Abstract
Approximately 10% to 20% of patients optimally treated for early Lyme disease develop persistent symptoms of unknown pathophysiology termed posttreatment Lyme disease syndrome (PTLDS). The objective of this study was to investigate associations between PTLDS and immune mediator levels during acute illness and at several time points following treatment. Seventy-six participants with physician-documented erythema migrans and 26 healthy controls with no history of Lyme disease were enrolled. Sixty-four cytokines, chemokines, and inflammatory markers were measured at each visit for a total of 6 visits over 1 year. An operationalized definition of PTLDS incorporating symptoms and functional impact was applied at 6 months and 1 year following treatment completion, and clinical outcome groups were defined as the return-to-health, symptoms-only, and PTLDS groups. Significance analysis of microarrays identified 7 of the 64 immune mediators to be differentially regulated by group. Generalized logit regressions controlling for potential confounders identified posttreatment levels of the T-cell chemokine CCL19 to be independently associated with clinical outcome group. Receiver operating characteristic analysis identified a CCL19 cutoff of >111.67 pg/ml at 1 month following treatment completion to be 82% sensitive and 83% specific for later PTLDS. We speculate that persistently elevated CCL19 levels among participants with PTLDS may reflect ongoing, immune-driven reactions at sites distal to secondary lymphoid tissue. Our findings suggest the relevance of CCL19 both during acute infection and as an immunologic risk factor for PTLDS during the posttreatment phase. Identification of a potential biomarker predictor for PTLDS provides the opportunity to better understand its pathophysiology and to develop early interventions in the context of appropriate and specific clinical information.
INTRODUCTION
Lyme disease is the most common tick-borne disease in temperate regions of the Northern Hemisphere (1). In North America, cases of Lyme disease are concentrated in the Northeast, upper Midwest, and Mid-Atlantic, and the causative agent is the spirochete Borrelia burgdorferi. Recently, CDC and state health investigators have estimated that approximately 300,000 new infections occur annually in the United States (2, 3). In certain high-risk communities, studies suggest that the seasonal incidence of new cases may be as high as 3% (4). As the geographic range of the tick vector and the mouse and deer hosts expands, the impact of Lyme and other tick-borne diseases is likely to grow (5).
The hallmark of early Lyme disease is a cutaneous lesion, erythema migrans (EM), which occurs with or without symptoms of infection, such as fever, arthralgias, fatigue, headache, or neck pain (6). Within days to weeks, B. burgdorferi may disseminate from the site of skin inoculation through the blood or tissues and spread systemically to other areas of the skin (called disseminated EM), as well as the musculoskeletal, cardiac, and neurologic systems. More than 50% of patients with EM are found to have positive PCR or blood culture results at this stage (7, 8).
Antibiotic treatment of early Lyme disease speeds the resolution of EM and is effective in preventing later objective manifestations. In the absence of effective antibiotic treatment, the adaptive immune response does not reliably eradicate infection, with small numbers of spirochetes being able to persist in certain tissues (9). If untreated, as many as 60% of patients will have a clinical relapse months to years later with manifestations of late Lyme arthritis or, less commonly in the United States, neurologic disease (10). Treatment at later stages may be more difficult. Approximately 10% to 20% of late Lyme arthritis patients develop persistent or recurrent objective findings termed posttreatment antibiotic-refractory disease. This is thought to be due to the autoimmune-mediated inflammation that occurs in genetically susceptible individuals, especially those expressing HLA-DR4 alleles (11).
Approximately 10% to 20% of patients with diagnosed and treated early Lyme disease have persistent symptoms of fatigue, arthralgias, sleep disruption, or cognitive complaints following antibiotic treatment (12). These posttreatment symptoms may be mild and limited or severe and chronic. When posttreatment symptoms last 6 months or longer and impair life functioning, patients meet the case definition for posttreatment Lyme disease syndrome (PTLDS) (1, 13, 14). Studies have suggested a severe initial illness (15, 16), delayed treatment, neurologic involvement (1, 17), or suboptimal antibiotic therapy (17, 18) to be potential risk factors for development of PTLDS.
The pathophysiology of PTLDS is currently unknown. Studies using rodent and primate models have suggested that the persistence of bacterial and/or spirochetal antigens after antibiotic therapy may drive disease (19, 20). In the case of antibiotic-refractory late Lyme arthritis, the persistence of spirochetal antigens has also been hypothesized to lead to immune dysregulation in CD4+ T-cell subsets (21). Lastly, PTLDS has been suggested to have an autoimmune component, and this is supported by the finding of antineural antibodies in one cohort of patients with PTLDS (22).
In a previous study, we identified a clear immune mediator signature for acute, untreated Lyme disease (23). In the current study, we extend our original observations across multiple time points and hypothesize that individuals with PTLDS have persistent elevations of specific immune mediators. Lastly, we sought to examine the diagnostic utility of any PTLDS-specific immune markers identified.
MATERIALS AND METHODS
Recruitment of study participants and visit timeline.
Lyme disease patients with a physician-documented EM of greater than 5 cm and evidence of dissemination (i.e., multiple skin lesions and/or at least one new-onset, concurrent systemic symptom) were enrolled at a suburban clinical practice in Maryland from 2008 to 2012. Patients with a known history of Lyme disease or preexisting, confounding medical conditions associated with prolonged fatigue, pain, or neurocognitive symptoms were excluded. Following Infectious Diseases Society of America (IDSA) treatment guidelines, all patients were treated with 3 weeks of oral doxycycline (14). They were seen regularly over the course of 1 year for a total of six study visits (at an acute-phase, pretreatment visit, 3 weeks later following treatment, and at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 1 year posttreatment).
Extensive clinical data and biological specimens were collected at each study visit. At the acute-phase, pretreatment visit, blood samples were drawn for a Lyme disease antibody screen by two-tier testing by enzyme-linked immunosorbent assay (ELISA) and Western blotting, and the results were interpreted according to CDC criteria (24). If the acute-phase test was negative, a repeat convalescent-phase test was performed at the second study visit following treatment completion. A symptom count was generated at each visit using an interviewer-administered checklist of 36 symptoms. Any self-reported, new-onset symptoms since the time of diagnosis of early Lyme disease that could not be explained by other causes were considered present and included in the count. Twenty-six healthy, seronegative controls with no known clinical history of Lyme disease were also enrolled from the same practice over the same period. This study was approved by the Johns Hopkins Medicine Institutional Review Board. Informed consent was obtained from all participants with early Lyme disease and controls prior to enrollment.
Generation of clinical outcome groups.
We applied a previously published definition of PTLDS (25) based on the IDSA's proposed case definition (14) to all patents in the study at the 6-month and 1-year study visits only. This definition includes two components: (i) the presence of persistent symptoms, defined by either fatigue, musculoskeletal pain in at least three areas of the body, or cognitive complaints of difficulty finding words, focusing, concentrating, or memory impairment, and (ii) functional impact, defined by a composite T score of less than 45 (a half standard deviation below the normative mean) on four previously identified subscales of the Short Form (36) Health Survey (SF-36). This cutoff was chosen on the basis of prior research demonstrating its sensitivity for determination of the impact of symptoms on daily life functioning in this population (25). Three clinical outcome groups were defined on the basis of this definition. Participants in the return-to-health group met neither the symptom nor the functional impact criteria, participants in the symptoms-only group met the symptoms but not the functional impact criteria, and participants who met both criteria at either the 6-month or 1-year time points were considered to have PTLDS.
Detection of immune markers by Bio-Plex bead array system.
The Bio-Plex bead array system was employed to perform multiplex analysis of 58 cytokines and chemokines and nine acute-phase markers (26). However, due to interbatch variation, three of the nine acute-phase markers were dropped, resulting in a final total of 58 cytokines and chemokines and six acute-phase markers. Data processing was completed using Bio-Plex manager software (version 5.0). The normalization of data between two batches was achieved by setting all values less than 1 pg/ml equal to 1 pg/ml, calculating the 10% trimmed mean for each batch, and then multiplying by a factor to equalize the trimmed mean values. The protocol and data generated were minimum information about a microarray experiment (MIAME) compliant and were deposited in the Gene Expression Omnibus repository. The final list of cytokines, chemokines, and inflammatory markers included in the analysis is shown in Table S1 in the supplemental material.
Statistical analyses.
To control for intrapersonal variability within the control group, samples were analyzed at two study visits 6 months apart, and a single mean value was generated for each individual. Univariate clinical outcome group tests for difference were performed using nonparametric Wilcoxon rank sum tests for continuous variables and the chi-square or Fisher's exact test for dichotomous variables. Statistical significance was set at a P value of <0.05 for univariate group comparisons. To examine the relationship between immune mediators and clinical outcome status, generalized logit regression models that also included predictor variables found to be associated (set at a P value of <0.25 for inclusion) with clinical outcome status in univariate analyses were generated. Receiver operating characteristic (ROC) analysis was performed to determine cutoff values and assess sensitivity and specificity. All calculations were performed using SAS (version 9.3) software (SAS Institute, Cary, NC).
For descriptive analyses of the multiplex data, values were analyzed using the log ratio to average. For subgroup comparisons, ratio-to-average values were analyzed by the use of significance analysis of microarrays (SAM; version 4.0) (27) and sorted on the basis of false discovery rates (represented by the q value) to identify cytokine or acute-phase proteins with the greatest differences between clinical outcome groups. The most stringent threshold for q (q < 0.1%) was employed to maximally minimize falsely discovered markers. Hierarchical clustering software (Cluster, version 3.0) was used to arrange the results, which were displayed using the Java Treeview (version 1.1.5r2) program.
Accession number(s).
The protocol and data generated in this study have been deposited in the Gene Expression Omnibus (GEO) repository under GEO accession number GSE84479.
RESULTS
Demographic and clinical characteristics of Lyme disease participant cohort and healthy controls.
The sex, age, and race of our sample of 76 participants with early Lyme disease and 26 healthy controls are shown in Table 1, as these biologic variables have been shown in previous studies to impact immune marker levels (28–30). There were no statistically significant differences between these two groups on any of the demographic variables examined. The PTLDS definition (25) was applied to our cohort of 76 Lyme disease participants. Eleven participants (14.47%) met the criteria for PTLDS at either 6 or 12 months, 29 (38.16%) met the criteria for the symptoms-only group, and 36 (47.37%) met the criteria for the return-to-health group. Participants in the PTLDS group were statistically significantly younger than those in the symptoms-only group but not those in the return-to-health group (Table 1). There were no other statistically significant differences found among the Lyme disease clinical outcome groups on any of the demographic variables examined (Table 1). Similarly, there were no statistically significant differences found among the three outcome groups (the PTLDS, symptoms-only, and return-to-health groups) on any of the clinical variables measured at the acute-phase, pretreatment visit (Table 2). As expected, the PTLDS and symptoms-only groups self-reported a higher number of symptoms at the 3-month, 6-month, and 1-year follow-up visits.
TABLE 1.
Demographic characteristics of 76 participants with early Lyme disease and 26 healthy controls
| Characteristic | Lyme disease patientsa |
Healthy controls (n = 26) | P value for all Lyme disease patients vs controlsb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PTLDS (n = 11) | Symptoms only (n = 29) | Return to health (n = 36) | Total (n = 76) |
P valueb |
|||||
| P vs S | P vs R | S vs R | |||||||
| % participants of female sex | 72.73 | 48.28 | 41.67 | 48.68 | 0.29 | 0.07 | 0.59 | 53.85 | 0.65 |
| Age (yr) | 0.04 | 0.24 | 0.29 | 0.17 | |||||
| Median | 43 | 54 | 53 | 53 | 57 | ||||
| IQRc | 29–53 | 46–66 | 34–62 | 38–63 | 46–66 | ||||
| Range | 20–64 | 20–75 | 20–77 | 20–77 | 22–73 | ||||
| % non-Hispanic white participants | 81.82 | 96.55 | 94.44 | 93.42 | 0.18 | 0.23 | 1.00 | 88.46 | 0.42 |
Lyme disease participants were grouped by clinical outcome (PTLDS [P], symptoms only [S], and return to health [R]) using a previously published definition incorporating both persistent symptoms and functional impact (25).
Significance was determined by the chi-square or Fisher's exact test for female sex and non-Hispanic white and the Wilcoxon rank sum test for age.
IQR, interquartile range.
TABLE 2.
Clinical characteristics of 76 participants with early Lyme disease grouped by clinical outcome
| Characteristic | Lyme disease patientsa |
P valueb |
|||||
|---|---|---|---|---|---|---|---|
| PTLDS (n = 11) | Symptoms only (n = 29) | Return to health (n = 36) | Total (n = 76) | P vs S | P vs R | S vs R | |
| % participants with physician-documented: | |||||||
| EM lesions | 100.00 | 100.00 | 100.00 | 100.00 | NA | NA | NA |
| Disseminated EM | 9.09 | 34.48 | 36.11 | 31.58 | 0.23 | 0.14 | 0.89 |
| Time from first symptom to initiation of antibiotic treatment (days) | 0.47 | 0.72 | 0.35 | ||||
| Median | 6 | 7 | 5 | 7 | |||
| IQRc | 3–10 | 5–10 | 3–14 | 4–11 | |||
| Range | 3–42 | 1–35 | 2–35 | 1–42 | |||
| % participants seropositived | 45.45 | 74.07 | 72.22 | 68.92 | 0.14 | 0.15 | 0.87 |
| No. of self-reported symptomse | |||||||
| Acute phase, pretreatment | 0.73 | 0.08 | 0.24 | ||||
| Median | 12 | 10 | 9 | 9 | |||
| IQR | 10–15 | 6–16 | 6–12 | 6–14 | |||
| Range | 4–19 | 0–22 | 1–17 | 0–22 | |||
| Posttreatment follow-up | 0.29 | 0.07 | 0.19 | ||||
| Median | 17 | 13 | 10 | 11 | |||
| IQR | 8–21 | 8–18 | 8–12 | 8–17 | |||
| Range | 4–24 | 4–24 | 3–24 | 3–24 | |||
| 1-mo follow-up | 0.05 | 0.02 | 0.79 | ||||
| Median | 10 | 3 | 4 | 4 | |||
| IQR | 2–14 | 1–7 | 0–6 | 1–8 | |||
| Range | 0–24 | 0–22 | 0–15 | 0–24 | |||
| 3-mo follow-up | 0.07 | 0.004 | 0.07 | ||||
| Median | 7 | 3 | 1 | 2 | |||
| IQR | 2–12 | 1–6 | 0–3 | 1–6 | |||
| Range | 1–22 | 0–21 | 0–15 | 0–22 | |||
| 6-mo follow-up | 0.22 | 0.002 | 0.002 | ||||
| Median | 6 | 4 | 2 | 3 | |||
| IQR | 3–9 | 3–7 | 0–3 | 1–6 | |||
| Range | 1–23 | 0–19 | 0–10 | 0–23 | |||
| 1-yr follow-up | 0.002 | <0.001 | <0.001 | ||||
| Median | 13 | 4 | 1 | 3 | |||
| IQR | 8–19 | 3–10 | 0–3 | 0–8 | |||
| Range | 6–34 | 0–16 | 0–12 | 0–34 | |||
Participants were grouped by clinical outcome using a previously published definition incorporating both persistent symptoms and functional impact (25).
Significance was determined by the chi-square or Fisher's exact test for disseminated erythema migrans lesions and seropositivity and the Wilcoxon rank sum test for time from first symptom to antibiotic treatment and number of symptoms. P, participants with PTLDS; S, participants with symptoms only; R, participants who returned to health; NA, not applicable.
IQR, interquartile range.
Interpreted according to CDC guidelines for acute- and convalescent-phase two-tier ELISA and reflex Western blot (IgG/IgM) testing (24). Complete serologic data were missing for two symptoms-only group participants.
Determined by use of an interviewer-administered checklist of 36 symptoms. Any new-onset symptoms since the time of diagnosis of early Lyme disease that were not explainable by other causes were considered present and included in the count. One participant from the symptoms-only group was missing complete symptom data at the posttreatment visit; three participants (one from the symptoms-only group, two from the return-to-health group) were missing complete symptom data at the 1-month follow-up visit, two participants from the return-to-health group were missing complete symptom data from the 3-month follow-up visit, and one participant from the PTLDS group was missing complete symptom data from the 1-year follow-up visit.
Identification of seven relevant immune markers through SAM analysis.
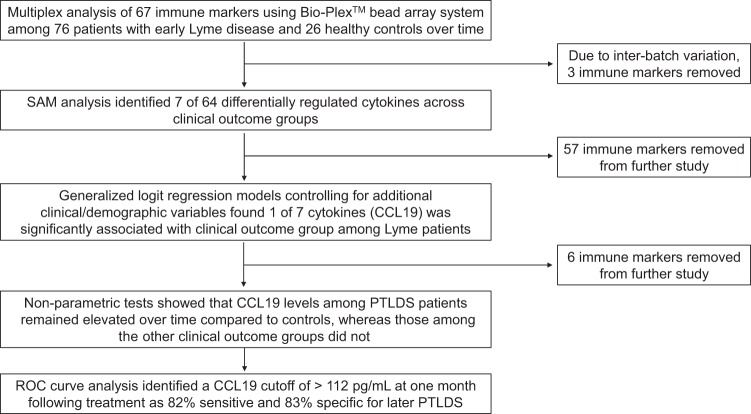
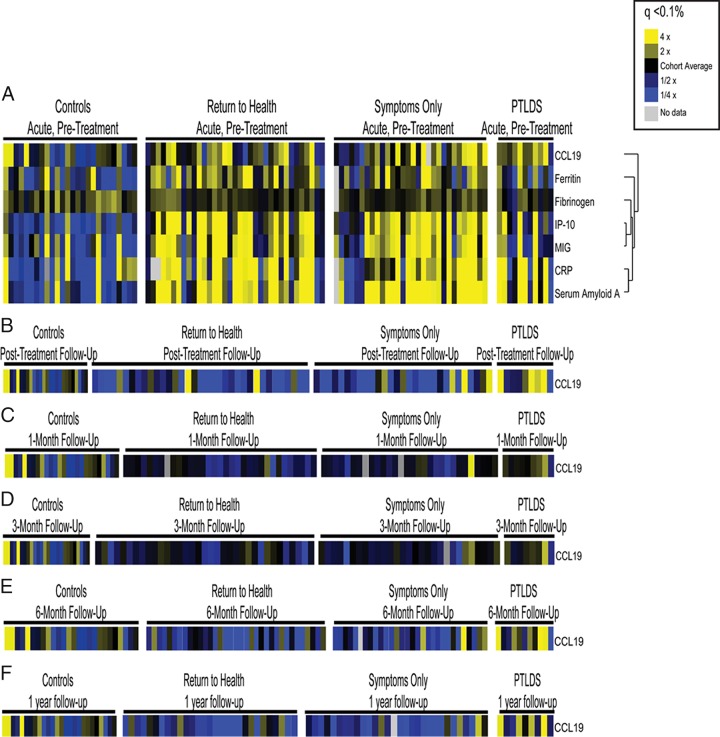
Figure 1 outlines the analytic stages presented here. First, SAM analysis was performed to compare the Lyme disease participant groups (the return-to-health, symptoms-only, and PTLDS groups) and healthy controls on the basis of all 64 immune mediators. At the initial, pretreatment visit, 7 cytokines were found to be differentially regulated among these groups (q < 0.10%; Fig. 2A): CCL19, ferritin, fibrinogen, gamma interferon-induced protein 10 (IP-10), monokine induced by gamma interferon (MIG), C-reactive protein, and serum amyloid A. This is consistent with the findings of our previous study with a smaller cohort (23). When the same SAM analysis was performed at the follow-up visits, of the 64 mediators, CCL19 was the only mediator found to differentiate the groups (q < 0.10%; Fig. 2B to F). In the next stage of the analysis, we focused only on these seven immune markers identified at the pretreatment or follow-up visits and removed the other 57 from future study.
FIG 1.
Immune marker analysis of early Lyme disease cases and controls over time. Three clinical outcome groups were defined using a previously published definition of PTLDS incorporating both persistent symptoms and functional impact (25).
FIG 2.
Identification of relevant immune mediators. Serum samples from participants with early Lyme disease and healthy controls were assayed for the presence of soluble mediators using an optimized multiplex-based assay system. Mediators with significant changes (q < 0.1%) are displayed as heat maps to visualize differences. All study time points are represented in panels A to F for healthy controls, those that returned to health, those that reported symptoms without functional impact, and those that met the criteria for PTLDS (25). (A) Mediators with significant changes (q < 0.1%) at the acute-phase, pretreatment visit; (B) mediators with significant changes (q < 0.1%) at the posttreatment follow-up visit 3 weeks later; (C) mediators with significant changes (q < 0.1%) at 1 month following treatment completion; (D) mediators with significant changes (q < 0.1%) at 3 months following treatment completion; (E) mediators with significant changes (q < 0.1%) at 6 months following treatment completion; (F) mediators with significant changes (q < 0.1%) at 1 year following treatment completion. CRP, C-reactive protein.
Controlling for potential confounders through generalized logit regression revealed posttreatment CCL19 to be the only differentially regulated immune marker.
Separate generalized logit regressions were used to predict Lyme disease clinical outcome group (the return-to-health, symptoms-only, and PTLDS groups) at each of the study time points. At each visit, we included as predictor variables in the model those immune mediators identified by SAM analysis to differ significantly between clinical outcome groups. Additionally, the variables presented in Tables 1 and 2 found to be associated with clinical outcome status (set at a P value of <0.25 for inclusion) were determined to be suitable for inclusion in the models as potential confounders. Sex, age, the presence of single versus multiple EM lesions, and final serologic status on two-tier testing were included. As symptoms at the later visits were associated with clinical outcome group by definition, they were not included in the models. Although the P value was <0.25 in univariate analysis, the small number of Hispanic and/or nonwhite participants resulted in quasiseparation of the data when that variable was included in the models and therefore was removed.
At the pretreatment visit, none of the immune mediators identified by SAM were significantly associated with an increased odds (P < 0.05) of later PTLDS after controlling for additional cytokine levels as well as other demographic and pretreatment clinical variables (Table 3). At all posttreatment follow-up visits, however, an increase in CCL19 of 0.5 standard deviation (SD; 49.96 pg/ml at the posttreatment visit, 51.73 pg/ml at the 1-month follow-up visit, 42.08 pg/ml at the 3-month follow-up visit, 30.99 pg/ml at the 6-month follow-up visit, and 45.97 pg/ml at the 1-year follow-up visit) did result in an increased odds of PTLDS (posttreatment visit, P = 0.02; 1-month follow-up visit, P = 0.03; 3-month follow-up visit, P = 0.04; 6-month follow-up visit, P = 0.02; 1-year follow-up visit, P = 0.02), indicating that participants in groups defined by symptoms and functional impact were expected to have higher CCL19 levels following treatment completion than those defined by symptoms alone, even after controlling for additional variables that may independently affect clinical outcome group status. As a result, we focused the remaining analyses specifically on CCL19.
TABLE 3.
Separate generalized logit regression models at all study visits to predict clinical outcome group statusa
| Characteristic | Unit increaseb | ORc (95% CI) |
|
|---|---|---|---|
| Return to health vs symptoms only | Return to health vs PTLDS | ||
| Acute phase, pretreatment | |||
| Fibrinogen | 443.74 ng/ml | 0.96 (0.65–1.44) | 0.78 (0.44–1.36) |
| MIG | 383.42 pg/ml | 0.91 (0.63–1.31) | 0.84 (0.35–2.02) |
| IP-10 | 509.06 pg/ml | 0.94 (0.67–1.32) | 0.85 (0.32–2.28) |
| CRPd | 57,140.56 ng/ml | 1.02 (0.68–1.53) | 1.57 (0.88–2.78) |
| Serum amyloid A | 3,173.89 ng/ml | 1.09 (0.78–1.51) | 1.11 (0.68–1.79) |
| Ferritin | 81,533.50 pg/ml | 0.86 (0.55–1.34) | 0.52 (0.15–1.82) |
| CCL19 | 78.81 pg/ml | 1.27 (0.87–1.85) | 1.36 (0.75–2.48) |
| Age | 1 yr | 1.01 (0.97–1.06) | 0.97 (0.90–1.03) |
| Sex | Female to male | 1.16 (0.33–4.15) | 0.32 (0.03–3.04) |
| Erythema migrans | Single to disseminated | 0.98 (0.28–3.36) | 0.30 (0.03–3.45) |
| Serology | Negative to positive | 1.12 (0.26–4.79) | 0.82 (0.12–5.67) |
| Posttreatment follow-up | |||
| CCL19 | 49.96 pg/ml | 1.49 (0.89–2.48) | 2.09 (1.12–3.88)e |
| Age | 1 year | 1.02 (0.98–1.06) | 0.98 (0.92–1.04) |
| Sex | Female to male | 0.96 (0.31–3.03) | 0.41 (0.06–2.97) |
| Presence of EM lesions | Single to disseminated | 0.85 (0.27–2.66) | 0.10 (0.01–1.96) |
| Serology | Negative to positive | 1.32 (0.38–4.60) | 0.72 (0.13–4.05) |
| 1-mo follow-up | |||
| CCL19 | 51.73 pg/ml | 1.47 (0.88–2.47) | 1.90 (1.06–3.39)e |
| Age | 1 year | 1.01 (0.97–1.05) | 0.96 (0.90–1.02) |
| Sex | Female to male | 1.05 (0.32–3.40) | 0.33 (0.05–2.26) |
| Presence of EM lesions | Single to disseminated | 0.86 (0.27–2.69) | 0.09 (0.01–2.33) |
| Serology | Negative to positive | 1.23 (0.33–4.60) | 0.48 (0.08–2.72) |
| 3-mo follow-up | |||
| CCL19 | 42.08 pg/ml | 1.20 (0.81–1.78) | 1.72 (1.03–2.88)e |
| Age | 1 year | 1.02 (0.98–1.06) | 0.98 (0.92–1.04) |
| Sex | Female to male | 1.07 (0.34–3.44) | 0.46 (0.06–3.43) |
| Presence of EM lesions | Single to disseminated | 0.89 (0.29–2.74) | 0.18 (0.01–2.24) |
| Serology | Negative to positive | 1.12 (0.32–3.87) | 0.52 (0.09–2.92) |
| 6 mo posttreatment | |||
| CCL19 | 30.99 pg/ml | 1.17 (0.82–1.67) | 1.84 (1.12–3.02)e |
| Age | 1 year | 1.02 (0.98–1.06) | 0.97 (0.91–1.03) |
| Sex | Female to male | 0.90 (0.29–2.75) | 0.26 (0.04–1.92) |
| Presence of EM lesions | Single to disseminated | 0.89 (0.28–2.83) | 0.10 (0.01–1.40) |
| Serology | Negative to positive | 1.34 (0.40–4.56) | 0.84 (0.15–4.72) |
| 1 yr posttreatment | |||
| CCL19 | 45.97 pg/ml | 1.03 (0.56–1.88) | 2.83 (1.15–6.94)e |
| Age | 1 year | 1.01 (0.97–1.05) | 0.98 (0.91–1.04) |
| Sex | Female to male | 0.77 (0.24–2.50) | 0.36 (0.04–3.57) |
| Presence of EM lesions | Single to disseminated | 1.07 (0.32–3.52) | 0.18 (0.01–2.30) |
| Serology | Negative to positive | 1.35 (0.37–4.97) | 1.17 (0.16–8.47) |
Clinical outcome group status (PTLDS, symptoms only, and return to health) was defined using a previously published definition incorporating both persistent symptoms and functional impact (25).
For immune markers, a unit increase was set at 0.5 SD for the Lyme disease participant sample.
OR, odds ratio.
CRP, C-reactive protein.
For significance of parameter estimate, P ≤ 0.05.
Elevated CCL19 levels are specific to the posttreatment Lyme disease syndrome clinical outcome group.
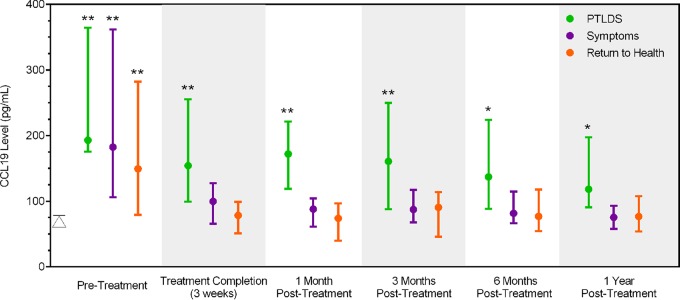
Displayed in Fig. 3 are median CCL19 levels over time by clinical group. Consistent with our findings from a previous study (23), the three clinical outcome groups had similarly elevated serum CCL19 levels at the time of acute infection, and all were significantly different (P < 0.01 for each) from those for the control group. However, following treatment completion and at each of the subsequent, posttreatment study visits, the median serum CCL19 levels for the PTLDS group remained significantly elevated (P ≤ 0.05 at each visit) compared to the levels for the control group. In contrast, the levels in the symptoms-only and return-to-health groups were not significantly elevated (P > 0.05 for both groups at each visit) compared to those in the control group.
FIG 3.
CCL19 levels are elevated in Lyme disease cases with PTLDS. Displayed are the median and interquartile range serum levels of CCL19 among 76 Lyme disease patients over time. Lyme disease-exposed participants are divided into the PTLDS, symptoms-only, and return-to-health clinical outcome groups on the basis of self-reported symptoms and survey measurements at 6 months and 1 year posttreatment (25). The median control value (79.28 pg/ml; interquartile range, 62.60 to 114.85 pg/ml) is represented by a triangle in the graph. *, P ≤ 0.05 for comparison of each group to the controls; **, P ≤ 0.01 for comparison of each group to the controls.
To further assess the sensitivity and specificity of the CCL19 level as a predictor of PTLDS clinical outcome status, visit-specific receiver operating characteristic (ROC) analyses were conducted. Sensitivity and specificity plots were generated (data not shown), and the intersection of these curves identified the cutoffs shown in Table 4. For each visit, the area under the curve obtained using these cutoffs ranged from 0.60 to 0.85. As predicted, the CCL19 level performed the worst as a predictor of PTLDS during acute infection, when the levels in all groups were similarly elevated to the levels in the controls (Fig. 3). At the 1-month follow-up visit, the identified cutoff (111.67 pg/ml) provided the highest sensitivity (82%) and specificity (83%) (Table 4). Lyme disease participants with levels above this cutoff at this time point had a 12.60 times (95% confidence interval [CI], 2.97 to 53.43 times) greater risk of meeting the criteria for PTLDS at the later 6- or 12-month posttreatment visit.
TABLE 4.
Results of visit-specific ROC curve analysisa
| Visit | Cutoff (pg/ml) | Area under the curve | Sensitivity (%) | Specificity (%) | Relative risk (95% CI) |
|---|---|---|---|---|---|
| Acute phase, pretreatment | 186.17 | 0.60 | 55 | 54 | 1.33 (0.44–4.00) |
| Posttreatment follow-up | 100.58 | 0.79 | 64 | 68 | 3.00 (0.96–9.35) |
| 1-mo follow-up | 111.67 | 0.85 | 82 | 83 | 12.60 (2.97–53.43) |
| 3-mo follow-up | 114.12 | 0.77 | 73 | 74 | 5.44 (1.58–18.75) |
| 6-mo follow-up | 104.94 | 0.76 | 64 | 65 | 2.68 (0.86–8.38) |
| 1-yr follow-up | 91.36 | 0.83 | 55 | 74 | 2.77 (0.94–8.15) |
The area under the curve, sensitivity, specificity, and relative risk of development of PTLDS are shown for each visit using cutoffs identified as the intersection of the sensitivity and specificity plots (not shown).
DISCUSSION
In a previous study, we examined the serum levels of 65 immune mediators among 44 participants with acute Lyme disease and identified a clear associated signature relative to the findings for the healthy controls (23). In the current study, we confirm the relevance of the T-cell chemokine CCL19 during acute infection and extend these observations to the posttreatment phase as well in an expanded cohort of participants. Specifically, the molecular finding of elevations in CCL19 levels was associated with functionally significant, persistent symptoms at 6 and 12 months after treatment of acute Lyme disease. This association remained significant even after controlling for potential demographic and clinical confounders, such as age, sex, the presence of single or disseminated EM lesions, and serologic status.
While CCL19 levels were elevated in most Lyme disease participants at the time of diagnosis, they frequently remained elevated immediately after completion of antibiotic therapy among those with the later clinical phenotype of PTLDS. While previous studies have indicated that the initial severity of illness may be predictive of persistent symptoms and, thus, that the biology of early infection may contain information related to long-term outcomes (15), our findings suggest the importance of the early posttreatment period as well. Individuals with ideally treated early Lyme disease have a greater than 12-fold higher risk of developing PTLDS by 6 or 12 months posttreatment if their CCL19 level is higher than 111.67 pg/ml at 1 month posttreatment. Lyme disease participants who return to normal health or have symptoms without an associated functional impact show a pattern of initial CCL19 elevation followed by a return to control levels after treatment, suggesting that CCL19 is specifically linked to PTLDS and is not a feature of mild subjective symptoms.
CCL19 (and the related chemokine CCL21) is largely produced by reticular stromal cells localized to secondary lymphoid tissues and functions to attract and position CCR7+ T cells, B cells, and dendritic cells to establish an optimal microenvironment for immune response generation (31, 32). The expression of CCL19 is thought to be constitutive, but activated dendritic cells produce high levels of CCL19 in order to increase immune cell trafficking in secondary lymphoid organs during active immune responses. This is likely responsible for the elevated levels of CCL19 and other immune mediators seen during acute Lyme disease (23). Consistent with this, in the mouse model of Lyme disease, CCL19 mRNA expression is increased in the lymph nodes of acutely infected mice (33). Elevated levels of CCL19 have also been observed during states of immune-mediated inflammation, including HIV infection, systemic lupus erythematosus, and rheumatoid arthritis (34–37).
The origin of persistent CCL19 levels among participants with PTLDS is less clear, as patients do not display signs of ongoing immune-mediated processes, such as joint synovitis. Interestingly, high levels of CCL19 expression have been found at sites of localized immune-driven reactions where ectopic lymphoid cell accumulation occurs, including the liver during chronic hepatitis C virus infection, the synovium in rheumatoid arthritis, the salivary glands in Sjogren's syndrome, and spinal fluid from patients with central nervous system inflammation, including that induced by Lyme neuroborreliosis (38–44). Based on this, we speculate that elevated CCL19 levels may reflect an ongoing, immune-driven reaction at sites distal to secondary lymphoid tissue. The observation that participants with PTLDS are defined by musculoskeletal pain and behavioral and neurologic symptoms suggests that the central nervous system may be a site for ectopic immune activity in PTLDS.
It has recently been reported that serum interleukin-23 (IL-23) levels are elevated during acute disease in patients that develop PTLDS and was proposed that Th-17-mediated immune responses may play a role in PTLDS (45). While we did not directly address the role of the Th-17 effector pathways in our longitudinal cohort, it has previously been shown that CCL19, along with IL-23, drive the development of pathogenic Th-17 cells in a murine model of encephalomyelitis (46). Therefore, CCL19 and IL-23 may identify an informative immune pathway.
Our findings raise the question of whether the subjective symptoms characteristic of PTLDS, such as fatigue, cognitive complaints, and mood changes (47, 48), may be related to a cytokine/chemokine effect, as has been hypothesized in other illnesses, such as hepatitis-associated fatigue (49) and multiple sclerosis-associated depression (50). If so, a range of disease management approaches may be helpful to patients and physicians. Medications used to treat depression may decrease cytokine levels and have been hypothesized to reverse symptoms induced by interferon alpha administration (51). The use of short-term antibiotic retreatment in the early, posttreatment phase of Lyme disease has yet to be formally tested, although it may be widely applied in clinical practice (52, 53).
There is strong evidence supporting the efficacy of behavioral interventions for pain and fatigue management (54, 55) and cognitive rehabilitation (56) in a variety of medical populations that may be applicable to patients with PTLDS. When such targeted symptom management and/or behavioral interventions are offered early in the recovery process, individuals with early Lyme disease may have a chance to learn how to adapt and adjust to persistent symptoms, thus helping to reduce interference with daily life functioning and possibly stave off emotional adjustment issues. Classifying immunologic risk factors associated with the development of PTLDS may provide opportunities to identify those at risk earlier than the current 6-month proposed case definition (14) and to provide closer follow-up, education, and early pharmacologic and behavioral interventions.
This study does have limitations, and the relationship between PTLDS and elevated CCL19 needs to be validated in other patient cohorts, as well as examined beyond the 1-year follow-up period used in the current study. Although we hypothesized that individuals with PTLDS would have persistent elevations of specific immune mediators, this was an exploratory analysis. Furthermore, different Borrelia species are associated with Eurasian Lyme disease; therefore, our results need to be tested in populations from other geographic areas. Study criteria limiting enrollment to those patients with EM and excluding those with preexisting conditions marked by subjective symptoms similar to those associated with PTLDS (such as fibromyalgia, chronic fatigue syndrome, or depression) may limit the generalizability of the findings. In addition, the current absence of reliable biomarkers or clinically available diagnostic tests for PTLDS compelled us to rely upon self-reported data in our analyses, which may have introduced bias in unknown ways. Finally, although all participants met the CDC case definition for confirmed early Lyme disease and the majority (68.42%) were positive on two-tier testing, the findings for those that remained seronegative were not confirmed by skin biopsy specimen PCR, and therefore, it is possible that they had other diseases, including southern tick-associated rash illness. Despite these limitations, the current study offers a foundational finding on how the immunologic response may contribute to clinical observations and identifies early posttreatment elevations of CCL19 levels to be a potential risk factor for PTLDS. This presents an opportunity not only to better understand the pathophysiology of PTLDS but also to design early interventions for disease management.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the physicians of Park Medical and Patient First for their patient referrals. We also acknowledge Alycia Williams and Rose Deegan for their assistance with sample processing, Eric Weinstein for assistance with the graph, and Xiangrong Kong for statistical assistance.
This work was supported by the Global Lyme Alliance (http://www.lymeresearchalliance.org), the Lyme Disease Research Foundation, Inc. (www.lymemd.org), the Gordon and Llura Gund Foundation, the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number P30AR05350, the Stabler Foundation (www.stablerfoundation.org), and a Synergy Award from the Johns Hopkins School of Medicine. We acknowledge support and funding from Edward St. John and the Brennan family.
The funders of this study had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00071-16.
REFERENCES
- 1.Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 2.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson C, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley A, Mead PS. 2015. Incidence of clinician-diagnosed Lyme disease, United States, 2005-2010. Emerg Infect Dis 21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tugwell P, Dennis DT, Weinstein A, Wells G, Shea B, Nichol G, Hayward R, Lightfoot R, Baker P, Steere AC. 1997. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med 127:1109–1123. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 5.Brownstein JS, Holford TR, Fish D. 2005. Effect of climate change on Lyme disease risk in North America. Ecohealth 2:38–46. doi: 10.1007/s10393-004-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wormser GP. 2006. Early Lyme disease. N Engl J Med 354:2794–2801. doi: 10.1056/NEJMcp061181. [DOI] [PubMed] [Google Scholar]
- 7.Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, Soloski MJ, Ecker DJ, Schutzer SE, Aucott JN. 2012. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One 7:e36825. doi: 10.1371/journal.pone.0036825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liveris D, Schwartz I, McKenna D, Nowakowski J, Nadelman R, Demarco J, Iyer R, Bittker S, Cooper D, Holmgren D, Wormser GP. 2012. Comparison of five diagnostic modalities for direct detection of Borrelia burgdorferi in patients with early Lyme disease. Diagn Microbiol Infect Dis 73:243–245. doi: 10.1016/j.diagmicrobio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai DM, Feng S, Hodzic E, Barthold SW. 2013. Dynamics of connective-tissue localization during chronic Borrelia burgdorferi infection. Lab Invest 93:900–910. doi: 10.1038/labinvest.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steere AC, Schoen RT, Taylor E. 1987. The clinical evolution of Lyme arthritis. Ann Intern Med 107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 11.Strle K, Shin JJ, Glickstein LJ, Steere AC. 2012. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum 64:1497–1507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques A. 2008. Chronic Lyme disease: a review. Infect Dis Clin North Am 22:341–360, vii–viii. doi: 10.1016/j.idc.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aucott JN, Rebman AW, Crowder LA, Kortte KB. 2013. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: is there something here? Qual Life Res 22:75–84. doi: 10.1007/s11136-012-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 15.Nowakowski J, Nadelman RB, Sell R, McKenna D, Cavaliere LF, Holmgren D, Gaidici A, Wormser GP. 2003. Long-term follow-up of patients with culture-confirmed Lyme disease. Am J Med 115:91–96. doi: 10.1016/S0002-9343(03)00308-5. [DOI] [PubMed] [Google Scholar]
- 16.Shadick NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, Berardi VP, Duray PH, Larson MG, Wright EA, Ginsburg KS, Katz JN, Liang MH. 1994. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann Intern Med 121:560–567. [DOI] [PubMed] [Google Scholar]
- 17.Kalish RA, Kaplan RF, Taylor E, Jones-Woodward L, Workman K, Steere AC. 2001. Evaluation of study patients with Lyme disease, 10-20-year follow-up. J Infect Dis 183:453–460. doi: 10.1086/318082. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro ED, Dattwyler R, Nadelman RB, Wormser GP. 2005. Response to meta-analysis of Lyme borreliosis symptoms. Int J Epidemiol 34:1437–1439. doi: 10.1093/ije/dyi241. [DOI] [PubMed] [Google Scholar]
- 19.Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, Jacobs MB, Hasenkampf NR, Martin DS, Narasimhan S, Phillippi-Falkenstein KM, Purcell JE, Ratterree MS, Philipp MT. 2012. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One 7:e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. 2012. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest 122:2652–2660. doi: 10.1172/JCI58813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen S, Shin JJ, Strle K, McHugh G, Li X, Glickstein LJ, Drouin EE, Steere AC. 2010. Treg cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis. Arthritis Rheum 62:2127–2137. doi: 10.1002/art.27468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandra A, Wormser GP, Klempner MS, Trevino RP, Crow MK, Latov N, Alaedini A. 2010. Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms. Brain Behav Immun 24:1018–1024. doi: 10.1016/j.bbi.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soloski MJ, Crowder LA, Lahey LJ, Wagner CA, Robinson WH, Aucott JN. 2014. Serum inflammatory mediators as markers of human Lyme disease activity. PLoS One 9:e93243. doi: 10.1371/journal.pone.0093243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 44:590–591. [PubMed] [Google Scholar]
- 25.Aucott JN, Crowder LA, Kortte KB. 2013. Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int J Infect Dis 17:e443–e449. doi: 10.1016/j.ijid.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Deane KD, O'Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, Gilliland WR, Edison JD, Norris JM, Robinson WH, Holers VM. 2010. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum 62:3161–3172. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tusher VG, Tibshirani R, Chu G. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oertelt-Prigione S. 2012. The influence of sex and gender on the immune response. Autoimmun Rev 11:A479–A485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Ness RB, Haggerty CL, Harger G, Ferrell R. 2004. Differential distribution of allelic variants in cytokine genes among African Americans and white Americans. Am J Epidemiol 160:1033–1038. doi: 10.1093/aje/kwh325. [DOI] [PubMed] [Google Scholar]
- 30.Ponnappan S, Ponnappan U. 2011. Aging and immune function: molecular mechanisms to interventions. Antioxid Redox Signal 14:1551–1585. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Comerford I, Harata-Lee Y, Bunting MD, Gregor C, Kara EE, McColl SR. 2013. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev 24:269–283. doi: 10.1016/j.cytogfr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Forster R, Davalos-Misslitz AC, Rot A. 2008. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 33.Hastey CJ, Ochoa J, Olsen KJ, Barthold SW, Baumgarth N. 2014. MyD88- and TRIF-independent induction of type I interferon drives naive B cell accumulation but not loss of lymph node architecture in Lyme disease. Infect Immun 82:1548–1558. doi: 10.1128/IAI.00969-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer JW, Baechler EC, Petri M, Batliwalla FM, Crawford D, Ortmann WA, Espe KJ, Li W, Patel DD, Gregersen PK, Behrens TW. 2006. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med 3:e491. doi: 10.1371/journal.pmed.0030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, Panoskaltsis-Mortari A, Gregersen PK, Behrens TW, Baechler EC. 2009. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum 60:3098–3107. doi: 10.1002/art.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontaine J, Poudrier J, Roger M. 2011. Short communication: persistence of high blood levels of the chemokines CCL2, CCL19, and CCL20 during the course of HIV infection. AIDS Res Hum Retroviruses 27:655–657. doi: 10.1089/aid.2010.0261. [DOI] [PubMed] [Google Scholar]
- 37.Sellam J, Rouanet S, Hendel-Chavez H, Miceli-Richard C, Combe B, Sibilia J, Le Loet X, Tebib J, Jourdan R, Dougados M, Taoufik Y, Mariette X. 2013. CCL19, a B cell chemokine, is related to the decrease of blood memory B cells and predicts the clinical response to rituximab in patients with rheumatoid arthritis. Arthritis Rheum 65:2253–2261. doi: 10.1002/art.38023. [DOI] [PubMed] [Google Scholar]
- 38.Bombardieri M, Pitzalis C. 2012. Ectopic lymphoid neogenesis and lymphoid chemokines in Sjogren's syndrome: at the interplay between chronic inflammation, autoimmunity and lymphomagenesis. Curr Pharm Biotechnol 13:1989–1996. doi: 10.2174/138920112802273209. [DOI] [PubMed] [Google Scholar]
- 39.Columba-Cabezas S, Serafini B, Ambrosini E, Aloisi F. 2003. Lymphoid chemokines CCL19 and CCL21 are expressed in the central nervous system during experimental autoimmune encephalomyelitis: implications for the maintenance of chronic neuroinflammation. Brain Pathol 13:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heydtmann M, Hardie D, Shields PL, Faint J, Buckley CD, Campbell JJ, Salmon M, Adams DH. 2006. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J Immunol 177:729–738. doi: 10.4049/jimmunol.177.1.729. [DOI] [PubMed] [Google Scholar]
- 41.Kowarik MC, Cepok S, Sellner J, Grummel V, Weber MS, Korn T, Berthele A, Hemmer B. 2012. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflammation 9:93. doi: 10.1186/1742-2094-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Mandelin AM II, Shahrara S. 2011. Characterization of CCL19 and CCL21 in rheumatoid arthritis. Arthritis Rheum 63:914–922. doi: 10.1002/art.30232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rupprecht TA, Plate A, Adam M, Wick M, Kastenbauer S, Schmidt C, Klein M, Pfister HW, Koedel U. 2009. The chemokine CXCL13 is a key regulator of B cell recruitment to the cerebrospinal fluid in acute Lyme neuroborreliosis. J Neuroinflammation 6:42. doi: 10.1186/1742-2094-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timmer TC, Baltus B, Vondenhoff M, Huizinga TW, Tak PP, Verweij CL, Mebius RE, van der Pouw Kraan TC. 2007. Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum 56:2492–2502. doi: 10.1002/art.22748. [DOI] [PubMed] [Google Scholar]
- 45.Strle K, Stupica D, Drouin EE, Steere AC, Strle F. 2014. Elevated levels of IL-23 in a subset of patients with post-Lyme disease symptoms following erythema migrans. Clin Infect Dis 58:372–380. doi: 10.1093/cid/cit735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuwabara T, Ishikawa F, Yasuda T, Aritomi K, Nakano H, Tanaka Y, Okada Y, Lipp M, Kakiuchi T. 2009. CCR7 ligands are required for development of experimental autoimmune encephalomyelitis through generating IL-23-dependent Th17 cells. J Immunol 183:2513–2521. doi: 10.4049/jimmunol.0800729. [DOI] [PubMed] [Google Scholar]
- 47.Hassett AL, Radvanski DC, Buyske S, Savage SV, Gara M, Escobar JI, Sigal LH. 2008. Role of psychiatric comorbidity in chronic Lyme disease. Arthritis Rheum 59:1742–1749. doi: 10.1002/art.24314. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan RF, Meadows ME, Vincent LC, Logigian EL, Steere AC. 1992. Memory impairment and depression in patients with Lyme encephalopathy: comparison with fibromyalgia and nonpsychotically depressed patients. Neurology 42:1263–1267. doi: 10.1212/WNL.42.7.1263. [DOI] [PubMed] [Google Scholar]
- 49.Thompson ME, Barkhuizen A. 2003. Fibromyalgia, hepatitis C infection, and the cytokine connection. Curr Pain Headache Rep 7:342–347. doi: 10.1007/s11916-003-0032-2. [DOI] [PubMed] [Google Scholar]
- 50.Pucak ML, Carroll KA, Kerr DA, Kaplin AI. 2007. Neuropsychiatric manifestations of depression in multiple sclerosis: neuroinflammatory, neuroendocrine, and neurotrophic mechanisms in the pathogenesis of immune-mediated depression. Dialogues Clin Neurosci 9:125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. 2002. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 52.Massarotti EM, Luger SW, Rahn DW, Messner RP, Wong JB, Johnson RC, Steere AC. 1992. Treatment of early Lyme disease. Am J Med 92:396–403. doi: 10.1016/0002-9343(92)90270-L. [DOI] [PubMed] [Google Scholar]
- 53.Nadelman RB, Luger SW, Frank E, Wisniewski M, Collins JJ, Wormser GP. 1992. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med 117:273–280. doi: 10.7326/0003-4819-117-4-273. [DOI] [PubMed] [Google Scholar]
- 54.Stanos S, Houle TT. 2006. Multidisciplinary and interdisciplinary management of chronic pain. Phys Med Rehabil Clin N Am 17:435–450, vii. doi: 10.1016/j.pmr.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Staud R. 2007. Treatment of fibromyalgia and its symptoms. Expert Opin Pharmacother 8:1629–1642. doi: 10.1517/14656566.8.11.1629. [DOI] [PubMed] [Google Scholar]
- 56.Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, Felicetti T, Laatsch L, Harley JP, Bergquist T, Azulay J, Cantor J, Ashman T. 2011. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil 92:519–530. doi: 10.1016/j.apmr.2010.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.