Abstract
Background
Body mass index (BMI), waist circumference (WC), visceral adiposity index (VAI), triglyceride glucose index (TyG), TyG-BMI, and TyG-WC have been reported as markers of insulin resistance or type 2 diabetes mellitus (T2DM). However, little is known about the associations between the aforementioned markers and the risk of prediabetes and diabetes in first-degree relatives (FDRs) of T2DM patients.
Methods
1544 FDRs of T2DM patients (635 men and 909 women) were enrolled in the initial cross-sectional study and all of them finished corresponding examinations. Logistic regression analysis and receiver operating characteristic (ROC) curve were used to compare and identify the associations of the six parameters (BMI, WC, VAI, TyG, TyG-BMI and TyG-WC) with the prevalence of prediabetes and diabetes. Subsequently, 452 of them were followed-up for an average of 5 years. Cox proportional hazard regression model was applied to confirm the predictive value of the optimal marker.
Results
Among the indices, TyG-WC was more strongly associated with the prevalence of prediabetes and diabetes. Compared with participants in the lowest quartile of TyG-WC, the adjusted odds ratio and 95 % CIs for prediabetes and diabetes was 11.19 (7.62–16.42) for those in the top quartile of TyG-WC. Moreover, the largest AUC was also observed in TyG-WC (0.765, 95 % CIs 0.741–0.789, P < 0.001). The robust predictive value of TyG-WC was further confirmed in the follow-up study (HR: 7.13, 95 % CIs 3.41–14.90, P < 0.001).
Conclusions
TyG-WC is a novel and clinically effective marker for early identifying the risks of prediabetes and diabetes in FDRs of T2DM patients.
Electronic supplementary material
The online version of this article (doi:10.1186/s12967-016-1020-8) contains supplementary material, which is available to authorized users.
Keywords: Type 2 diabetes mellitus, First-degree relative, Triglyceride glucose index, Visceral adiposity index, TyG-WC
Background
The occurrence rate of type 2 diabetes mellitus (T2DM) is quite astonishing worldwide, of which is a major risk factor for cardiovascular disease and even premature mortality [1]. Thus, it is of utmost significance to early identify and treat subjects at high risk of developing T2DM, though the unclear etiologies and pathological process of it. Familial clustering phenomenon of T2DM may back the genetic susceptibility to T2DM. Ma et al. [2] demonstrated that first-degree relatives (FDRs) of patients with T2DM may have a higher prevalence of diabetes than those without a family history of T2DM (26.6 versus 9.2 %). Therefore, it is more important to early determine the susceptible population vulnerable to T2DM via simple and effective diagnostic tools, considering the enormous population of FDRs.
Previous literature indicated that several effective and inexpensive variables, ranging from simple anthropometric measures to more complex models, are closely related to insulin resistance (IR) or diabetes. Body mass index (BMI) and waist circumference (WC), two clinical indices for body fat assessment, are commonly used for detecting prediabetes and diabetes risk [3, 4]. Moreover, visceral adiposity index (VAI), a mathematical model based on BMI, WC, triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C), is a more effective tool for prediabetes and diabetes prediction [5, 6]. In addition, triglyceride glucose index (TyG) as well as TyG-related indicators (TyG-BMI and TyG-WC) have been reported as excellent surrogate markers of IR, which is deemed to be the vital pathological mechanism of T2DM [7, 8]. To our knowledge, little is known about the accuracy and predictability of these indicators in suffering prediabetes and diabetes in FDRs of T2DM patients.
The objectives of the present study were to investigate the corresponding associations of the aforementioned indicators with the prevalence of prediabetes and diabetes in FDRs of T2DM patients and identify the excellent one firstly. Subsequently, a follow-up study was conducted to evaluate the incidence of diabetes in this population and further assess the performance of the optimal indicator in predicting the risk of T2DM.
Methods
Participants
Stratified random sampling was performed to select T2DM patients from the database of Renji hospital from January 1995 to 2005. The family of each randomly selected subject were contacted by telephone or door-to-door visit. Only one of the FDRs (including parents, children and full siblings) of each T2DM patient was randomly selected and invited to our study from September 2005 to August 2009. A total of 2392 FDRs of these T2DM patients were invited to the survey. After excluding ineligible subjects, 2018 FDRs were recruited to the study and finished structured questionnaires on their first visit. Next, 474 subjects were further excluded according to the exclusion criteria including self-reported diabetes diagnosis and/or regular diabetic medication use, less than 18 or more than 90 years old, pregnant, chronic renal or hepatic failure, cancer, taking regular medication for dyslipidemia and/or hypertension. Finally, 1544 subjects (635 men and 909 women) were enrolled in the cross-sectional study.
To further test whether the optimal marker identified through cross-sectional study is useful for predicting incident diabetes, we conducted a 5-year prospective cohort study including FDRs of T2DM patients diagnosed with NGT or prediabetes in the initial study. After excluding ineligible participants, 452 of the 1544 FDRs completed the annual examinations with the average duration of 5 years (Fig. 1).
Fig. 1.

Trial profile. OGTT oral glucose tolerance test, FDR first degree relative, NGT normal glucose tolerance
The study protocol was in compliance with the declaration of Helsinki and approved by the Ethical Committee of Renji Hospital, School of Medicine, Shanghai Jiaotong University. Written informed consents were signed from all participants included in the study.
Measurements
Body height, weight, WC and blood pressure (BP) were measured by trained survey personnel. Both height and weight measurements were taken in light clothing without shoes. The smallest abdominal circumference was measured as WC, which was taken twice and the mean value was recorded. Blood pressure was measured three times in each subject on the right arm after 5 min resting in a sitting position, and the mean value was recorded.
Laboratory analysis
Each participant received a 75 g OGTT after at least 10 h of overnight fasting. Blood samples were collected at 0, 30, 60, 120 and 180 min after the glucose load. Plasma glucose levels were measured using the glucose oxidase method. Serum insulin levels were obtained using a bio-antibody technique (Linco, St Louis, MO, USA). Serum lipid profiles were tested with an automated biochemical instrument by radioimmunoassay (RIA) based on the double-antibody technique (DPC, Los Angeles, CA, USA). HbA1c was measured by the high-performance liquid chromatography (HPLC) method with a BIO-RAD analyzer (Bio-Rad Variant II; Bio-Rad Laboratories, Hercules, CA, USA).
Diagnostic criteria and definition
The 1999 World Health Organization (WHO) diagnostic criteria for T2DM was adopted [9].
Normal glucose tolerance (NGT) was defined as fasting plasma glucose <6.1 mmol/l and 2-h plasma glucose <7.8 mmol/l. Prediabetes includes isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT) and combined glucose intolerance (CGI). IFG was defined as fasting plasma glucose between 6.1 and 7.0 mmol/l and 2-h plasma glucose <7.8 mmol/l; IGT was defined as fasting plasma glucose <6.1 mmol/l and 2-h plasma glucose between 7.8 and 11.1 mmol/l; CGI was defined as fasting plasma glucose between 6.1 and 7.0 mmol/l and 2-h plasma glucose between 7.8 and 11.1 mmol/l. Diabetes mellitus (DM) was defined as fasting plasma glucose ≥7.0 mmol/l and/or 2-h plasma glucose ≥11.1 mmol/l.
BMI was calculated as the body weight (kg) divided by the square of body height (m2). VAI and TyG were calculated using the former formula [10]. VAI: Men: [WC/(39.68 + 1.88 × BMI)] × (TG/1.03) × (1.31/HDL); Women: [WC/(36.58 + 1.89 × BMI)] × (TG/0.81) × (1.52/HDL), where both TG and HDL levels are expressed in mmol/L. The TyG index: Ln [TG (mg/dl) × FPG (mg/dl)/2]. TyG-BMI: TyG index × BMI. TyG-WC: TyG index × WC (cm).
Incidence was calculated as the number of T2DM cases per 100 person years of follow-up starting from the date of finishing the initial examination in 2005–2009 to the occurrence of diabetes or the final follow-up visit in the 5th year.
Statistical analysis
All data were analyzed using SPSS version 17.0 for Windows (SPSS, Chicago, IL, USA). Continuous data were shown as medians and interquartile ranges (IQR) by virtue of the skewed distribution and compared utilizing Kruskal–Wallis H test or Mann–Whitney U test. Adjusted means were calculated and compared with general linear models. Categorical variables were expressed as percentages and compared with Chi square test. Multinomial logistic regression was conducted to determine the correlations between different factors and the risk of prediabetes and diabetes after controlling potential confounding factors. For each indicator, odds ratios and 95 % CIs of quartiles 2–4 were calculated and compared using quartile 1 as the reference. Receiver operating characteristic (ROC) curves were applied to compare the relative diagnostic strengths of these indicators for correctly discriminating prediabetes and diabetes. The area under the ROC curve (AUC) was utilized to quantify the overall diagnostic accuracy. Indicator with the largest AUC was considered as the best marker. The cutoff point of the optimal indictor was calculated according to Youden Index and the corresponding sensitivity, specificity, positive and negative predictive values were further assessed in the cohort study. Cox proportional hazard regression was taken to evaluate the predictive power of the optimal marker for incident diabetes after adjusting for confounding factors. Probability value less than 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 1544 participants were enrolled in the cross-sectional study, including 657 with NGT, 423 with prediabetes and 464 with previously undiagnosed diabetes. Baseline characteristics of participants, stratified by glucose tolerance status, were presented in Table 1. The median ages of subjects with NGT, prediabetes and diabetes were 47.0, 52.0 and 59.0 years old, respectively (P < 0.05). After adjusting for age, subjects with prediabetes and diabetes had higher levels of blood pressure, VAI, BMI, WC, TyG, TyG-BMI, TyG-WC, TG, LDL-C and lower levels of HDL-C than those with NGT.
Table 1.
Baseline characteristics of study population
| Characteristics | NGT | Prediabetes | Diabetes |
|---|---|---|---|
| Number | 657 | 423 | 464 |
| Sex (M/F) | 256/401 | 174/249 | 205/259 |
| Age (years) | 47.0 (36.0, 54.0) | 52.0 (44.0, 58.0)a | 59.0 (50.0, 65.0)a, b |
| SBP (mmHg) | 121.0 (119.0, 122.0) | 126.0 (124.0, 127.0)a | 129.0 (127.0, 131.0)a, b |
| DBP (mmHg) | 76.0 (75.0, 77.0) | 79.0 (78.0, 80.0)a | 79.0 (78.0, 80.0)a |
| BMI (kg/m2) | 24.39 (24.03, 24.75) | 25.12 (24.68, 25.55)a | 25.26 (24.81, 25.70)a |
| WC (cm) | 83.0 (82.1, 83.9) | 88.4 (87.3, 89.5)a | 92.2 (91.1, 93.3)a, b |
| VAI | 1.70 (1.53, 1.87) | 2.39 (2.19, 2.59)a | 2.82 (2.62, 3.03)a, b |
| TyG | 8.43 (8.38, 8.48) | 8.81 (8.75, 8.87)a | 9.21 (9.15, 9.27)a, b |
| TyG-BMI | 206.47 (202.86, 210.08) | 222.05 (217.69, 226.42)a | 233.45 (228.99, 237.91)a, b |
| TyG-WC | 702.07 (692.13, 712.00) | 780.36 (768.35, 792.37)a | 851.94 (839.66, 864.22)a, b |
| TG (mmol/L) | 1.33 (1.23, 1.43) | 1.71 (1.59, 1.82)a | 1.94 (1.82, 2.07)a, b |
| TC (mmol/L) | 4.92 (4.83, 5.00) | 5.01 (4.91, 5.11) | 5.09 (4.98, 5.19)a |
| HDL-C (mmol/L) | 1.47 (1.44, 1.50) | 1.31 (1.27, 1.35)a | 1.31 (1.27, 1.34)a |
| LDL-C (mmol/L) | 2.93 (2.87, 2.99) | 3.03 (2.96, 3.10)a | 3.06 (2.98, 3.13)a |
| FPG (mmol/L) | 5.10 (4.97, 5.22) | 5.74 (5.59, 5.89)a | 8.65 (8.50, 8.81)a, b |
| 2hPG (mmol/L) | 6.06 (5.83, 6.29) | 8.65 (8.38, 8.93)a | 17.11 (16.83, 17.39)a, b |
| FINS (μU/ml) | 9.23 (8.55, 9.92) | 11.19 (10.36, 12.03)a | 12.58 (11.73, 13.43)a, b |
| 2hINS (μU/ml) | 38.70 (36.09, 41.31) | 65.52 (62.36, 68.68)a | 43.02 (39.80, 46.25)b |
| HbA1c (%) | 5.50 (5.42, 5.58) | 5.75 (5.65, 5.84)a | 7.36 (7.27, 7.46)a, b |
Data were expressed as median (Interquartile range 25–75 %)
Comparisons among NGT, Prediabetes and Diabetes groups were performed after adjusting for age
SBP systolic blood pressure, DBP diastolic blood pressure, BMI body mass index, WC waist circumference, VAI visceral adiposity index, TyG triglyceride glucose index, TyG-BMI combined TyG and BMI, TyG-WC combined TyG and WC, TG triglyceride, TC total cholesterol, HDL-C high density lipoprotein cholesterol, LDL-C low density lipoprotein cholesterol, FPG fasting plasma glucose, 2hPG 2 h postload plasma glucose, FINS fasting serum insulin, 2hINS 2 h postload serum insulin, HbA1c glycated hemoglobin A1c
aP < 0.05 versus NGT group
bP < 0.05 versus Prediabetes group
Associations of indicators with prediabetes and diabetes risk
The ORs and 95 % CIs for prediabetes and/or diabetes were progressively increased across quartiles of each index after adjusting for age, sex, SBP and DBP (Table 2). After direct comparison, TyG-WC presented the highest ORs and 95 % CIs for prediabetes and diabetes, reaching 11.19 (95 % CIs 7.62–16.42) for the top quartile as compared with the bottom quartile (P < 0.001), followed by TyG index (Q4 11.04, 95 % CIs 7.57–16.09) and WC (Q4 5.65, 95 % CIs 3.97–8.04).
Table 2.
Adjusted odds ratios (OR) for prediabetes and diabetes in quartiles of each index
| Parameters | Prediabetes OR (95 % CI) | P value | Diabetes OR (95 % CI) | P value | Prediabetes + diabetes OR (95 % CI) | P value |
|---|---|---|---|---|---|---|
| VAI | ||||||
| Q1 (≤0.981) | 1 | – | 1 | – | 1 | – |
| Q2 (−1.630) | 1.26 (0.86–1.83) | 0.239 | 1.31 (0.89–1.93) | 0.172 | 1.28 (0.94–1.74) | 0.123 |
| Q3 (−2.633) | 3.16 (2.19–4.57) | <0.001 | 2.28 (1.54–3.38) | <0.001 | 2.72 (1.98–3.75) | <0.001 |
| Q4 (≥2.634) | 4.07 (2.76–6.00) | <0.001 | 4.57 (3.07–6.81) | <0.001 | 4.23 (3.03–5.90) | <0.001 |
| BMI | ||||||
| Q1 (≤22.49) | 1 | – | 1 | – | 1 | – |
| Q2 (−24.61) | 1.40 (0.97–2.01) | 0.069 | 2.05 (1.38–3.03) | <0.001 | 1.64 (1.21–2.24) | 0.002 |
| Q3 (−26.82) | 1.41 (0.99–2.02) | 0.057 | 2.09 (1.40–3.10) | <0.001 | 1.66 (1.21–2.27) | 0.002 |
| Q4 (≥26.83) | 1.75 (1.21–2.53) | 0.003 | 2.45 (1.63–3.68) | <0.001 | 2.03 (1.46– 2.80) | <0.001 |
| WC | ||||||
| Q1 (≤79.0) | 1 | – | 1 | – | 1 | – |
| Q2 (−87.0) | 1.64 (1.16–2.33) | 0.005 | 4.64 (2.85–7.56) | <0.001 | 2.27 (1.66–3.10) | <0.001 |
| Q3 (−94.0) | 2.72 (1.88–3.95) | <0.001 | 11.16 (6.83–18.23) | <0.001 | 4.50 (3.22–6.28) | <0.001 |
| Q4 (≥94.1) | 3.05 (2.05–4.52) | <0.001 | 15.36 (9.27–25.43) | <0.001 | 5.65 (3.97–8.04) | <0.001 |
| TyG | ||||||
| Q1 (≤8.269) | 1 | – | 1 | – | 1 | – |
| Q2 (−8.724) | 2.07 (1.45–2.97) | <0.001 | 2.69 (1.68–4.33) | <0.001 | 2.21 (1.60–3.04) | <0.001 |
| Q3 (−9.186) | 3.19 (2.19–4.64) | <0.001 | 6.66 (4.18–10.63) | <0.001 | 4.16 (2.98–5.80) | <0.001 |
| Q4 (≥9.187) | 6.54 (4.29–9.97) | <0.001 | 23.04 (14.03–37.82) | <0.001 | 11.04 (7.57–16.09) | <0.001 |
| TyG-BMI | ||||||
| Q1 (≤190.27) | 1 | – | 1 | – | 1 | – |
| Q2 (−214.53) | 1.87 (1.29–2.72) | 0.001 | 2.41 (1.56–3.72) | <0.001 | 2.06 (1.50–2.81) | <0.001 |
| Q3 (−243.30) | 1.92 (1.35–2.73) | <0.001 | 3.79 (2.47–5.82) | <0.001 | 2.50 (1.81–3.46) | <0.001 |
| Q4 (≥243.31) | 3.52 (2.37–5.23) | <0.001 | 9.04 (5.76–14.18) | <0.001 | 5.27 (3.72–7.47) | <0.001 |
| TyG-WC | ||||||
| Q1 (≤668.00) | 1 | – | 1 | – | 1 | – |
| Q2 (−758.52) | 1.67 (1.17–2.38) | 0.004 | 4.39 (2.56–7.50) | <0.001 | 2.19 (1.59–3.02) | <0.001 |
| Q3 (−849.62) | 4.12 (2.83–6.01) | <0.001 | 15.33 (8.92–26.33) | <0.001 | 6.25 (4.42–8.84) | <0.001 |
| Q4 (≥849.63) | 4.99 (3.25–7.66) | <0.001 | 38.69 (22.01–68.02) | <0.001 | 11.19 (7.62–16.42) | <0.001 |
All indices were divided into quartiles and examined by multinomial logistic analysis. P value was adjusted for age, sex, systolic blood pressure and diastolic blood pressure
VAI visceral adiposity index, BMI body mass index, WC waist circumference, TyG triglyceride glucose index, TyG-BMI combined TyG and BMI, TyG-WC combined TyG and WC
The results of ROC analyses and AUCs with their corresponding 95 % CIs for VAI, BMI, WC, TyG, TyG-BMI and TyG-WC were shown in Fig. 2. For prediabetes, the largest AUC was observed in VAI (AUC = 0.600, 95 % CIs 0.569–0.631, Grade: D), followed by TyG (AUC = 0.557, 95 % CIs 0.526–0.587, Grade: F) and TyG-WC (AUC = 0.544, 95 % CIs 0.513–0.575, Grade: F). For diabetes, the largest AUC was showed in TyG-WC (AUC = 0.767, 95 % CIs 0.743–0.791, Grade: C), followed by TyG (AUC = 0.748, 95 % CIs 0.722–0.774, Grade: C) and WC (AUC = 0.709, 95 % CIs: 0.682–0.735, Grade: C). For mixed prediabetes and diabetes, the largest AUC was also showed in TyG-WC (AUC = 0.765, 95 % CIs 0.741–0.789, Grade: C), followed by TyG (AUC = 0.759, 95 % CIs 0.735–0.783, Grade: C) and WC (AUC = 0.703, 95 % CIs 0.677–0.730, Grade: C). Taking the odds ratio and the AUC value into consideration, TyG-WC may be regarded as an optimal marker for predicting prediabetes and diabetes in those participants.
Fig. 2.
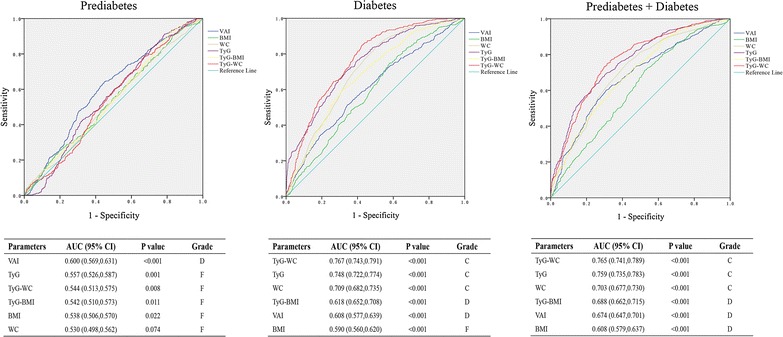
Receiver operating characteristic (ROC) curve analysis of each index. Area under the ROC curve (AUC) and 95 % CIs of each index was shown in tables below. VAI visceral adiposity index, BMI body mass index, WC waist circumference, TyG triglyceride glucose index, TyG-BMI combined TyG and BMI, TyG-WC combined TyG and WC. Grade: 0.90–1 = excellent (A), 0.80–0.90 = good (B), 0.70–0.80 = fair (C), 0.60–0.70 = poor (D) and 0.50–0.60 = fail (F)
Clinical outcomes at the final follow-up
Data from 184 men and 268 women with a median age at baseline of 51.0 and 48.0 years respectively were observed for an average of 5 years (4.62 ± 0.99). During the 2013 person-years of follow-up, 75 of the 452 participants were identified as newly occurred diabetes patients and the total incidence of diabetes was 3.7 per 100 person-years. When stratified by quartiles of TyG-WC, the incidences of diabetes, from Quartile1 to 4, were 1.2, 2.1, 5.1 and 9.6 per 100 person-years, respectively. In addition, the cumulative rates of incident diabetes from Q1 to Q4 were 5.9, 9.8, 21.9 and 35.9 %, respectively (Table 3).
Table 3.
Incidence of diabetes according to TyG-WC quartiles
| TyG-WC | Subjects N | New DM N | Person-years | DM Incidence/100 person-years | Cumulative incidence of DM (%) |
|---|---|---|---|---|---|
| Q1 (≤668.00) | 153 | 9 | 740 | 1.2 | 5.9 |
| Q2 (−758.52) | 102 | 10 | 477 | 2.1 | 9.8 |
| Q3 (−849.62) | 105 | 23 | 451 | 5.1 | 21.9 |
| Q4 (≥849.63) | 92 | 33 | 345 | 9.6 | 35.9 |
DM diabetes mellitus, TyG-WC triglyceride glucose-waist circumference index
The predictive value of TyG-WC
As compared to individuals with the lowest TyG-WC (Table 4), those who had the highest TyG-WC were at 7.13-fold risk of diabetes (95 % CIs 3.41–14.90). The positive trend between TyG-WC level and diabetes risk was attenuated but still remarkable after adjusting for age, sex, SBP, DBP, TC and LDL-C (HR: 3.69, 95 % CIs 1.65–8.28). Additionally, according to the results of ROC curve and the Youden Index, the optimal cutoff point of TyG-WC was 760.06, with the sensitivity of 74.7 % and the specificity of 63.1 %. Meanwhile, the positive and negative predictive values at this point were 28.7 and 92.6 %, respectively.
Table 4.
Hazard Ratio (95 % CI) of diabetes risk according to TyG-WC quartiles
| TyG-WC | Crude | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Q1 (≤668.00) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| Q2 (−758.52) | 1.70 (0.69–4.19) | 1.58 (0.64–3.92) | 1.33 (0.53–3.30) | 1.21 (0.48–3.05) |
| Q3 (−849.62) | 4.01 (1.86–8.66)*** | 3.55 (1.60–7.88)** | 2.71 (1.21–6.05)* | 2.34 (1.01–5.42)* |
| Q4 (≥849.63) | 7.13 (3.41–14.90)*** | 6.41 (2.96–13.90)*** | 4.87 (2.23–10.65)*** | 3.69 (1.65–8.28)** |
Model 1 adjusted for age and sex
Model 2 adjusted for Model 1 added SBP, DBP
Model 3 adjusted for Model 2 added TC, LDL-C
* P < 0.05
** P < 0.01
*** P < 0.001
Discussion
In the cross-sectional study, we directly compared six parameters (BMI, WC, VAI, TyG, TyG-BMI and TyG-WC) as predictors of prediabetes and diabetes in FDRs of T2DM patients. Overall, we found that TyG-WC outperformed other predictors with a higher OR and a larger AUC. Moreover, in the prospective study, we observed that subjects in the highest quartile of TyG-WC had 3.7-fold risk of diabetes for those in the lowest quartile even after the adjustment of potential compounders, which indicated that TyG-WC was an independent predictor of diabetes in FDRs of T2DM patients.
Previous studies have indicated that both genetic and environmental factors contribute to the development of diabetes [11–13]. Of note, the prevalence of the multifactorial disease and potential population of FDRs of T2DM patients are increasing obviously with the change of lifestyle [14]. FDRs of T2DM patients are regarded as high-risk diabetic populations, considering the genetic predisposition and the similar lifestyle [15, 16]. In the current study, the crude prevalence of diabetes in FDRs was 30.1 % and the age-standardized prevalence was 15.6 %, which was higher than the national prevalence of diabetes in China (9.7 %) [17]. Furthermore, Du et al. [18] demonstrated that the prevalence of diabetes was independently associated with an increasing family history risk level. Hence, more attention to FDRs of T2DM patients should be paid in the clinical diagnosis and treatment of diabetes in an early stage, though the contribution of genetic factors to the pathological development of diabetes remains obscure.
The strong relationship between obesity and diabetes has been mentioned in many studies [19–21]. Oti et al. [22] found that obesity is closely associated with high blood glucose. Matsuda et al. [23] maintained that adipose tissue is the main source of reactive oxygen species, which may contribute to a variety of metabolic problems, including obesity-associated IR and T2DM. As simple, cheap and noninvasive anthropometric parameters, BMI and WC are commonly adopted as useful indicators of obesity and metabolic risk. However, recent studies indicated that some populations show unexpected metabolic profiles that deviate from the typical dose-response relationship between BMI and metabolic disturbances [24–26]. In the current study, we also found the association between BMI and abnormal glucose metabolism was weaker than that of WC when considering the lower odds ratios and AUCs of BMI. These results may be explained by the different roles of BMI and WC in the evaluation of adiposity status [27]. BMI, a measure of body fat based on weight and height, stands for general obesity, while WC, a measure of abdominal fat, stands for central obesity. The National Cholesterol Education Program-Adult Treatment Panel-III suggested that central obesity is an independent risk factor for T2DM, and measuring WC is an inexpensive tool to screen risk of diabetes [28]. Therefore, WC may be more effective than BMI. However, WC cannot sufficiently discriminate between visceral and subcutaneous fat. Accumulating evidence has demonstrated that visceral adipose tissue plays more critical roles in the development of insulin resistance and diabetes than subcutaneous fat. Molecular mechanisms responsible for the differences are still under discussion. It has been suggested that visceral fat produces more free fatty acid than subcutaneous fat, thus increases the risk of IR and diabetes [29]. Moreover, visceral adipose secretes various inflammatory cytokines and adipokines, which may also promote the occurrence of IR and diabetes [30, 31].
Besides obesity, increased FPG levels have also been demonstrated as an independent risk factor for developing T2DM [32–34]. Moreover, elevated TG levels over time also enhance the risk of developing diabetes in various populations [35–38]. Additionally, Guerrero-Romero et al. suggested that, TyG index, the product of FPG and TG, could be a surrogate index of insulin resistance due to its high sensitivity similar to euglycemic-hyperinsulinemic clamp test [6]. Meanwhile, it is also proposed that TyG index is a valuable marker for predicting the risk of future diabetes in both men and women [39]. Given that insulin resistance is the core pathological mechanism of T2DM and always occurs before the diagnosis of T2DM [40, 41], surrogate indices of insulin resistance might aid in the prediction of incident diabetes. In our study, we found TyG-WC, the combination of adiposity status and TyG, was a better marker for early predicting the risk of prediabetes and diabetes. The superiority of TyG-WC might be achieved as TG, FPG and obesity are well validated for their roles in IR and the development of diabetes. These results also support that both glucotoxicity and lipotoxicity play crucial roles in the pathogenesis of diabetes.
The visceral adiposity index (VAI), a mathematical model based on BMI, WC, TG and HDL-C, is another predictor of prediabetes and diabetes demonstrated by several research, although the relationship might differ by ethnicity [42, 43]. In our study, we found the association between VAI and diabetes was weaker than that of TyG related parameters. However, it was noteworthy that VAI was better correlated with prediabetes than with diabetes, which was commensurate with the study of Yang et al. [44]. Underlying mechanisms are still unclear. Possible explanation might be that subjects with prediabetes have better glucose regulation than those with diabetes, so the effect of glucotoxicity was slight in this stage. Thus, VAI, an index stands for the condition of obesity and lipid levels, is closely related to prediabetes while TyG and related parameters are well associated with diabetes.
In the follow-up study, we further evaluated the clinical outcomes of participants and confirmed the predictive value of TyG-WC. Notably, the incidences of diabetes were significantly increased in sequence of quartiles of TyG-WC. Furthermore, compared with participants in the highest quartile of TyG-WC value, the hazard ratio of incident diabetes was more than threefold for those in the lowest quartile after adjusting for age, gender, blood pressure and other potential compounders, which demonstrated the close association between TyG-WC and diabetes risk.
Several limitations may exist in this study. First, the results might have potential bias due to the single-center design. Second, some potential bias from socio-economic background and general diet intake were not well controlled. Third, the number of participants in the follow-up study was relative small. Fourth, the results were obtained from FDRs of diabetes patients, and further investigations were required in other populations.
Conclusions
TyG-WC is a valuable marker for predicting the risk of prediabetes and diabetes in FDRs of T2DM patients. Because it can be easily calculated from routine laboratory data, we suggest the possibility of applying this index in risk assessment in real clinical practice or epidemiologic survey.
Authors’ contributions
SZ, SS and XR attended the data collection, statistical analysis, data interpretation, manuscript writing and revision. TH, YL and YC contributed to the acquisition and interpretation of the data. WL and PH contributed to the revision of the paper. YH contributed to acquisition of funding, study design, and revision of the paper. All authors revised and approved the final manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the staff of the Endocrinology and Metabolism Laboratory and the nursing staff for their dedicated assistance in patient sample collection.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its Additional file 1.
Ethics approval and consent to participate
The study protocol was in agreement with Helsinki declaration and the study was approved by the Ethical Committee of Renji Hospital, School of Medicine, Shanghai Jiaotong University. Written informed consents were signed from all participants included in the study.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81270946, 81170758, 30670988) and the Foundation from Renji Hospital, School of Medicine, Shanghai Jiaotong University (RJZZ14-003).
Abbreviations
- BMI
body mass index
- CGI
combined glucose intolerance
- HDL-C
high density lipoprotein cholesterol
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- LDL-C
low density lipoprotein cholesterol
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- TC
total cholesterol
- TG
triglyceride
- TyG
triglyceride glucose index
- VAI
visceral adiposity index
- WC
waist circumference
Additional file
10.1186/s12967-016-1020-8 Clinical dataset.
Footnotes
Shuang Zheng, Sheng Shi and Xingxing Ren contributed equally to this work
Contributor Information
Shuang Zheng, Email: 75548043@qq.com.
Sheng Shi, Email: shisheng273@163.com.
Xingxing Ren, Email: 1275163770@qq.com.
Tingting Han, Email: htt86226@163.com.
Yangxue Li, Email: 1756366571@qq.com.
Yawen Chen, Email: kat0401@163.com.
Wei Liu, Email: sue_liuwei@163.com.
Peter C. Hou, Email: phou@bwh.harvard.edu
Yaomin Hu, Email: amin1031@sina.cn.
References
- 1.Keating ST, Plutzky J, El-Osta A. Epigenetic changes in diabetes and cardiovascular risk. Circ Res. 2016;118(11):1706–1722. doi: 10.1161/CIRCRESAHA.116.306819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma H, Gong Y, Liu YY, Song J, Tian HM, Chen T, et al. Prevalence of diabetes and prediabetes mellitus in the first-degree relatives of patients with type 2 diabetes in Chengdu. Sichuan Da Xue Xue Bao Yi Xue Ban. 2011;42(2):264–268. [PubMed] [Google Scholar]
- 3.Hunter GR, Chandler-Laney PC, Brock DW, Lara-Castro C, Fernandez JR, Gower BA. Fat distribution, aerobic fitness, blood lipids, and insulin sensitivity in African–American and European–American women. Obesity (Silver Spring). 2010;18(2):274–281. doi: 10.1038/oby.2009.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryoo JH, Park SK, Ye S, Choi JM, Oh CM, Kim SY, et al. Estimation of risk for diabetes according to the metabolically healthy status stratified by degree of obesity in Korean men. Endocrine. 2015;50(3):650–658. doi: 10.1007/s12020-015-0635-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Xu Y, Guo ZR, Yang J, Wu M, Hu XS. The application of visceral adiposity index in identifying type 2 diabetes risks based on a prospective cohort in China. Lipids Health Dis. 2014;13:108. doi: 10.1186/1476-511X-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du T, Sun X, Huo R, Yu X. Visceral adiposity index, hypertriglyceridemic waist and risk of diabetes: the China Health and Nutrition Survey 2009. Int J Obes (Lond). 2014;38(6):840–847. doi: 10.1038/ijo.2013.181. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic–hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 8.Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11(3):e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melnik BC, John SM, Schmitz G. Milk consumption during pregnancy increases birth weight, a risk factor for the development of diseases of civilization. J Transl Med. 2015;13:13. doi: 10.1186/s12967-014-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Tian H, Zhang F, Zhang C, Li Y, Leng J, et al. A randomised translational trial of lifestyle intervention using a 3-tier shared care approach on pregnancy outcomes in Chinese women with gestational diabetes mellitus but without diabetes. J Transl Med. 2014;12:290. doi: 10.1186/s12967-014-0290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soldati L, Bertoli S, Terranegra A, Brasacchio C, Mingione A, Dogliotti E, et al. Relevance of Mediterranean diet and glucose metabolism for nephrolithiasis in obese subjects. J Transl Med. 2014;12:34. doi: 10.1186/1479-5876-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 15.de Visser KL, Landman GW, Kleefstra N, Meyboom-de Jong B, de Visser W, te Meerman GJ, et al. Familial aggregation between the 14th and 21st century and type 2 diabetes risk in an isolated Dutch population. PLoS ONE. 2015;10(7):e0132549. doi: 10.1371/journal.pone.0132549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Yang Z, Xiao J, Xing X, Lu J, Weng J, et al. Association between family history risk categories and prevalence of diabetes in Chinese population. PLoS ONE. 2015;10(2):e0117044. doi: 10.1371/journal.pone.0117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 18.Du X, Zhang Y, Gao F, Lu H, Shen Y, Chen R, et al. The influence of family history risk levels of diabetes on disease prevalence in a high-risk diabetic Chinese population. Diabetes Technol Ther. 2016 doi: 10.1089/dia.2016.0023. [DOI] [PubMed] [Google Scholar]
- 19.Ishola AF, Gerstein HC, Engert JC, Mohan V, Diaz R, Anand SS, et al. Longitudinal relationships between glycemic status and body mass index in a multiethnic study: evidence from observational and genetic epidemiology. Sci Rep. 2016;6:30744. doi: 10.1038/srep30744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma S, Hussain ME. Obesity and diabetes: an update. Diabetes Metab Syndr. 2016 doi: 10.1016/j.dsx.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Raghavan S, Pachucki MC, Chang Y, Porneala B, Fox CS, Dupuis J, et al. Incident type 2 diabetes risk is influenced by obesity and diabetes in social contacts: a social network analysis. J Gen Intern Med. 2016 doi: 10.1007/s11606-016-3723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oti SO, van de Vijver SJ, Agyemang C, Kyobutungi C. The magnitude of diabetes and its association with obesity in the slums of Nairobi, Kenya: results from a cross-sectional survey. Trop Med Int Health. 2013;18(12):1520–1530. doi: 10.1111/tmi.12200. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7(5):e330–e341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89(6):2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 25.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91(8):2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 26.Malara M, Keska A, Tkaczyk J, Lutoslawska G. Body shape index versus body mass index as correlates of health risk in young healthy sedentary men. J Transl Med. 2015;13:75. doi: 10.1186/s12967-015-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansego ML, De Marco G, Ivorra C, Lopez-Izquierdo R, Morcillo S, Rojo-Martinez G, et al. The nutrigenetic influence of the interaction between dietary vitamin E and TXN and COMT gene polymorphisms on waist circumference: a case control study. J Transl Med. 2015;13:286. doi: 10.1186/s12967-015-0652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotto AM. NCEP ATP III guidelines incorporate global risk assessment. Am J Manag Care. 2003;1:3. [PubMed] [Google Scholar]
- 29.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 30.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 31.Savastano S, Tarantino G, D’Esposito V, Passaretti F, Cabaro S, Liotti A, et al. Bisphenol-A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: a cross-sectional study on adult male population. J Transl Med. 2015;13:169. doi: 10.1186/s12967-015-0532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353(14):1454–1462. doi: 10.1056/NEJMoa050080. [DOI] [PubMed] [Google Scholar]
- 33.Brambilla P, La Valle E, Falbo R, Limonta G, Signorini S, Cappellini F, et al. Normal fasting plasma glucose and risk of type 2 diabetes. Diabetes Care. 2011;34(6):1372–1374. doi: 10.2337/dc10-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121(6):519–524. doi: 10.1016/j.amjmed.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Hjellvik V, Sakshaug S, Strom H. Body mass index, triglycerides, glucose, and blood pressure as predictors of type 2 diabetes in a middle-aged Norwegian cohort of men and women. Clin Epidemiol. 2012;4:213–224. doi: 10.2147/CLEP.S31830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tirosh A, Shai I, Bitzur R, Kochba I, Tekes-Manova D, Israeli E, et al. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care. 2008;31(10):2032–2037. doi: 10.2337/dc08-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maturu A, DeWitt P, Kern PA, Rasouli N. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of beta-cell function in African American women. Metabolism. 2015;64(5):561–565. doi: 10.1016/j.metabol.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Zheng S, Zhou H, Han T, Li Y, Zhang Y, Liu W, et al. Clinical characteristics and beta cell function in Chinese patients with newly diagnosed type 2 diabetes mellitus with different levels of serum triglyceride. BMC Endocr Disord. 2015;15:21. doi: 10.1186/s12902-015-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS ONE. 2014;9(2):e90430. doi: 10.1371/journal.pone.0090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J. 2011;35(2):107–116. doi: 10.4093/dmj.2011.35.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu PJ, Ma F, Lou HP, Chen Y. Visceral adiposity index is associated with pre-diabetes and type 2 diabetes mellitus in Chinese adults aged 20–50. Ann Nutr Metab. 2016;68(4):235–243. doi: 10.1159/000446121. [DOI] [PubMed] [Google Scholar]
- 43.Bozorgmanesh M, Hadaegh F, Azizi F. Predictive performance of the visceral adiposity index for a visceral adiposity-related risk: type 2 diabetes. Lipids Health Dis. 2011;10:88. doi: 10.1186/1476-511X-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y, Feng Y, Ma X, Chen K, Wu N, Wang D, et al. Visceral adiposity index and insulin secretion and action in first-degree relatives of subjects with type 2 diabetes. Diabetes Metab Res Rev. 2015;31(3):315–321. doi: 10.1002/dmrr.2615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its Additional file 1.


