Abstract
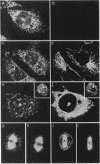
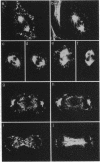
Casein kinase I (CKI) is a class of protein kinases ubiquitous to all eukaryotic cells. Recently, cDNA clones encoding several bovine CKI isoforms have been sequenced that show high sequence identity to the HRR25 gene product of the budding yeast Saccharomyces cerevisiae; HRR25 is required for normal cellular growth, nuclear segregation, DNA repair, and meiosis. We have raised polyclonal antibodies to a human erythroid 34-kDa CKI and have sequenced a portion of this kinase. The amino acid sequence identifies the CKI as the alpha-CKI isoform, which is 62% identical to the HRR25 protein kinase. By use of immunofluorescence, the alpha-CKI has been localized to vesicular cytosolic structures and to the centrosome in interphase cells. As cells progress into mitosis, centrospheric staining increases and, in mitosis, alpha-CKI associates with kinetochore fibers. This localization suggests that alpha-CKI, like HRR25, plays a role in the segregation of chromosomes during mitosis and may be cell cycle-regulated both in humans and in yeast.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostinis P., Vandenheede J. R., Goris J., Meggio F., Pinna L. A., Merlevede W. The ATP,Mg-dependent protein phosphatase: regulation by casein kinase-1. FEBS Lett. 1987 Nov 30;224(2):385–390. doi: 10.1016/0014-5793(87)80489-1. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Marchesi V. T. Regulation of the association of membrane skeletal protein 4.1 with glycophorin by a polyphosphoinositide. Nature. 1985 Nov 21;318(6043):295–298. doi: 10.1038/318295a0. [DOI] [PubMed] [Google Scholar]
- Bailly E., Dorée M., Nurse P., Bornens M. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989 Dec 20;8(13):3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazenet C. E., Brockman J. L., Lewis D., Chan C., Anderson R. A. Erythroid membrane-bound protein kinase binds to a membrane component and is regulated by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1990 May 5;265(13):7369–7376. [PubMed] [Google Scholar]
- Bazenet C. E., Ruano A. R., Brockman J. L., Anderson R. A. The human erythrocyte contains two forms of phosphatidylinositol-4-phosphate 5-kinase which are differentially active toward membranes. J Biol Chem. 1990 Oct 15;265(29):18012–18022. [PubMed] [Google Scholar]
- Brockman J. L., Anderson R. A. Casein kinase I is regulated by phosphatidylinositol 4,5-bisphosphate in native membranes. J Biol Chem. 1991 Feb 5;266(4):2508–2512. [PubMed] [Google Scholar]
- Chauhan A., Chauhan V. P., Deshmukh D. S., Brockerhoff H. Phosphatidylinositol 4,5-bisphosphate competitively inhibits phorbol ester binding to protein kinase C. Biochemistry. 1989 Jun 13;28(12):4952–4956. doi: 10.1021/bi00438a007. [DOI] [PubMed] [Google Scholar]
- Chu A., Stefani E. Phosphatidylinositol 4,5-bisphosphate-induced Ca2+ release from skeletal muscle sarcoplasmic reticulum terminal cisternal membranes. Ca2+ flux and single channel studies. J Biol Chem. 1991 Apr 25;266(12):7699–7705. [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Ahmad Z., Roach P. J. Characterization of a rabbit skeletal muscle protein kinase (PC0.7) able to phosphorylate glycogen synthase and phosvitin. J Biol Chem. 1981 Sep 10;256(17):8955–8962. [PubMed] [Google Scholar]
- Fiol C. J., Wang A., Roeske R. W., Roach P. J. Ordered multisite protein phosphorylation. Analysis of glycogen synthase kinase 3 action using model peptide substrates. J Biol Chem. 1990 Apr 15;265(11):6061–6065. [PubMed] [Google Scholar]
- Flotow H., Graves P. R., Wang A. Q., Fiol C. J., Roeske R. W., Roach P. J. Phosphate groups as substrate determinants for casein kinase I action. J Biol Chem. 1990 Aug 25;265(24):14264–14269. [PubMed] [Google Scholar]
- Flotow H., Roach P. J. Role of acidic residues as substrate determinants for casein kinase I. J Biol Chem. 1991 Feb 25;266(6):3724–3727. [PubMed] [Google Scholar]
- Flotow H., Roach P. J. Synergistic phosphorylation of rabbit muscle glycogen synthase by cyclic AMP-dependent protein kinase and casein kinase I. Implications for hormonal regulation of glycogen synthase. J Biol Chem. 1989 Jun 5;264(16):9126–9128. [PubMed] [Google Scholar]
- Gorbsky G. J., Borisy G. G. Microtubules of the kinetochore fiber turn over in metaphase but not in anaphase. J Cell Biol. 1989 Aug;109(2):653–662. doi: 10.1083/jcb.109.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Bretscher A., Esch F. S., Hunter T. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 1989 Dec 20;8(13):4133–4142. doi: 10.1002/j.1460-2075.1989.tb08598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grankowski N., Issinger O. G. Subcellular localization of casein kinase I. Biochem Biophys Res Commun. 1990 Mar 16;167(2):471–476. doi: 10.1016/0006-291x(90)92047-4. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Helms J. B., de Vries K. J., Wirtz K. W. Synthesis of phosphatidylinositol 4,5-bisphosphate in the endoplasmic reticulum of Chinese hamster ovary cells. J Biol Chem. 1991 Nov 15;266(32):21368–21374. [PubMed] [Google Scholar]
- Hirokawa N., Sato-Yoshitake R., Yoshida T., Kawashima T. Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J Cell Biol. 1990 Sep;111(3):1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra M. F., Liskay R. M., Ou A. C., DeMaggio A. J., Burbee D. G., Heffron F. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science. 1991 Aug 30;253(5023):1031–1034. doi: 10.1126/science.1887218. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Gelsolin-polyphosphoinositide interaction. Full expression of gelsolin-inhibiting function by polyphosphoinositides in vesicular form and inactivation by dilution, aggregation, or masking of the inositol head group. J Biol Chem. 1989 Mar 25;264(9):4825–4831. [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kolarov J., Kulpa J., Baijot M., Goffeau A. Characterization of a protein serine kinase from yeast plasma membrane. J Biol Chem. 1988 Aug 5;263(22):10613–10619. [PubMed] [Google Scholar]
- Krek W., Maridor G., Nigg E. A. Casein kinase II is a predominantly nuclear enzyme. J Cell Biol. 1992 Jan;116(1):43–55. doi: 10.1083/jcb.116.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M. L., Haimo L. T. Cytoplasmic dynein is a vesicle protein. J Biol Chem. 1992 Mar 5;267(7):4793–4798. [PubMed] [Google Scholar]
- Lee M. H., Bell R. M. Mechanism of protein kinase C activation by phosphatidylinositol 4,5-bisphosphate. Biochemistry. 1991 Jan 29;30(4):1041–1049. doi: 10.1021/bi00218a023. [DOI] [PubMed] [Google Scholar]
- Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990 Jun 1;61(5):743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- Maller J. L. Mitotic control. Curr Opin Cell Biol. 1991 Apr;3(2):269–275. doi: 10.1016/0955-0674(91)90151-n. [DOI] [PubMed] [Google Scholar]
- Maniotis A., Schliwa M. Microsurgical removal of centrosomes blocks cell reproduction and centriole generation in BSC-1 cells. Cell. 1991 Nov 1;67(3):495–504. doi: 10.1016/0092-8674(91)90524-3. [DOI] [PubMed] [Google Scholar]
- Matsudaira P., Janmey P. Pieces in the actin-severing protein puzzle. Cell. 1988 Jul 15;54(2):139–140. doi: 10.1016/0092-8674(88)90542-9. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Schäfer G., Hilz H., Eppenberger H. M. Cyclic-AMP-dependent protein kinase type II is associated with the Golgi complex and with centrosomes. Cell. 1985 Jul;41(3):1039–1051. doi: 10.1016/s0092-8674(85)80084-2. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Ohba T., Miyamoto E. Ca2+/calmodulin-dependent protein kinase II: localization in the interphase nucleus and the mitotic apparatus of mammalian cells. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5341–5345. doi: 10.1073/pnas.87.14.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr C. M., Coue M., Grissom P. M., Hays T. S., Porter M. E., McIntosh J. R. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature. 1990 May 17;345(6272):263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Wagner M. C., Stenoien D. L., Brady S. T., Bloom G. S. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989 Apr;108(4):1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991 Oct;115(1):1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. J., Ades S. E., Singer S. J., Hynes R. O. Sequence and domain structure of talin. Nature. 1990 Oct 18;347(6294):685–689. doi: 10.1038/347685a0. [DOI] [PubMed] [Google Scholar]
- Roach P. J. Multisite and hierarchal protein phosphorylation. J Biol Chem. 1991 Aug 5;266(22):14139–14142. [PubMed] [Google Scholar]
- Roach P. J. Multisite and hierarchal protein phosphorylation. J Biol Chem. 1991 Aug 5;266(22):14139–14142. [PubMed] [Google Scholar]
- Robinson L. C., Hubbard E. J., Graves P. R., DePaoli-Roach A. A., Roach P. J., Kung C., Haas D. W., Hagedorn C. H., Goebl M., Culbertson M. R. Yeast casein kinase I homologues: an essential gene pair. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):28–32. doi: 10.1073/pnas.89.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowles J., Slaughter C., Moomaw C., Hsu J., Cobb M. H. Purification of casein kinase I and isolation of cDNAs encoding multiple casein kinase I-like enzymes. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9548–9552. doi: 10.1073/pnas.88.21.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer E. R., Wordeman L., Schroer T. A., Sheetz M. P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990 May 17;345(6272):266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Yang Q., Tonks N. K. Isolation of a cDNA clone encoding a human protein-tyrosine phosphatase with homology to the cytoskeletal-associated proteins band 4.1, ezrin, and talin. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5949–5953. doi: 10.1073/pnas.88.14.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I. J., Spector D. L., Bae Y. S., Marshak D. R. Immunocytochemical localization of casein kinase II during interphase and mitosis. J Cell Biol. 1991 Sep;114(6):1217–1232. doi: 10.1083/jcb.114.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]