficking of immune cells to tissue-specific sites of inflammation. The data presented here demonstrate that cAMP levels consistent with GPCR-mediated signaling can modulate chemokine receptor-dependent chemotaxis of T cells without inhibiting other T cell effector functions. These findings also suggest that mechanisms that induce relatively low-level cAMP production in T cells could play a role in T cell tissue retention by inhibiting CCR7-dependent egress through lymph vessels. Finally, we suggest that these cAMP signals could be induced through the activation of ORs expressed on T cells.
There is a great deal of literature examining the impact of cAMP signaling on T cell activation and function. Overall, T cell activities are generally thought to be negatively impacted by cAMP [36]. Indeed, several studies suggest that regulatory T cells may use transfer of cAMP to inhibit the activity of effector cells [37, 38]. However, studies that detail the inhibition of T cell effector functions by cAMP have often treated T cells with high concentrations of cAMP (≥250 μM) or with strong cAMP inducers (e.g., forskolin, Bordetella pertussis toxin CyaA) [39, 40]. Interestingly, there have also been previous suggestions that lower concentrations of cAMP, and/or cAMP inducers can result in modulation of T cell function without generalized T cell inhibition [41, 42]. Our results extend these earlier reports detailing concentration-separable effects of cAMP analogs by showing that chemotaxis is particularly sensitive to cAMP-mediated inhibition. Overall, we conclude that the effects of cAMP on T cell functions are highly concentration dependent, and as such, cAMP could play multiple roles in the regulation of T cell activity.
The capacity of low concentrations of cAMP to modulate T cell responses to chemokines without inhibiting alternate effector function makes this second messenger a strong candidate to modulate tissue retention. It was long thought that T cell egress from nonlymphoid tissue was a result of the same hydrostatic pressures that drive lymph through afferent lymphatics to the lymph node [43]. However, it is now clear that T cell egress from both the skin and lung requires chemokine receptor activation, specifically through CCR7 [2, 3]. These findings suggest that modulation of CCR7 activity may be responsible for the long-term T cell tissue retention reported in a variety of inflammatory conditions [44]. However, the stimuli and receptors that induce tissue retention are unknown.
The observation that cAMP signaling can modulate T cell chemotaxis suggested that 1 or more GPCRs could couple extracellular retention signals to cAMP production. Among GPCRs, the Olfr gene superfamily constitutes a massive component of a mammal’s total genetic pool, representing ∼3% of mouse genes [45, 46]. In the main olfactory epithelium, ORs are the principal receptors for volatile odorants and thus, are critical for detecting chemical cues related to food, conspecifics, predators, and prey [12, 13, 33, 47, 48]. Recently, ORs have also been implicated in functions outside of the olfactory system, suggesting that ORs may be used by multiple cell types to detect exogenous or endogenous odorant cues [14–16]. Furthermore, a recent study indicated that extranasal expression of ORs is partially conserved between humans and chimpanzees and correlates with constrained evolutionary divergence of the ORs, suggesting that these extranasal ORs may be functionally selected [49].
Individual OSNs each appear to express stably only a single Olfr gene product, at least at high levels, out of the ∼1200 Olfr genes in the mouse genome. As the odorant selectivity of an individual OSN is dictated by the OR it expresses, and individual ORs display distinct (although sometimes overlapping) odorant response profiles, one would predict a great diversity in odorant tuning across the OSN population. Indeed, this is the case [12]. In contrast, it is not yet clear how Olfr gene expression is regulated within T cells. The low expression levels of individual ORs observed by qPCR within T cells would suggest that either expression occurs at very low levels in all or at least a significant minority of CD4+ T cells or that a higher-level expression occurs in a small subset(s) of T cells. Although we find evidence for the expression of >100 different Olfr genes in a mixed T cell population, our data demonstrate that single odorants can evoke responses in large numbers of T cells. These results suggest that individual T cells express multiple ORs or alternatively, that a limited number of ORs, biased toward a small group of related stimuli, are expressed by T cells. The latter possibility could reflect the known promiscuity of OR ligand recognition.
Previous studies found that only a fraction of ORs tested was responsive to AzA [33], suggesting a level of specificity consistent with other ORs/odorants. However, it is certainly possible that additional ORs within our CD4 T lymphocyte subsets contribute to the overall functional responses described here. This suggestion is supported by the finding that equivalent effects could be achieved through treatment with NA, an odorant found to stimulate several, but not all, ORs tested in the same study [33]. Combined together, our capacity to predict the degree of cAMP production following treatment with multiple defined odorants, in conjunction with the expression of OR signaling components necessary to generate cAMP, strongly suggests that 1 or more ORs expressed in CD4 T cells are capable of functional activation following recognition of cognate agonists.
Unfortunately, the expression of multiple ORs, combined with the complexity of OR ligand recognition and subsequent signaling activities, makes the establishment of individual OR activation as the definitive response to our putative OR ligands problematic. As such, it also remains possible that the effects described in this study are associated with an unknown alternate mechanism for the induction of cAMP. Even so, the data reported here extend the findings of other reports that suggested ORs are functional in mouse pulmonary macrophages [20] and in human B and T lymphocytes, as well as granulocytes [21].
Our data suggest that odorant-induced cAMP signaling via PKA is critical for the inhibition of chemotaxis, a finding that fits well with the previously reported impact of PKA signaling on multiple components of cell migration [50]. Our data also demonstrated that activation of Epac was neither sufficient nor necessary for chemotaxis inhibition induced by a cAMP signal. These data bolster earlier reports that suggested that Epac had little or no contribution to cAMP signaling in T cells [51, 52].
In this study, we made use of AzA as a previously characterized ligand for Olfr544 and Olfr642. AzA has very low toxicity and has been used as a therapeutic agent for inflammatory acne and rosacea, making it an attractive potential therapeutic [53]. Potential anti-inflammatory activities associated with AzA treatment have been proposed [54]. However, to the best of our knowledge, this is the first description of AzA-dependent cAMP signaling. Whether AzA or other unidentified cAMP-inducing agents can act as an endogenous T cell retention signal is unknown. Be that as it may, ligands for T cell-expressed ORs are intriguing, new candidates for therapeutic modulation of T cell activity.
AUTHORSHIP
A.A.C. assisted with study design, performed experiments, analyzed data, and contributed to the writing of this study. S.N. assisted with study design, performed experiments, and contributed to the writing of this study. X.L. performed experiments and contributed to the writing of this study. S.D.M. assisted in the conception of the study and study design, analyzed data, and contributed to the writing of this study. J.R.L. assisted in the conception of the study and study design, performed experiments, analyzed data, and contributed to the writing of this study.
ACKNOWLEDGMENTS
This work was supported, in part, by the U.S. National Multiple Sclerosis Society Research Grants RG 4305A1/T and RG 4492-A-2; and by the U.S. National Institutes of Health National Institute on Deafness and Other Communication Disorders Grant R01 DC005633.
Glossary
- 8-pCPT-2′-O-Me-cAMP
8-(4-chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate monosodium hydrate
- ADCY3
adenylyl cyclase III
- ADRB2
β2 adrenergic receptor
- a.k.a.
also known as
- AzA
azelaic acid
- cBiMPS
Sp-5,6-dichloro-1-β-d- ribofuranosylbenzimidazole-3′,5′-monophosphorothioate
- CNG
cyclic nucleotide-gated
- EAE
experimental autoimmune encephalomyelitis
- Epac
exchange protein directly activated by cAMP
- FSC
forward-scatter
- GNAL
G protein α subunit Gαolf
- GPCR
G protein-coupled receptor
- NA
nonanoic acid
- OR
odorant receptor
- OSN
olfactory sensory neuron
- PKA
protein kinase A
- qPCR
quantitative real-time PCR
- Rp-cAMPS
adenosine 3′,5′-cyclic monophosphorothioate, Rp-isomer
- SSC
side-scatter
DISCLOSURES
The content and views expressed in this paper are the sole responsibility of the authors and do not necessarily reflect the views or policies of the Department of Defense or the U.S. government. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. government. The authors declare no conflicts of interest.
REFERENCES
- 1.Charo I. F., Ransohoff R. M. (2006) The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621. [DOI] [PubMed] [Google Scholar]
- 2.Debes G. F., Arnold C. N., Young A. J., Krautwald S., Lipp M., Hay J. B., Butcher E. C. (2005) Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 6, 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bromley S. K., Thomas S. Y., Luster A. D. (2005) Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6, 895–901. [DOI] [PubMed] [Google Scholar]
- 4.Ledgerwood L. G., Lal G., Zhang N., Garin A., Esses S. J., Ginhoux F., Merad M., Peche H., Lira S. A., Ding Y., Yang Y., He X., Schuchman E. H., Allende M. L., Ochando J. C., Bromberg J. S. (2008) The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat. Immunol. 9, 42–53. [DOI] [PubMed] [Google Scholar]
- 5.Lees J. R., Sim J., Russell J. H. (2010) Encephalitogenic T-cells increase numbers of CNS T-cells regardless of antigen specificity by both increasing T-cell entry and preventing egress. J. Neuroimmunol. 220, 10–16. [DOI] [PubMed] [Google Scholar]
- 6.Flügel A., Berkowicz T., Ritter T., Labeur M., Jenne D. E., Li Z., Ellwart J. W., Willem M., Lassmann H., Wekerle H. (2001) Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity 14, 547–560. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami N., Nägerl U. V., Odoardi F., Bonhoeffer T., Wekerle H., Flügel A. (2005) Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J. Exp. Med. 201, 1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archambault A. S., Sim J., Gimenez M. A., Russell J. H. (2005) Defining antigen-dependent stages of T cell migration from the blood to the central nervous system parenchyma. Eur. J. Immunol. 35, 1076–1085. [DOI] [PubMed] [Google Scholar]
- 9.Bartholomäus I., Kawakami N., Odoardi F., Schläger C., Miljkovic D., Ellwart J. W., Klinkert W. E., Flügel-Koch C., Issekutz T. B., Wekerle H., Flügel A. (2009) Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98. [DOI] [PubMed] [Google Scholar]
- 10.Skålhegg B. S., Landmark B. F., Døskeland S. O., Hansson V., Lea T., Jahnsen T. (1992) Cyclic AMP-dependent protein kinase type I mediates the inhibitory effects of 3′,5′-cyclic adenosine monophosphate on cell replication in human T lymphocytes. J. Biol. Chem. 267, 15707–15714. [PubMed] [Google Scholar]
- 11.Jimenez J. L., Punzón C., Navarro J., Muñoz-Fernández M. A., Fresno M. (2001) Phosphodiesterase 4 inhibitors prevent cytokine secretion by T lymphocytes by inhibiting nuclear factor-kappaB and nuclear factor of activated T cells activation. J. Pharmacol. Exp. Ther. 299, 753–759. [PubMed] [Google Scholar]
- 12.Spehr M., Munger S. D. (2009) Olfactory receptors: G protein-coupled receptors and beyond. J. Neurochem. 109, 1570–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck L., Axel R. (1991) A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187. [DOI] [PubMed] [Google Scholar]
- 14.Spehr M., Gisselmann G., Poplawski A., Riffell J. A., Wetzel C. H., Zimmer R. K., Hatt H. (2003) Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299, 2054–2058. [DOI] [PubMed] [Google Scholar]
- 15.Griffin C. A., Kafadar K. A., Pavlath G. K. (2009) MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev. Cell 17, 649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pluznick J. L., Zou D. J., Zhang X., Yan Q., Rodriguez-Gil D. J., Eisner C., Wells E., Greer C. A., Wang T., Firestein S., Schnermann J., Caplan M. J. (2009) Functional expression of the olfactory signaling system in the kidney. Proc. Natl. Acad. Sci. USA 106, 2059–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pluznick J. L., Protzko R. J., Gevorgyan H., Peterlin Z., Sipos A., Han J., Brunet I., Wan L. X., Rey F., Wang T., Firestein S. J., Yanagisawa M., Gordon J. I., Eichmann A., Peti-Peterdi J., Caplan M. J. (2013) Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 110, 4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldmesser E., Olender T., Khen M., Yanai I., Ophir R., Lancet D. (2006) Widespread ectopic expression of olfactory receptor genes. BMC Genomics 7, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blache P., Gros L., Salazar G., Bataille D. (1998) Cloning and tissue distribution of a new rat olfactory receptor-like (OL2). Biochem. Biophys. Res. Commun. 242, 669–672. [DOI] [PubMed] [Google Scholar]
- 20.Li J. J., Tay H. L., Plank M., Essilfie A. T., Hansbro P. M., Foster P. S., Yang M. (2013) Activation of olfactory receptors on mouse pulmonary macrophages promotes monocyte chemotactic protein-1 production. PLoS One 8, e80148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malki A., Fiedler J., Fricke K., Ballweg I., Pfaffl M. W., Krautwurst D. (2015) Class I odorant receptors, TAS1R and TAS2R taste receptors, are markers for subpopulations of circulating leukocytes. J. Leukoc. Biol. 97, 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao W., Ho L., Varghese M., Yemul S., Dams-O’Connor K., Gordon W., Knable L., Freire D., Haroutunian V., Pasinetti G. M. (2013) Decreased level of olfactory receptors in blood cells following traumatic brain injury and potential association with tauopathy. J. Alzheimers Dis. 34, 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lees J. R., Iwakura Y., Russell J. H. (2008) Host T cells are the main producers of IL-17 within the central nervous system during initiation of experimental autoimmune encephalomyelitis induced by adoptive transfer of Th1 cell lines. J. Immunol. 180, 8066–8072. [DOI] [PubMed] [Google Scholar]
- 24.Chisari F. V., Edgington T. S. (1974) Human T lymphocyte “E” rosette function. I. A process modulated by intracellular cyclic AMP. J. Exp. Med. 140, 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vischer T. L. (1976) The differential effect of cyclic AMP on lymphocyte stimulation by T- or B-cell mitogens. Immunology 30, 735–739. [PMC free article] [PubMed] [Google Scholar]
- 26.Gray L. S., Gnarra J., Hewlett E. L., Engelhard V. H. (1988) Increased intracellular cyclic adenosine monophosphate inhibits T lymphocyte-mediated cytolysis by two distinct mechanisms. J. Exp. Med. 167, 1963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L., Yee C., Beavo J. A. (1999) CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science 283, 848–851. [DOI] [PubMed] [Google Scholar]
- 28.Cheng X., Ji Z., Tsalkova T., Mei F. (2008) Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim. Biophys. Sin. (Shanghai) 40, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirshev S. V. (2011) Role of Epac proteins in mechanisms of cAMP-dependent immunoregulation. Biochemistry. (Mosc). 76, 981–998. [DOI] [PubMed] [Google Scholar]
- 30.Almahariq M., Tsalkova T., Mei F. C., Chen H., Zhou J., Sastry S. K., Schwede F., Cheng X. (2013) A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol. Pharmacol. 83, 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enserink J. M., Christensen A. E., de Rooij J., van Triest M., Schwede F., Genieser H. G., Døskeland S. O., Blank J. L., Bos J. L. (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4, 901–906. [DOI] [PubMed] [Google Scholar]
- 32.Munger S. D., Leinders-Zufall T., Zufall F. (2009) Subsystem organization of the mammalian sense of smell. Annu. Rev. Physiol. 71, 115–140. [DOI] [PubMed] [Google Scholar]
- 33.Malnic B., Hirono J., Sato T., Buck L. B. (1999) Combinatorial receptor codes for odors. Cell 96, 713–723. [DOI] [PubMed] [Google Scholar]
- 34.Abaffy T., Malhotra A., Luetje C. W. (2007) The molecular basis for ligand specificity in a mouse olfactory receptor: a network of functionally important residues. J. Biol. Chem. 282, 1216–1224. [DOI] [PubMed] [Google Scholar]
- 35.Atkinson J. P., Sullivan T. J., Kelly J. P., Parker C. W. (1977) Stimulation by alcohols of cyclic AMP metabolism in human leukocytes. Possible role of cyclic AMP in the anti-inflammatory effects of ethanol. J. Clin. Invest. 60, 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosenden R., Taskén K. (2011) Cyclic AMP-mediated immune regulation—overview of mechanisms of action in T cells. Cell. Signal. 23, 1009–1016. [DOI] [PubMed] [Google Scholar]
- 37.Bopp T., Becker C., Klein M., Klein-Hessling S., Palmetshofer A., Serfling E., Heib V., Becker M., Kubach J., Schmitt S., Stoll S., Schild H., Staege M. S., Stassen M., Jonuleit H., Schmitt E. (2007) Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 204, 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodor J., Bopp T., Vaeth M., Klein M., Serfling E., Hünig T., Becker C., Schild H., Schmitt E. (2012) Cyclic AMP underpins suppression by regulatory T cells. Eur. J. Immunol. 42, 1375–1384. [DOI] [PubMed] [Google Scholar]
- 39.Paccani S. R., Dal Molin F., Benagiano M., Ladant D., D’Elios M. M., Montecucco C., Baldari C. T. (2008) Suppression of T-lymphocyte activation and chemotaxis by the adenylate cyclase toxin of Bordetella pertussis. Infect. Immun. 76, 2822–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez G., Ross J. A., Nagy Z. S., Kirken R. A. (2013) Forskolin-inducible cAMP pathway negatively regulates T-cell proliferation by uncoupling the interleukin-2 receptor complex. J. Biol. Chem. 288, 7137–7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi Paccani S., Benagiano M., Capitani N., Zornetta I., Ladant D., Montecucco C., D’Elios M. M., Baldari C. T. (2009) The adenylate cyclase toxins of Bacillus anthracis and Bordetella pertussis promote Th2 cell development by shaping T cell antigen receptor signaling. PLoS Pathog. 5, e1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albert D., Kowalski J., Nodzenski E., Micek M., Wu P. (1990) The dose dependent effect of cyclic AMP on ribonucleotide reductase in mitogen stimulated mononuclear cells. Biochem. Biophys. Res. Commun. 167, 383–390. [DOI] [PubMed] [Google Scholar]
- 43.Seabrook T., Au B., Dickstein J., Zhang X., Ristevski B., Hay J. B. (1999) The traffic of resting lymphocytes through delayed hypersensitivity and chronic inflammatory lesions: a dynamic equilibrium. Semin. Immunol. 11, 115–123. [DOI] [PubMed] [Google Scholar]
- 44.Woodland D. L., Kohlmeier J. E. (2009) Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 9, 153–161. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X., Firestein S. (2009) Genomics of olfactory receptors. Results Probl. Cell Differ. 47, 25–36. [DOI] [PubMed] [Google Scholar]
- 46.Young J. M., Shykind B. M., Lane R. P., Tonnes-Priddy L., Ross J. A., Walker M., Williams E. M., Trask B. J. (2003) Odorant receptor expressed sequence tags demonstrate olfactory expression of over 400 genes, extensive alternate splicing and unequal expression levels. Genome Biol. 4, R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H., Ivic L., Otaki J. M., Hashimoto M., Mikoshiba K., Firestein S. (1998) Functional expression of a mammalian odorant receptor. Science 279, 237–242. [DOI] [PubMed] [Google Scholar]
- 48.Krautwurst D., Yau K. W., Reed R. R. (1998) Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell 95, 917–926. [DOI] [PubMed] [Google Scholar]
- 49.De la Cruz O., Blekhman R., Zhang X., Nicolae D., Firestein S., Gilad Y. (2009) A signature of evolutionary constraint on a subset of ectopically expressed olfactory receptor genes. Mol. Biol. Evol. 26, 491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howe A. K. (2004) Regulation of actin-based cell migration by cAMP/PKA. Biochim. Biophys. Acta 1692, 159–174. [DOI] [PubMed] [Google Scholar]
- 51.Kawasaki H., Springett G. M., Mochizuki N., Toki S., Nakaya M., Matsuda M., Housman D. E., Graybiel A. M. (1998) A family of cAMP-binding proteins that directly activate Rap1. Science 282, 2275–2279. [DOI] [PubMed] [Google Scholar]
- 52.Bjørgo E., Moltu K., Taskén K. (2011) Phosphodiesterases as targets for modulating T-cell responses. Handbook Exp. Pharmacol. 345–363. [DOI] [PubMed] [Google Scholar]
- 53.Graupe K., Cunliffe W. J., Gollnick H. P., Zaumseil R. P. (1996) Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis 57 (1 Suppl), 20–35. [PubMed] [Google Scholar]
- 54.Mastrofrancesco A., Ottaviani M., Aspite N., Cardinali G., Izzo E., Graupe K., Zouboulis C. C., Camera E., Picardo M. (2010) Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARgamma activation. Exp. Dermatol. 19, 813–820. [DOI] [PubMed] [Google Scholar]