Abstract
Ellagic acid (EA) is a naturally occurring polyphenol found in some fruits and nuts, including berries, pomegranates, grapes, and walnuts. EA has been investigated extensively because of its antiproliferative action in some cancers, along with its anti-inflammatory effects. A growing body of evidence suggests that the intake of EA is effective in attenuating obesity and ameliorating obesity-mediated metabolic complications, such as insulin resistance, type 2 diabetes, nonalcoholic fatty liver disease, and atherosclerosis. In this review, we summarize how intake of EA regulates lipid metabolism in vitro and in vivo, and delineate the potential mechanisms of action of EA on obesity-mediated metabolic complications. We also discuss EA as an epigenetic effector, as well as a modulator of the gut microbiome, suggesting that EA may exert a broader spectrum of health benefits than has been demonstrated to date. Therefore, this review aims to suggest the potential metabolic benefits of consumption of EA-containing fruits and nuts against obesity-associated health conditions.
Keywords: ellagic acid, urolithins, obesity, inflammation, polyphenols, epigenetic regulation
Introduction
Obesity and its associated metabolic complications, such as type 2 diabetes, have reached epidemic proportions in the United States (1). Studies show an inverse relation between an increased intake of fruits and vegetables and the incidence of obesity (2, 3), emphasizing the significance of fruit and vegetable intake in the pathogenesis of obesity. The metabolic benefit of fruit and vegetable intake likely is dependent on the synergistic effects of numerous dietary bioactive components, including fiber, vitamins, minerals, and polyphenolic compounds. Dietary polyphenols have received attention for their ability to modulate lipid and glucose metabolism (4, 5). In addition to the health benefits from flavonoids, it is also notable that several nonflavonoid polyphenolic compounds are considered to be important to metabolic health, including resveratrol, unique to grapes and red wine (6); curcumin, a strong antioxidant from turmeric (7); and now perhaps ellagic acid (EA; 2,3,7,8-tetrahydroxy-chromeno[5,4,3-cde]chromene-5,10-dione)6, found in berries.
We and others have demonstrated that EA is a promising dietary nonflavonoid polyphenolic compound that could modulate the development of obesity and the associated metabolic consequences. EA occurs naturally in some fruits, such as berries (strawberries and red and black raspberries), tree nuts (walnuts, pistachios, cashews, acorns, and pecans), pomegranates, and muscadine grapes (8–12). In particular, berries provide EA in the form of ellagitannins, which constitute ∼60% (red raspberry) to 80% (cloudberry) of the total phenolic compounds present (13). There is emerging evidence that EA may decrease symptoms of chronic metabolic diseases, including dyslipidemia, insulin resistance, type 2 diabetes, and nonalcoholic fatty liver disease. Despite the growing amount of information on EA, a definitive mechanism of action has not been established. This may be attributed to the complexity of EA metabolism, which is governed by various factors, including 1) the dietary source of EA and the EA-to-ellagitannin ratio, 2) the existence of other phytochemicals in a specific food, 3) the absorption rate, 4) interactions with gut microbes, and 5) the health status of the host. Therefore, an integrative view that considers all of the above is required for a complete assessment of a potential beneficial role of EA-containing foods on obesity-mediated chronic diseases. The objective of this review is to consolidate recent advances in EA research related to obesity and its metabolic consequences, especially from the most recently published reports. In this review, we first address EA absorption in the digestive system, summarizing how EA enters circulation, then separately delineate the metabolic effects of EA in vitro and in vivo. For in vitro studies, we included studies that examined the effects of EA on lipid metabolism in adipocytes or hepatocytes, but excluded studies in breast- or colon-cancer cells. For in vivo studies, we included studies that used obesity-prone rodent models with dietary supplementation with pure EA or EA-enriched polyphenolic extracts. To be included in this review, cited reports indicated that EA or ellagitannins were the primary polyphenolic component, or demonstrated that EA or ellagitannins were the most responsible polyphenol for target outcomes. The term “ellegic acid” was used in a PubMed search in combination with each of the following terms: “urolithins, obesity, hepatic lipids, diabetes, metabolic syndrome, bioavailability, antioxidant, and inflammation.”
In summary, this review is composed of 3 sections: 1) bioavailability of EA, 2) in vitro and in vivo evidence of EA regulation of lipid and glucose metabolism, and 3) proposed mechanisms of action by which EA counteracts obesity and its associated metabolic complications.
Bioavailability of EA
EA (2,3,7,8-tetrahydroxy[1]-benzopyranol[5,4,3-cde]benzopyran-5,10-dione; MW = 302 g/mol) was discovered by Braconnot in 1831 (14). EA possesses both a lipophilic moiety with four 6-member hydrocarbon rings and a hydrophilic moiety with 4 hydroxyl groups and 2 lactone groups (15). This unique structure makes EA able to accept electrons from various substrates, thereby participating in antioxidant redox reactions (16). EA is present in several forms, including free, glycosylated and/or acylated, or a hydrolyzable ellagitannin polymer usually esterified with glucose molecules. ellagitannins can be hydrolyzed, spontaneously releasing free EA. Here, we summarize the factors that regulate the entry of EA into circulation rather than providing a more global discussion of bioavailability.
It is suggested that the absorption sites of free EA into circulation are either the stomach or small intestine. A small proportion of free EA from the diet is absorbed in the stomach, whereas ellagitannins are resistant to acid hydrolysis, as well as degradation in the stomach (17). Hydrolysis of ellagitannins and release of EA occur in the small intestine at a neutral to slightly basic pH, which in turn may allow some freed EA to be absorbed in the small intestine. The uptake process for EA is thought to be via passive diffusion driven by a concentration gradient, with no specific transporters having been identified yet that facilitate EA uptake across the gut epithelium (18).
Supporting this presumption, the appearance and clearance of EA in plasma is largely dependent on the EA-to-ellagitannin ratio. When a human subject consumed pomegranate juice containing 318 mg ellagitannins and 25 mg free EA, plasma concentrations of EA reached maximum serum concentration (Cmax) = 0.106 μmol/L (32 ng/mL) at time of maximum concentration observed (Tmax) = 1.0 h, which reached a plateau at 4 h (19). After drinking a single dose of pomegranate juice that contained the same amount of ellagitannins but one-half of the free EA (i.e., 318 mg ellagitannins and 12 mg free EA), plasma concentrations of EA reached Cmax = 0.06 μmol/L (18 ng/mL) at Tmax = 0.98 h, and the elimination half-time was 0.71 h (20). In a similar study, when human subjects were given 400 mg pomegranate extract that contained 330 mg ellagitannins and 22 mg free EA, the Cmax of EA was found to be 0.11 μmol/L (33 ng/mL) at Tmax = 1 h (21). In a crossover pharmacokinetic study, human subjects were given either 130 mg punicalagin (the ellagitannin form found in pomegranate) plus 524 mg free EA, or 279 mg punicalagin plus 25 mg free EA. Intriguingly, the high dose of free EA did not enhance EA bioavailability compared with the lower dose, suggesting that absorption saturation is reached in the small intestine (22). The short Tmax suggested that EA was primarily absorbed from the stomach and the upper region of the small intestine. In addition, the rapid elimination of EA from systemic circulation suggests an efficient first-pass metabolism of absorbed EA in the intestine and liver and a weak enterohepatic recirculation. During first-pass metabolism, absorbed EA is converted to methyl esters, dimethyl esters, and glucuronides. These metabolites were detected in human plasma and urine 1–5 h after ingestion of ellagitannins (20, 21).
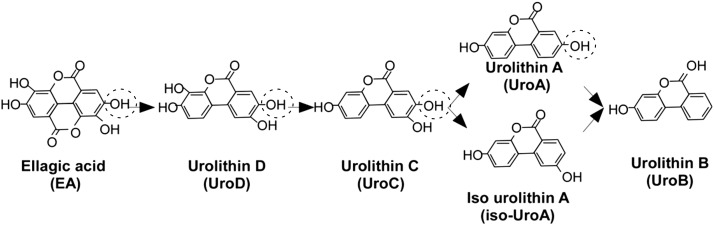
When unabsorbed ellagitannins and free EA reach the colon, they are metabolized by gut microbiota to yield a family of microbial metabolites called urolithins. Urolithin D is produced from EA upon the opening of a lactone ring and removal of a carboxyl group. Urolithin D loses 1, 2, or 3 hydroxyl groups via dehydroxylation to form urolithin C, urolithin A/isourolithin A, and urolithin B, respectively (23) (Figure 1). Urolithins were detectable in vitro after anaerobic incubation of ellagitannin or EA with human fecal suspension (24). However, urolithins were not detected in the urine of human ileostomy subjects, confirming the essential role of gut mirobiota in urolithins production (18). Compared with free EA, urolithins had a much higher absorption rate, presumably because of increased lipophilicity (25). Accordingly, urolithins are present in the circulation in much higher concentrations. When healthy human volunteers were given 1 L of pomegranate juice that contained 4.37 g punicalagins/L daily for 5 d, urolithin A, urolithin B, and urolithin C were detected in plasma, and their total concentration ranged from 0.5 to 18.6 μmol/L (117.5 ng/mL to 4.4 μg/mL) (26). In a separate study, when human subjects were given a single dose of pomegranate juice or pomegranate extracts, plasma concentrations of urolithins reached >2.0 μmol/L (470 ng/mL) after 24 h and persisted through 48 h. In contrast, the observed Cmax of EA in plasma in the same study was in the range of 0.02–0.06 μmol/L (6.4–18.1 ng/mL) (27), demonstrating that urolithins are ∼25–80-fold more bioavailable than is EA. In addition to their microbial metabolism in the colon, the longer residence time of urolithins than EA in the plasma may suggest active phase-II metabolism and enterohepatic circulation (25). The majority of circulating urolithins (<8.6% of ingested total EA/ellagitannin) were glucuronide conjugates (28) that may exert lower antiproliferative activity than do their aglycone counterparts, based on in vitro studies with the use of colon cancer cells (29). There is huge variability in microbial metabolism of EA between humans. Some human subjects produce a substantial amount of urolithins, whereas others produce little or none. It was proposed that humans largely can be stratified into 3 phenotypes according to the type and amount of urolithin production after intake of mixtures of ellagitannin and EA. Phenotype 0 does not produce any urolithin. Phenotype A produces only urolithin A, whereas phenotype B produces urolithin A, isourolithin A, and urolithin B (30). This variability in microbial metabolism of EA was attributed to differences in gut microbial ecology (31), which will be addressed in further detail in a later section.
FIGURE 1.
Chemical structures of ellagic acid and urolithins. A series of metabolites called urolithins are formed from ellagic acid via the enzymatic action of gut microbes. The hydroxyl moiety at the C8 position of ellagic acid (-OH; dotted circle) is lost in isourolithin A via isomerization and, in urolithin B, via a cleavage reaction during metabolic conversion in the gut.
As with many other polyphenolic compounds, low bioavailability of EA (EA, 0.1–0.4 μmol/L, equivalent to 30–120 ng/mL; urolithins, 0.5–18.6 μmol/L, equivalent to 0.1–4 μg/mL) may be a major limiting factor for exhibiting metabolic activities in vivo. The bioavailability range of EA is comparable with that of resveratrol. For example, free resveratrol concentration in the circulation can reach only ∼40 nmol/L (∼9.1 ng/mL), but its metabolites can be found in the ∼0.3–2 μmol/L (∼68.4–456 ng/mL) range after 25–1000 mg resveratrol intake (32, 33). A recent study by González-Sarrías et al. (22) reported that the Cmax of EA in human plasma can reach close to 0.4 μmol/L (120 ng/mL), which is much higher than previously reported. The bioavailability of EA is regulated by multiple factors, including the EA-to-ellagitannin ratio, pH, gut microbiota composition, and conjugating counterparts (i.e., glucuronide, aglycone moiety, and sulfate, among others) (22). In summary, EA and its metabolites enter into circulation via 3 major processes: 1) free EA in diets is first absorbed in the stomach and proximal small intestine, 2) ellagitannins are hydrolyzed to release EA for absorption in the small intestine, and 3) unabsorbed EA and ellagitannins may be metabolized by gut microbes, generating urolithins in the colon, where urolithins should be expected to be passed into the circulatory system.
Effects of EA on Obesity
In vitro evidence.
A growing number of studies demonstrate the potential role of EA in the regulation of lipid metabolism in adipocyte models (Table 1). One of the most popular cells used is the 3T3-L1 cell, a well-established murine preadipocyte cell line. With the use of 3T3-L1 cells, Mejia-Meza et al. (42) reported that EA-containing raspberry products altered adipocyte differentiation. In their study, 250 μg/mL fresh and dried red raspberries, containing 3.7 mg EA/g on a dry basis, inhibited adipogenesis and reduced lipid accumulation in 3T3-L1 cells. Wang et al. (34) found that 15–20 μmol EA/L (4.5–6 μg/mL) significantly reduced adipogenesis through the inhibition of the cell cycle at the G1→S transition without causing apoptosis in 3T3-L1 cells. Consistently, Woo et al. (35) also demonstrated that EA significantly reduced lipid accumulation and early adipogenic markers such as Kruppel-like factor 4, Kruppel-like factor 5, and early growth response protein 2, as well as peroxisome proliferator–activated receptor γ (PPARG) and CCAAT/enhancer-binding protein α (C/EBPα), via cell cycle arrest.
TABLE 1.
In vitro studies carried out with pure EA or EA-rich plant extracts in relation to lipid metabolism1
| Test material | Test model | Dose (EA content) | Duration | Metabolic responses | References |
| EA | 3T3-L1 preadipocytes | 20 μmol/L | 2 d | ↓ Adipocyte differentiation | Wang et al., 2013 (34) |
| ↓ Clonal expansion | |||||
| Inhibition of G1/S cell cycle progression | |||||
| ↓ Cyclin A and Rb phosphorylation | |||||
| EA | 3T3-L1 preadipocytes | 5–50 μmol/L | 6–8 d | ↓ Lipid accumulation, PPARG, C/EBPα | Woo et al., 2015 (35) |
| ↓ Early adipogenic markers KLF4, KLF5, Krox20, C/EBPβ within 24 h | |||||
| EA | Huh7 cells | 10 μmol/L | 2 d | ↓ De novo TG synthesis and TG esterification | Okla et al., 2015 (36) |
| ↑ FA oxidation | |||||
| EA | hASCs | 10 μmol/L | 7 d | ↓ De novo lipogenesis | Okla et al., 2015 (36) |
| EA | hASCs | 10 μmol/L | 7 d | ↓ Adipogenic genes and protein (PPARG, C/EBPα, aP2, Fas) | Kang et al., 2014 (37) |
| Alteration of epigenetic markers | |||||
| (↑ HDAC9, ↓ CARM1 enzyme activity) | |||||
| 3,3′-di-O-methylellagic acid and EA | 3T3-L1 preadipocytes | 1,3,10 μmol/L | 8 d | ↓ TGs, and GPDH activity | Yang et al., 2011 (38) |
| Chinese sweet leaf tea extract | 3T3-L1 preadipocytes | 10 μg/mL | 24 h | ↓ VEGF expression | Koh et al., 2011 (39) |
| Pomegranate fruit extract and EA | 3T3-L1 adipocytes | 20–70 μmol/L | 12 h | ↓ Resistin release | Makino-Wakagi et al., 2012 (40) |
| EA | J774A1 macrophages | 5 μmol/L | 18 h | ↓ Lipid accumulation and SR-BI induction | Park et al., 2011 (41) |
| ↓ Macrophage lipid uptake to block foam cells | |||||
| ↑ Cholesterol efflux |
aP2, fatty acid–binding protein 4; CARM1, coactivator arginine methyltransferase 1; C/EBP, CCAAT/enhancer-binding protein; EA, ellagic acid; Fas, fatty acid synthase; GPDH, glycerol-3-phosphate dehydrogenase; hASC, human adipose–derived stem cell; HDAC9, histone deacetylase 9; KLF, Kruppel-like factor; Krox20, early growth response protein 2; PPARG, peroxisome proliferator–activated receptor gamma; SR-BI, scavenger receptor class B member 1; VEGF, vascular endothelial growth factor; ↓, decrease; ↑, increase.
The antiadipogenic role of 15–50 μmol EA/L (4.5–15.1 μg/mL) in 3T3-L1 cells was confirmed in primary cultures of human adipocytes by using <10 μmol EA/L (3 μg/mL), which provides some degree of human relevance. Primary human adipose–derived stem cells (hASCs), isolated from scavenged adipose tissue after liposuction or abdominoplasty surgery (43), have been used extensively to investigate adipocyte lipid metabolism in humans. Okla et al. (36) reported that muscadine grape polyphenols (MGPs), which contain 18.2 mg free EA/g, were able to reduce lipid accumulation during adipogenesis in hASCs. It was further determined that only the nonanthocyanin fraction (5–25 μg/mL), but not the anthocyanin fraction (5–25 μg/mL), was able to inhibit adipogenesis. Subsequently, of the 4 major polyphenolic compounds present in the nonanthocyanin fraction (i.e., EA, quercetin, myricetin, and kaempferol), EA was identified as the polyphenol that was solely responsible for repressing adipogenesis by decreasing the expression of adipogenic genes, e.g., PPARG, fatty acid synthase (Fas), fatty acid–binding protein 4 (aP2), and C/EBPα (36, 37). These results were similar to those of Yang et al. (38), who showed that EA from Euphorbia lunulata decreased TG accumulation in 3T3-L1 cells, which is attributed at least partly to hydrolyzable tannins.
Most in vitro studies have been focused on EA inhibition of new fat cell formation, whereas few studies have investigated the role of EA on the terminal stages of differentiation or lipogenesis. Interestingly, Okla et al. (36) showed that incubation of mature human adipocytes or hepatocytes with 10 μmol EA/L (3 μg/mL) for 3 d reduced intracellular lipid accumulation. By using a radiolabeled precursor, it was demonstrated that EA significantly reduced de novo lipogenesis and TG esterification, while enhancing FA oxidation. The activation of AMP-activated protein kinase (AMPK) likely is involved in this link, because AMPK activation has been shown to regulate energy homeostasis (44) by inhibiting adipogenesis and de novo TG synthesis, and by augmenting FA oxidation (45). Supporting this notion, Poulose et al. (46) demonstrated that exposure to a relatively low concentration of EA (100 nmol/L, equivalent to 30 ng/mL) for 30 min induced AMPK activation in fully differentiated 3T3-L1 cells. Consistently, Kang et al. (47) also showed that activation of AMPK is triggered by EA, as well as by urolithin A, C, and D, in cultured human adipocytes by inhibiting differentiation of hASCs into adipocytes. Until now, to our knowledge, only a few studies have been conducted to determine whether AMPK is activated by urolithins in vivo. Therefore, further research is required to validate the potential activation of AMPK by both EA and urolithins.
In addition to the antiadipogenic/lipogenic effects of EA, with Chinese sweet leaf tea extract that contained 10 μg EA/mL (equivalent to 30 μmol/L), angiogenic gene expression of the vascular endothelial growth factor was attenuated in 3T3-L1 cells (39). Pomegranate fruit extract that contained 10–100 μg EA/mL (30–300 μmol/L) or a 20–70 μmol pure EA/L treatment of 3T3-L1 cells reduced the release of resistin, an anti-insulin–sensitizing adipokine (40). Other evidence also shows that EA not only influences TG metabolism in adipocytes and hepatocytes, but also regulates cholesterol metabolism in macrophages. In the J774 macrophage cell line, treatment with 5 μmol EA/L for 18 h reduced cholesterol ester accumulation, presumably by a reduction in scavenger receptor class B member 1 (SR-BI) and oxidized LDL uptake, and increased cholesterol efflux (41). This study implies that EA may be involved in reverse cholesterol transport from plaque areas, thus attenuating cardiovascular disease risk.
An important caveat of these in vitro studies is the high EA concentration that was used. Most studies used ∼10–50 μmol EA/L, which is not attainable under human physiologic conditions. The use of higher concentrations of polyphenols may be necessary to simulate chronic dietary responses with the use of a short-term cell-based platform. Therefore, caution should be used when extrapolating these cell-based results to humans. In addition, future studies are warranted to confirm whether physiologically relevant doses of EA (∼0.1–0.4 μmol/L) for a prolonged time period can recapitulate the lipid-lowering effects of higher-dose EA short-term treatments of adipocytes and hepatocytes.
In vivo evidence.
The TG-lowering function of EA observed with in vitro studies is well supported by animal studies establishing the physiologic role of EA against the pathogenesis of obesity. Data from in vivo studies suggest that “EA-enriched fractions from whole food [5–150 mg EA · kg body weight (BW)−1 · d−1]” are effective in attenuating adiposity and plasma markers of metabolic syndrome, although the degree of fat mass attenuation is variable, depending on the source and content of EA, as well as the supplementation duration (Table 2). Animals that consumed EA-enriched fractions of pomegranate peel extract (11 mg EA/kg BW) (58), pomegranate leaf extract (85 mg EA/kg BW) (48), blueberry extracts (15 mg EA/kg BW) (59), Chinese sweet leaf tea (20 mg EA/kg BW) (39), chestnut inner shell extract (150 mg EA/kg BW) (60), and hydroalcoholic extract of Persea americana avocado (5 mg gallic acid and EA/kg BW) (49), exhibited a significant reduction in BW, as well as in white adipose tissue mass. Also, delivery of EA through nanoparticles effectively attenuated hyperlipidemia in rats fed a high-fat diet (50).
TABLE 2.
In vivo studies carried out with EA or an EA-enriched fraction in relation to obesity and its metabolic complications1
| Results |
|||||||
| Test material | Test model/diet | Dose (EA content) | Duration, wk | ∆BW | ∆Fat | Metabolic responses | References |
| Muscadine grape polyphenols | C57BL/6J/HFD (60%)* | 0.4% MGP (216 mg/kg BW) | 15 | — | ↓ | ↑ Glucose and insulin tolerance | Gourineni et al., 2012 (10) |
| ↓ FFA, TGs, TC, and CRP in plasma | |||||||
| Chinese sweet leaf tea extract | SD rats/HFD (60%) | 0.22 g/kg BW | 12 | ↓ | ↓ | ↓ Glucose, TGs, and TC in plasma | Koh et al., 2011 (39) |
| PE | ddY mice (ovariectomized) | 30 mg/kg BW | 12 | — | ↓ | ↓ Serum resistin | Makino-Wakagi et al., 2012 (40) |
| PLE | ICR mice/HFD (60%) | 400 or 800 mg PLE/kg BW | 5 | ↓ | ↓ | ↓ Serum glucose and TC, TGs | Lei et al., 2007 (48) |
| ↑ Serum HDL cholesterol | |||||||
| HFEA | SD rats/HFD (40%) | 100 mg HFEA/kg BW | 11 | ↓ | ↓ | ↓ TGs, TC, LDL and leptin in plasma | Monika and Geetha, 2015 (49) |
| ↓ FAS, LPL, and leptin in adipocytes | |||||||
| ↑ FGF21 in adipocytes | |||||||
| EA nanoparticle | SD rats/HFD (40%) | 10% (wt:wt of polymer) | 4 | — | — | ↓ Glucose and TGs | Ratnam et al., 2009 (50) |
| ↑ Endothelial function | |||||||
| PE | SD rats/HFD (45%) | 150 mg PE/kg BW | 8 | ↓ | — | ↓ Cardiac lipid accumulation | Cao et al., 2015 (51) |
| ↓ Myocardial damage | |||||||
| ↑ Mitochondrial biogenesis | |||||||
| ↓ Oxidative stress in heart | |||||||
| RSF | C57BL/6 HFHS diet (40% fat, 37% sucrose) | 100 mg RSF/kg BW | 8 | ↓ | — | ↓TC, LDL cholesterol, TGs | Kang et al., 2016 (52) |
| ↑ Insulin sensitivity | |||||||
| ↓ Hepatic ER stress | |||||||
| ↓ Adipose inflammation | |||||||
| EA | KKAy mice/HFD (30%) | 100 mg/kg BW | ∼10 | — | — | ↓ Serum resistin | Yoshimura et al., 2013 (53) |
| ↓ Lipid accumulation in hepatocytes | |||||||
| ↑ Hepatic apoA-I, LDLr, CPT1, and PPARG | |||||||
| EA | Wistar rats/HFD (48%) | 800 mg/kg BW | 16 | ↓ | ↓ | ↓ Cardiovascular abnormalities | Panchal et al., 2013 (54) |
| ↑ Ventricular function | |||||||
| ↑ Glucose tolerance | |||||||
| ↑ Nrf2 and CPT1 in heart and liver | |||||||
| EA | Isoproterenol-injected MI-induced Wistar rats | 7.5–15 mg/kg BW | 1.5 | — | — | ↓ Arrhythmias, ventricular hypertrophy, lipid peroxidation | Kannan and Quine, 2013 (55) |
| ↓ Myocardial necrosis | |||||||
| ↓ TC, LDL cholesterol, VLDL cholesterol, FFA, TGs | |||||||
| EA | streptozotocin-injected Wistar rats/HFD (60%) | 40 mg/kg BW | 16 | — | — | ↓ TC, LDL cholesterol, VLDL cholesterol, FFA, TGs | Ahad et al., 2014 (56) |
| ↑ HDL cholesterol | |||||||
| EA | streptozotocin-injected Wistar rats | 2000 mg/kg BW | 12 | — | — | ↓ Glucose, TGs | Rani et al., 2013 (57) |
| ↓ Lipid and collagen deposition in the aortic arch | |||||||
| ↓ Cyclin D1 expression in medial smooth muscle cells | |||||||
*Expressed in percentage of total calories from fat. BW, body weight; CPT1, carnitine palmitoyltransferase 1; CRP, C-reactive protein; EA, ellagic acid; ER, endoplasmic reticulum; FAS, fatty acid synthase; FGF21, fibroblast growth factor 21; HFD, high-fat diet; HFEA, hydroalcoholic fruit extract of avocado; HFHS, high fat and high sucrose; LDLr, LDL receptor; LPL, lipoprotein lipase; MGP, muscadine grape polyphenol; MI, myocardial infarction; Nrf2, nuclear factor erythroid 2–related factor 2; PE, pomegranate extract; PLE, pomegranate leaf extract; PPARG, peroxisome proliferator–activated receptor gamma; RSF, red raspberry seed flour; SD, Sprague–Dawley; TC, total cholesterol; ↓, decrease; ↑, increase.
Moreover, despite similar BW changes, loss of visceral fat was found in some studies, including ovariectomized ddY female mice fed 30 mg pomegranate fruit extract/kg BW (40) and C57BL/6 mice fed 0.4% (wt:wt) MGPs (containing ∼18 mg EA/kg BW) (10). In the latter case, the lipid-lowering effects of MGPs were evident in both hepatic TG content and adipose tissue mass. In contrast, muscadine wine polyphenols having a similar polyphenolic composition, but devoid of EA from precipitation, showed reduced metabolic improvements, suggesting that EA might be a key component in producing metabolic benefits (10). The reduction of TG in the liver was associated with increased expression of hepatic genes related to FA oxidation (36), implying that decreased TG accumulation is linked to energy expenditure. To confirm this hypothesis, Cao et al. (51) recently reported that oral gavage of punicalagin (150 mg/kg BW), a major ellagitannin in pomegranate, reduced high-fat diet–induced obesity in rats via mechanisms associated with AMPK activation. Pomegranate flour at a concentration of 0.5% (wt:wt) in the diet reduced epididymal fat mass in aged ddY mice (61). The loss of BW or fat mass were accompanied by an improvement in plasma lipid profiles (reduced FFA, TGs, total cholesterol, LDL cholesterol, and VLDL cholesterol, but increased HDL cholesterol) and glucose and insulin tolerance (reduced hyperglycemia and hyperinsulinemia). In addition, Kang et al. (52) reported that supplementation with raspberry seed flour (equivalent to 100 mg EA/kg BW) in the diet of C57BL/6 mice 1) normalized high-fat– and high-sugar–induced dyslipidemia, 2) significantly enhanced hepatic and systemic insulin sensitivity, 3) reduced hepatic endoplasmic reticulum and oxidative stress, and 4) inhibited proinflammatory adipose tissue remodeling and adipose dysfunction.
As with EA-enriched fractions of whole foods, it has also been shown that pure EA attenuates obesity or obesity-mediated metabolic complications in animals. In KK-Ay mice, a model of type 2 diabetes, supplementation with 0.1% EA (equivalent to ∼100 mg EA/kg BW) ameliorated dysregulation of serum lipid profiles and resistin, and upregulated hepatic gene expression of apoA-I, LDL receptor (LDLr), carnitine palmitoyltransferase 1 (CPT1), and PPARG gene expression, resulting in decreased hepatic steatosis (53). Panchal et al. (54) and Kannan et al. (55) showed that EA (80 mg/kg BW and 15 mg/kg BW, respectively) not only ameliorated lipid and glucose metabolism, but also attenuated obesity-associated myocardiac dysfunction and cardiovascular remodeling by altering myocardial necrosis and upregulation of nuclear factor erythroid 2–related factor 2 (Nrf2) and CPT1 gene expression in the heart and liver. Interestingly, Ahad et al. (56) demonstrated that low-dose oral administration of EA (40 mg/kg BW for 16 wk) significantly prevented the onset of dyslipidemia and diabetic nephropathy in streptozotocin-induced diabetes in Wistar albino rats. This effect presumably was through the inactivation of renal nuclear transcription factor κB (NF-κB) (56). Collectively, these studies suggest that the intake of EA may be beneficial to diabetes-associated microvascular diseases (such as cardiovascular disease and diabetic kidney disease) by decreasing chronic inflammation.
In summary, chronic supplementation of EA/ellagitannin-enriched extracts or pure EA were effective in attenuating obesity and metabolic syndrome in rodents. In some cases, the improvement in plasma lipid profiles and/or glucose and insulin sensitivity was evident despite the absence of measurable differences in total BW. The variability of EA bioavailability depending on the source of EA and confounding factors of other polyphenols is likely contributing to these different effects. Also, it should be noted that EA-rich extracts contain numerous other phytochemicals, and the observed effects cannot be attributed solely to EA. Further research is warranted to determine whether the intake of EA along with other phytochemicals facilitates or perhaps impedes EA uptake at the intestinal epithelium barrier.
In terms of safety of EA supplementation in humans, little information is available. When the effective dose determined in the mouse (5–150 mg EA/kg BW) is converted into a human equivalent dose based on body surface area and metabolic rate, a scaling factor of 12.3 is applied to appropriately compare these 2 species (62). The estimated effective human dose would therefore be 0.4–12.2 mg EA/kg BW; thus, for an average 70-kg individual, the dose is ∼30–850 mg EA/d. To determine EA toxicity, a subchronic toxicity study (90 d) performed in F344 rats fed them up to ∼40 g EA/kg BW (5% in the diet) (63). There were no adverse effects observed with an estimated no-observed-adverse-effect level (NOAEL) of ∼3000 mg/kg BW (63). When this NOAEL value is applied to humans, the anticipated EA value is ∼500 mg EA/kg BW, with the use of a scaling factor of 6.2 for rat-to-human conversion. This suggests that daily consumption of ∼30–850 mg EA for a human is predicted to be safe and is probably consumed at that amount by individuals who use commercially available EA-containing dietary supplements. However, further safety studies of chronic supplementation conditions should be conducted before recommending EA supplementation for humans.
Mechanisms Involved in Metabolic Benefits of EA
Oxidative stress and inflammation.
Obesity is associated with chronic low-grade inflammation and increased oxidative stress (64). In the obese state, inflamed adipose tissue is associated directly with the activation of inflammatory signaling and abnormal production of proinflammatory adipokines (65). Proinflammatory signaling pathways are related closely to oxidative stress via reactive oxygen species, including free radicals (66). It has been reported that EA reduces gene expression levels of tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), and chemokine C-C motif ligand-2 (CCL-2) secretion in LPS-stimulated macrophages and adipocytes, indicating that EA may directly reduce adipose inflammation in vitro (67). Ahad et al. (56) demonstrated that EA inhibits NF-κB, the major transcription factor for the proinflammatory response, ameliorating dyslipidemia and diabetic nephropathy in rats. With the administration of an increasing dose of EA (20, 40, 80, and 100 mg/kg BW; 14 d) there was substantial inhibition of NF-κB–p65, transforming growth factor β (TGF-β), and fibronectin, with an improvement in insulin resistance. Moreover, a study showed that EA (0.1% in the diet) (68) or EA-containing pomegranate fruit extract (30 mg/kg BW) (40) improved hepatic steatosis and dyslipidemia (53), which is closely associated with obesity and chronic inflammation. Reduction of resistin release was confirmed in 3T3-L1 cells with treatment with 20–70 μM EA (40). The direct anti-inflammatory effects of EA in white adipose tissue, including alternative M2 macrophage polarization and changes in innate or adaptive immune responses, have not been identified yet. Despite the fact that EA does not appear to accumulate in adipose tissue, our recent study clearly demonstrated that raspberry seed EA decreases proinflammatory cytokine secretion in adipose tissue and decreases macrophage infiltration during the consumption of a high-fat, high-sugar diet (52).
In addition to the attenuation of inflammation, EA supplementation (∼100 mg/kg BW) appears to decrease oxidative stress. Consumption of a high-cholesterol, high-fat diet with EA supplementation (1% wt:wt diet) improved lipid profiles and decreased lipid peroxidation by reducing malondialdehyde production and caspase-8, caspase-9, and Fas ligand concentrations in aortic arches (69). Consistently, EA supplementation (30 mg EA/kg BW) for 14 wk attenuated oxidative stress–induced endothelial dysfunction and atherosclerosis through Nrf2 activation in high-fat diet–fed apoE knockout mice (70). The antioxidative effects of EA-containing fruits also were confirmed in several human studies. Chen et al. (71) demonstrated that supplementing pomegranate juice (1%) for 6–30 d in pregnant women significantly decreased oxidative stress and apoptotic cell death in the placenta. Higher doses of EA might be effective in delaying or alleviating existing inflammatory disease symptoms. The cardioprotective effects of 2% EA (2 g EA/kg BW) also were observed in the streptozotocin-induced type 1 diabetes model of rat (57). Intriguingly, 2.5–5% EA supplementation (9–18 g EA/kg BW) for 12 wk reduced streptozotocin-induced type 1 diabetes symptoms, decreasing protein glycation levels and inflammation in male Balb/cA mice (72). However, in considering these therapeutic effects with the use of high doses of EA close to the NOAEL value (5% EA, 40 g EA/kg BW) (63), caution must be used in translating to clinical trials in humans.
Epigenetic regulation.
Accumulating evidence suggests that consuming a high-fat diet in early life can influence the obese phenotype via mechanisms associated with epigenetic modification (73). Conversely, epidemiologic studies show that fruit and vegetable consumption has a reciprocal correlation with the incidence of obesity (3). Studies conducted to determine whether the dietary components of fruits and vegetables participate in the epigenetic regulation of obesity are gradually increasing. Boque et al. (74) reported that apple polyphenol consumption increases DNA methylation by attenuating adipocyte hypertrophy in a diet-induced obesity model of rat. Moreover, Okla et al. (36) also showed that a high-fat diet with MGP supplementation (18 mg EA/kg BW) upregulates the expression of histone deacetylase 9 (HDAC9), a negative regulator of adipogenesis. These data implied that dietary polyphenols may affect chromatin remodeling to regulate obesity outcomes. Interestingly, Kang et al. (37) found that EA is the polyphenolic compound in MGPs responsible for increasing HDAC9 expression, and further identified that EA inhibits histone arginine methyltransferase 4 (CARM1), an enzyme necessary for adipogenesis, during the differentiation of hASCs. Even though there is no direct evidence that EA metabolites alter epigenetic enzymes to regulate obesity-associated adipose expansion, urolithin C was reported to reduce TNFα-induced inflammation through the inhibition of histone acetyltransferase (HAT) activity in monocytes (75). This implies that EA metabolites, like EA, also may modulate chromatin remodeling during adipogenesis. In addition to EA, several other dietary phytochemicals, including curcumin (76), genistein (77), and isoflavones (78), are reported to improve the obesity-associated metabolic index (dyslipidemia, hyperglycemia, and hyperinsulinemia) by altering DNA methylation and/or histone acetylation. An increasing number of studies are revealing that fruit and vegetable consumption in pregnancy may result in metabolic benefits to offspring (77). It is unknown whether the polyphenolic constituents found in fruits and vegetables (including EA) may positively affect chromatin reprogramming of the fetus, which may exert lifelong metabolic benefits against obesity and its associated metabolic dysfunction. Further studies in this area would be important to provide novel insights into the prevention of childhood obesity, as well as for adults.
Potential effects of EA consumption on gut microbes.
The gut microbial community has been proposed as a crucial environmental factor to control obesity by altering the host’s energy homeostasis (79, 80). The colonic microbiota is also responsible for the extensive breakdown of polyphenols into a series of phenolic metabolites that may be responsible for human health effects. Thus, recently, many researchers have investigated the relation between polyphenol supplementation and changes in the microbiome. Neyrinck et al. (58) reported that mice fed a high-fat diet with pomegranate peel extract reduces inflammation and hypercholesterolemia by promoting the growth of Bifidobacterium spp. in the ceca. Anhê et al. (81) also described how the consumption of polyphenol-rich cranberry extract protects from high-fat and high-sugar diet–induced obesity and insulin resistance through the modulation of microbiota ecology, and particularly Akkermansia spp. abundance in mice. These data suggested the potential implication of the manipulation of host metabolism by gut microbiota in response to dietary polyphenols. There is growing evidence that EA or EA-enriched food consumption may attenuate obesity and metabolic complications by altering gut microflora–associated metabolism, thus altering urolithin production by gut microbes. Tomas-Barberan et al. (30) suggested that different urolithins are produced in response to a host’s metabolic health and that each urolithin may potentiate or nullify the health benefits of EA. It indeed may be that metabolically healthy individuals possess microbiota that are able to generate mainly active urolithins, such as urolithin A [i.e., belonging to the so-called metabotype A (30)]. In contrast, metabolically unhealthy humans may have bacterial communities that produce urolithin A but also other less-active urolithins, such as isourolithin A and urolithin B [i.e., subjects with metabotype B (30)]. García-Villalba et al. (23) reported that there were compositional differences in the gut microbiome between human subjects who produce urolithin A (an effective urolithin) and who produce isourolithins A and B (less-active urolithins). Li et al. (82) demonstrated that a 4-wk supplementation with 1000 mg pomegranate extract altered microbiota by increasing the diversity of the microbiome and by decreasing the Firmicutes population only in urolithin producers, but not in nonproducers of urolithin. Because the decreased Firmicutes:Bacteroidetes ratio is tightly associated with the improvement of obesity phenotypes (83), it strongly suggests that urolithin production may be associated directly with modulating metabolic outcomes partly through gut microbes. It is important to note that subjects who have a higher risk of chronic illness produce isourolithin A and urolithin B (30), the 2 inactive EA metabolites in our own trials (47). This differential impact of urolithins in humans was confirmed by Kang et al. (47), who showed that urolithin A, urolithin C, and urolithin D, but not urolithin B and isourolithin A, reduce adipogenesis in primary human adipocyte cultures. Based on these results, Kang et al. (47) suggested that the “structure–function relation,” i.e., the absence of the -OH moiety at the C8 position of urolithins because of isomerism (isourolithin A) or removal (urolithin B) (Figure 1), is inversely correlated with the ability to downregulate adipogenic differentiation. Recently, the Gordonibacter urolithinfaciens species nova has been described as a novel species that is responsible for converting EA into urolithin M5 and urolithin C (84). This bacterium belongs to the family Coriobacteriaceae, a family that is associated with benefits in obesity (85). Because the bacterial phylum that specifically transforms EA or its intermediate metabolites into urolithin A compared with isourolithin A or urolithin B has not yet been identified, this is of substantial interest for future studies. Also, further investigation is warranted to determine whether urolithin metabotypes could serve as biomarkers of obesity-induced metabolic diseases and altered microbiota (86).
Conclusions
The excessive expansion of white adipose tissue during obesity triggers complex and multifactorial conditions of chronic inflammation and oxidative stress. In this review, we summarized current knowledge on the absorption of EA and its metabolites throughout the gastrointestinal system, in vitro and in vivo evidence that EA may attenuate adipocyte expansion (hyperplastic and hypertrophic), and proposed mechanisms of action.
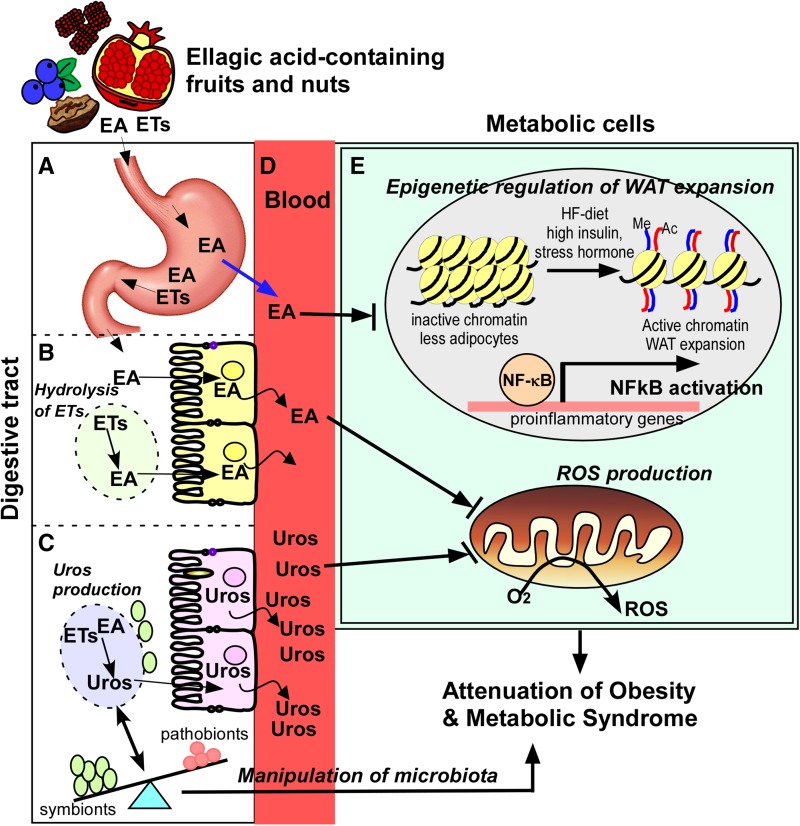
The overall bioavailability of EA and its metabolites in humans is low (typical plasma concentrations of EA at ∼0.1–0.4 μmol/L and urolithins at ∼2–10 μmol/L), although these values may vary depending on the EA-to-ellagitannin ratio and the surrounding microenvironment, such as pH, enzymes, gut microbes, and other conjugating counterparts. The entry points of EA into the circulatory system include the stomach and small intestine, whereas urolithins enter the systemic circulation primarily via the colon. In terms of mode of action, the antioxidative and anti-inflammatory characteristics of EA are key contributors to the lipid-lowering and ameliorattion of obesity-mediated inflammatory responses. It has also been discussed that EA may function to modify epigenetic markers in chromatin by altering histone methylation and acetylation levels. Further elucidation is necessary to identify the target DNA regions and relevant genes in pertinent metabolic cells, such as adipocytes. In addition, we summarized the most recent findings to determine whether urolithins, gut-produced metabolites of EA, may participate directly in lipid metabolism, and perhaps serve as biomarkers of the health status of their hosts (Figure 2).
FIGURE 2.
Working model explaining how the intake of EA-rich fruits and vegetables promotes metabolic benefits. A proportion of free EA from some fruits and nuts, such as raspberries, blueberries, walnuts, and pomegranates, will be absorbed in the stomach (A). EA that is released from the hydrolysis of ETs (dotted circle) is absorbed in the small intestine (B). Gut microbes metabolize the unabsorbed EA and ETs, generating different types of urolithins (dotted circle), which are more efficiently absorbed in the colon (C). It is suggested that EA and its metabolic derivatives may manipulate the microbiota, promoting the metabolic activities of hosts. As a result, EA can reach ∼0.1–0.4 μmol/L and urolithins can reach ∼2–10 μmol/L in circulation maximally (D). EA and its gut metabolites, urolithins, may play a synergistic role in regulating epigenetic factors to control white adipose tissue formation (i.e., histone acetylation and methylation levels), inhibiting NF-κB activation and attenuating ROS production, thereby promoting metabolic activities against diet-induced obesity (E). Ac, histone acetylation; EA, ellagic acid; ET, ellagitannin; HF, high fat; Me, histone methylation; ROS, reactive oxygen species; Uro, urolithin; WAT, white adipose tissue.
To translate this working model into humans, it should be noted that the estimated effective human dose of EA (30–850 mg/d for a healthy individual; see in vivo evidence section) is not attainable easily by the typical American dietary pattern. According to Murphy et al. (87), the estimated mean EA intake in adults is highly variable in relation to the intake of fruits and vegetables. The estimated mean EA intake was 5.3 (in men) to 5.9 (in women) mg · 1000 kcal−1 · d−1 for adults not meeting the recommended amounts of fruits and vegetables, whereas mean EA intake was 17.9 (in men) to 27.6 (in women) mg · 1000 kcal−1 · d−1 for adults meeting the recommendation of fruits and vegetables in response to the Dietary Guidelines for Americans. This analysis suggests that average Americans who conform to dietary guidelines for fruits and vegetables barely meet the lower end of the effective human dose of EA for metabolic benefits (44 mg/2500 kcal for men and 55 mg/2000 kcal for women), and only a small proportion of adults consume the minimum recommended amounts of EA through the typical American diet. “Placing greater emphasis on recommending EA-concentrated fruits and nuts” would be one way to narrow this gap (87).
In addition to the extrapolation of animal studies to humans to predict optimal EA intake, it is necessary to validate the metabolic benefits and proposed mechanisms in humans. Hitherto, to our knowledge, very few human clinical studies have been available in terms of assessing how EA consumption affects obesity-mediated inflammation and metabolic disease progression. Therefore, conducting human clinical research in healthy volunteers as well as participants susceptible to metabolic disease risk should be the next-step challenge to establish the metabolic benefits of EA-containing fruits and nuts in managing obesity and its metabolic complications.
In conclusion, recommending the inclusion of EA-containing fruits and nuts in our daily diets (with the top 5 sources of EA in American diet being raspberries, strawberries, apples, walnuts, and pecans) may be a promising strategy to lessen adiposity and/or improve obesity-mediated metabolic complication even without substantial weight loss.
Acknowledgments
We thank Juan Carlos Espín and Francisco A Tomás-Barberán at the Spanish National Research Council (CEBAS-CSIC), Murcia, Spain, for their collaboration and constructive discussion. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AMPK, AMP-activated protein kinase; aP2, fatty acid–binding protein 4; BW, body weight; CARM1, histone arginine methyltransferase 4; C/EBPα, CCAAT/enhancer-binding protein α CPT1, carnitine palmitoyltransferase 1; EA, ellagic acid; Fas, fatty acid synthase; hASC, human adipose–derived stem cell; HAT, histone acetyltransferase; HDAC9, histone deacetylase 9; LDLr, LDL receptor; MGP, muscadine grape polyphenol; NOAEL, no-observed-adverse-effect level; Nrf2, nuclear factor erythroid 2–related factor 2.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He K, Hu FB, Colditz GA, Manson JE, Willett WC, Liu S. Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int J Obes Relat Metab Disord 2004;28:1569–74. [DOI] [PubMed] [Google Scholar]
- 3.Epstein LH, Gordy CC, Raynor HA, Beddome M, Kilanowski CK, Paluch R. Increasing fruit and vegetable intake and decreasing fat and sugar intake in families at risk for childhood obesity. Obes Res 2001;9:171–8. [DOI] [PubMed] [Google Scholar]
- 4.Amiot MJ, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev 2016;17:573–86. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun I, Shen CL. Novel insights of dietary polyphenols and obesity. J Nutr Biochem 2014;25:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Ligt M, Timmers S, Schrauwen P. Resveratrol and obesity: Can resveratrol relieve metabolic disturbances? Biochim Biophys Acta 2015;1852:1137–44. [DOI] [PubMed] [Google Scholar]
- 7.Bradford PG. Curcumin and obesity. Biofactors 2013;39:78–87. [DOI] [PubMed] [Google Scholar]
- 8.Anderson KJ, Teuber SS, Gobeille A, Cremin P, Waterhouse AL, Steinberg FM. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J Nutr 2001;131:2837–42. [DOI] [PubMed] [Google Scholar]
- 9.Zafrilla P, Ferreres F, Tomas-Barberan FA. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J Agric Food Chem 2001;49:3651–5. [DOI] [PubMed] [Google Scholar]
- 10.Gourineni V, Shay NF, Chung S, Sandhu AK, Gu L. Muscadine grape (Vitis rotundifolia) and wine phytochemicals prevented obesity-associated metabolic complications in C57BL/6J mice. J Agric Food Chem 2012;60:7674–81. [DOI] [PubMed] [Google Scholar]
- 11.Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem 2000;48:4581–9. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Johnson JV, Talcott ST. Identification of ellagic acid conjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. J Agric Food Chem 2005;53:6003–10. [DOI] [PubMed] [Google Scholar]
- 13.Kähkönen M, Kylli P, Ollilainen V, Salminen JP, Heinonen M. Antioxidant activity of isolated ellagitannins from red raspberries and cloudberries. J Agric Food Chem 2012;60:1167–74. [DOI] [PubMed] [Google Scholar]
- 14.Law DJ. Synthetic tannins: their synthesis, industrial products and application. Georg Grasser. Translated by F.G.A. Enna. Pp. vi+143. (London: Crosby Lockwood and Son. 1922.) Journal of the Society of Chemical Industry. 1922;41:R141–R.
- 15.Rossi M, Erlebacher J, Zacharias DE, Carrell HL, Iannucci B. The crystal and molecular structure of ellagic acid dihydrate: a dietary anti-cancer agent. Carcinogenesis 1991;12:2227–32. [DOI] [PubMed] [Google Scholar]
- 16.Clifford MN, Scalbert A. Ellagitannins—nature, occurrence and dietary burden. J Sci Food Agric 2000;80:1118–25. [Google Scholar]
- 17.Lipińska L, Klewicka E, Sojka M. The structure, occurrence and biological activity of ellagitannins: a general review. Acta Sci Pol Technol Aliment 2014;13:289–99. [DOI] [PubMed] [Google Scholar]
- 18.González-Barrio R, Borges G, Mullen W, Crozier A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J Agric Food Chem 2010;58:3933–9. [DOI] [PubMed] [Google Scholar]
- 19.Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta 2004;348:63–8. [DOI] [PubMed] [Google Scholar]
- 20.Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr 2006;136:2481–5. [DOI] [PubMed] [Google Scholar]
- 21.Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem 2006;54:8956–61. [DOI] [PubMed] [Google Scholar]
- 22.González-Sarrías A, García-Villalba R, Núñez-Sánchez MÁ, Tomé-Carneiro J, Zafrilla P, Mulero J, Tomás-Barberán FA, Espín JC. Identifying the limits for ellagic acid bioavailability: a crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J Funct Foods 2015;19, Part A:225–35. [Google Scholar]
- 23.García-Villalba R, Beltran D, Espin JC, Selma MV, Tomas-Barberan FA. Time course production of urolithins from ellagic acid by human gut microbiota. J Agric Food Chem 2013;61:8797–806. [DOI] [PubMed] [Google Scholar]
- 24.González-Barrio R, Edwards CA, Crozier A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: in vivo and in vitro studies. Drug Metab Dispos 2011;39:1680–8. [DOI] [PubMed] [Google Scholar]
- 25.Espín JC, González-Barrio R, Cerdá B, López-Bote C, Rey AI, Tomás-Barberán FA. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem 2007;55:10476–85. [DOI] [PubMed] [Google Scholar]
- 26.Cerdá B, Espín JC, Parra S, Martínez P, Tomás-Barberán FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy–6H–dibenzopyran–6– one derivatives by the colonic microflora of healthy humans. Eur J Nutr 2004;43:205–20. [DOI] [PubMed] [Google Scholar]
- 27.Seeram NP, Zhang Y, McKeever R, Henning SM, Lee R-p, Suchard MA, Li Z, Chen S, Thames G, Zerlin A, et al. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J Med Food 2008;11:390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tulipani S, Urpi-Sarda M, García-Villalba R, Rabassa M, López-Uriarte P, Bulló M, Jáuregui O, Tomás-Barberán F, Salas-Salvadó J, Espín JC, et al. Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J Agric Food Chem 2012;60:8930–40. [DOI] [PubMed] [Google Scholar]
- 29.González-Sarrías A, Gimenez-Bastida JA, Nunez-Sanchez MA, Larrosa M, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur J Nutr 2014;53:853–64. [DOI] [PubMed] [Google Scholar]
- 30.Tomás-Barberán FA, Garcia-Villalba R, Gonzalez-Sarrias A, Selma MV, Espin JC. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem 2014;62:6535–8. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Muñoz C, Vaillant F. Metabolic fate of ellagitannins: implications for health, and research perspectives for innovative functional foods. Crit Rev Food Sci Nutr 2014;54:1584–98. [DOI] [PubMed] [Google Scholar]
- 32.Tomé-Carneiro J, Larrosa M, Gonzalez-Sarrias A, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des 2013;19:6064–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gambini J, Ingles M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, Mas-Bargues C, Abdelaziz KM, Gomez-Cabrera MC, Vina J, et al. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev 2015;2015:837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Li L, Ran X, Long M, Zhang M, Tao Y, Luo X, Wang Y, Ma X, Halmurati U, et al. Ellagic acid reduces adipogenesis through inhibition of differentiation-prevention of the induction of rb phosphorylation in 3T3–L1 adipocytes. Evid Based Complement Alternat Med 2013;2013:287534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo MS, Choi HS, Seo MJ, Jeon HJ, Lee BY. Ellagic acid suppresses lipid accumulation by suppressing early adipogenic events and cell cycle arrest. Phytother Res 2015;29:398–406. [DOI] [PubMed] [Google Scholar]
- 36.Okla M, Kang I, Kim da M, Gourineni V, Shay N, Gu L, Chung S. Ellagic acid modulates lipid accumulation in primary human adipocytes and human hepatoma Huh7 cells via discrete mechanisms. J Nutr Biochem 2015;26:82–90. [DOI] [PubMed] [Google Scholar]
- 37.Kang I, Okla M, Chung S. Ellagic acid inhibits adipocyte differentiation through coactivator-associated arginine methyltransferase 1-mediated chromatin modification. J Nutr Biochem 2014;25:946–53. [DOI] [PubMed] [Google Scholar]
- 38.Yang ZG, Jia LN, Shen Y, Ohmura A, Kitanaka S. Inhibitory effects of constituents from Euphorbia lunulata on differentiation of 3T3–L1 cells and nitric oxide production in RAW264.7 cells. Molecules 2011;16:8305–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh GY, McCutcheon K, Zhang F, Liu D, Cartwright CA, Martin R, Yang P, Liu Z. Improvement of obesity phenotype by Chinese sweet leaf tea (Rubus suavissimus) components in high-fat diet-induced obese rats. J Agric Food Chem 2011;59:98–104. [DOI] [PubMed] [Google Scholar]
- 40.Makino-Wakagi Y, Yoshimura Y, Uzawa Y, Zaima N, Moriyama T, Kawamura Y. Ellagic acid in pomegranate suppresses resistin secretion by a novel regulatory mechanism involving the degradation of intracellular resistin protein in adipocytes. Biochem Biophys Res Commun 2012;417:880–5. [DOI] [PubMed] [Google Scholar]
- 41.Park SH, Kim JL, Lee ES, Han SY, Gong JH, Kang MK, Kang YH. Dietary ellagic acid attenuates oxidized LDL uptake and stimulates cholesterol efflux in murine macrophages. J Nutr 2011;141:1931–7. [DOI] [PubMed] [Google Scholar]
- 42.Mejia-Meza EI, Yanez JA, Remsberg CM, Takemoto JK, Davies NM, Rasco B, Clary C. Effect of dehydration on raspberries: polyphenol and anthocyanin retention, antioxidant capacity, and antiadipogenic activity. J Food Sci 2010;75:H5–12. [DOI] [PubMed] [Google Scholar]
- 43.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods 2008;45:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 2012;13:251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25. [DOI] [PubMed] [Google Scholar]
- 46.Poulose N, Prasad C, Haridas P, Anilkumar G. Ellagic acid stimulates glucose transport in adipocytes and muscles through AMPK mediated pathway. J Diabetes Metab 2011;2:149. [Google Scholar]
- 47.Kang I, Kim Y, Tomas-Barberan FA, Espin JC, Chung S, Urolithin A. C and D, but not iso-Urolithin A and Urolithin B, attenuate triglyceride accumulation in human cultures of adipocytes and hepatocytes. Mol Nutr Food Res 2016;60:1129–38. [DOI] [PubMed] [Google Scholar]
- 48.Lei F, Zhang XN, Wang W, Xing DM, Xie WD, Su H, Du LJ. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes (Lond) 2007;31:1023–9. [DOI] [PubMed] [Google Scholar]
- 49.Monika P, Geetha A. The modulating effect of Persea americana fruit extract on the level of expression of fatty acid synthase complex, lipoprotein lipase, fibroblast growth factor-21 and leptin–a biochemical study in rats subjected to experimental hyperlipidemia and obesity. Phytomedicine 2015;22:939–45. [DOI] [PubMed] [Google Scholar]
- 50.Ratnam DV, Chandraiah G, Meena AK, Ramarao P, Kumar MN. The co-encapsulated antioxidant nanoparticles of ellagic acid and coenzyme Q10 ameliorates hyperlipidemia in high fat diet fed rats. J Nanosci Nanotechnol 2009;9:6741–6. [DOI] [PubMed] [Google Scholar]
- 51.Cao K, Xu J, Pu W, Dong Z, Sun L, Zang W, Gao F, Zhang Y, Feng Z, Liu J. Punicalagin, an active component in pomegranate, ameliorates cardiac mitochondrial impairment in obese rats via AMPK activation. Sci Rep 2015;5:14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang I, Espin JC, Carr T, Tomas-Barberan FA, Chung S. Raspberry seed flour attenuates high sucrose diet-mediated hepatic stress and adipose tissue inflammation. J Nutr Biochem 2016;32:64–72. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura Y, Nishii S, Zaima N, Moriyama T, Kawamura Y. Ellagic acid improves hepatic steatosis and serum lipid composition through reduction of serum resistin levels and transcriptional activation of hepatic PPARa in obese, diabetic KK-A(y) mice. Biochem Biophys Res Commun 2013;434:486–91. [DOI] [PubMed] [Google Scholar]
- 54.Panchal SK, Ward L, Brown L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur J Nutr 2013;52:559–68. [DOI] [PubMed] [Google Scholar]
- 55.Kannan MM, Quine SD. Ellagic acid inhibits cardiac arrhythmias, hypertrophy and hyperlipidaemia during myocardial infarction in rats. Metabolism 2013;62:52–61. [DOI] [PubMed] [Google Scholar]
- 56.Ahad A, Ganai AA, Mujeeb M, Siddiqui WA. Ellagic acid, an NF-kappaB inhibitor, ameliorates renal function in experimental diabetic nephropathy. Chem Biol Interact 2014;219:64–75. [DOI] [PubMed] [Google Scholar]
- 57.Rani UP, Kesavan R, Ganugula R, Avaneesh T, Kumar UP, Reddy GB, Dixit M. Ellagic acid inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and prevents atheroma formation in streptozotocin-induced diabetic rats. J Nutr Biochem 2013;24:1830–9. [DOI] [PubMed] [Google Scholar]
- 58.Neyrinck AM, Van Hee VF, Bindels LB, De BF, Cani PD, Delzenne NM. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota. Br J Nutr 2013;109:802–9. [DOI] [PubMed] [Google Scholar]
- 59.Song Y, Park HJ, Kang SN, Jang SH, Lee SJ, Ko YG, Kim GS, Cho JH. Blueberry peel extracts inhibit adipogenesis in 3T3–L1 cells and reduce high-fat diet-induced obesity. PLoS One 2013;8:e69925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noh J, Kim Y, Gang G, Yang K, Lee H, Oh W, Song K, Lee C. Chestnut (Castanea crenata) inner shell extract inhibits development of hepatic steatosis in C57BL/6 mice fed a high-fat diet. Food Chem 2012;121:437–42. [Google Scholar]
- 61.Wang J, Rong X, Um IS, Yamahara J, Li Y. 55-week treatment of mice with the unani and ayurvedic medicine pomegranate flower ameliorates ageing-associated insulin resistance and skin abnormalities. Evid Based Complement Alternat Med 2012;2012:350125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.USDA Food and Drug Administration, Center for Drug Evaluation and Research [Internet]. Guidance for industry on estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers [updated 2005 July 22] [cited 2016 May 1]. Available from: http://www.fda.gov/downloads/Drugs/.../Guidances/UCM078932.pdf.
- 63.Tasaki M, Umemura T, Maeda M, Ishii Y, Okamura T, Inoue T, Kuroiwa Y, Hirose M, Nishikawa A. Safety assessment of ellagic acid, a food additive, in a subchronic toxicity study using F344 rats. Food Chem Toxicol 2008;46:1119–24. [DOI] [PubMed] [Google Scholar]
- 64.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D’Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002;105:804–9. [DOI] [PubMed] [Google Scholar]
- 65.Frühbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab 2001;280:E827–47. [DOI] [PubMed] [Google Scholar]
- 66.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci 2009;84:705–12. [DOI] [PubMed] [Google Scholar]
- 67.Winand J, Schneider YJ. The anti-inflammatory effect of a pomegranate husk extract on inflamed adipocytes and macrophages cultivated independently, but not on the inflammatory vicious cycle between adipocytes and macrophages. Food Funct 2014;5:310–8. [DOI] [PubMed] [Google Scholar]
- 68.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature 2001;409:307–12. [DOI] [PubMed] [Google Scholar]
- 69.Yu YM, Chang WC, Wu CH, Chiang SY. Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid. J Nutr Biochem 2005;16:675–81. [DOI] [PubMed] [Google Scholar]
- 70.Ding Y, Zhang B, Zhou K, Chen M, Wang M, Jia Y, Song Y, Li Y, Wen A. Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: role of Nrf2 activation. Int J Cardiol 2014;175:508–14. [DOI] [PubMed] [Google Scholar]
- 71.Chen B, Tuuli MG, Longtine MS, Shin JS, Lawrence R, Inder T, Michael Nelson D. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am J Physiol Endocrinol Metab 2012;302:E1142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chao CY, Mong MC, Chan KC, Yin MC. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol Nutr Food Res 2010;54:388–95. [DOI] [PubMed] [Google Scholar]
- 73.Wu Q, Suzuki M. Parental obesity and overweight affect the body-fat accumulation in the offspring: the possible effect of a high-fat diet through epigenetic inheritance. Obes Rev 2006;7:201–8. [DOI] [PubMed] [Google Scholar]
- 74.Boqué N, de la Iglesia R, de la Garza AL, Milagro FI, Olivares M, Banuelos O, Soria AC, Rodriguez-Sanchez S, Martinez JA, Campion J. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol Nutr Food Res 2013;57:1473–8. [DOI] [PubMed] [Google Scholar]
- 75.Kiss AK, Granica S, Stolarczyk M, Melzig MF. Epigenetic modulation of mechanisms involved in inflammation: influence of selected polyphenolic substances on histone acetylation state. Food Chem 2012;131:1015–20. [Google Scholar]
- 76.Yun JM, Jialal I, Devaraj S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J Nutr Biochem 2011;22:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect 2006;114:567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Howard TD, Ho SM, Zhang L, Chen J, Cui W, Slager R, Gray S, Hawkins GA, Medvedovic M, Wagner JD. Epigenetic changes with dietary soy in cynomolgus monkeys. PLoS One 2011;6:e26791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004;101:15718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 2007;104:979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anhê FF, Roy D, Pilon G, Dudonne S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015;64:872–83. [DOI] [PubMed] [Google Scholar]
- 82.Li Z, Henning SM, Lee RP, Lu QY, Summanen PH, Thames G, Corbett K, Downes J, Tseng CH, Finegold SM, et al. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct 2015;6:2487–95. [DOI] [PubMed] [Google Scholar]
- 83.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022–3. [DOI] [PubMed] [Google Scholar]
- 84.Selma MV, Beltran D, Garcia-Villalba R, Espin JC, Tomas-Barberan FA. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct 2014;5:1779–84. [DOI] [PubMed] [Google Scholar]
- 85.Clavel T, Desmarchelier C, Haller D, Gérard P, Rohn S, Lepage P, Daniel H. Intestinal microbiota in metabolic diseases. Gut Microbes 2014;5:544–51. [DOI] [PubMed] [Google Scholar]
- 86.Selma MV, Romo-Vaquero M, Garcia-Villalba R, Gonzalez-Sarrias A, Tomas-Barberan FA, Espin JC. The human gut microbial ecology associated with overweight and obesity determines ellagic acid metabolism. Food Funct 2016;7:1769–74. [DOI] [PubMed] [Google Scholar]
- 87.Murphy MM, Barraj LM, Herman D, Bi X, Cheatham R, Randolph RK. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J Acad Nutr Diet 2012;112:222–9. [DOI] [PubMed] [Google Scholar]