Abstract
Background
Kawasaki disease (KD) is a pediatric vasculitis with coronary artery aneurysm (CAA) as a major complication. Controversy exists about cardiovascular risk later in life. The aim of our study was to evaluate whether KD patients are at increased risk, as assessed by carotid intima‐media thickness (cIMT).
Methods and Results
We measured cIMT over 15 years by B‐mode ultrasonography in KD patients during follow‐up and in unaffected controls (mostly siblings). A multilevel, repeated‐measures, linear mixed‐effects model was used to evaluate the association between KD and cIMT. A total of 319 patients with 528 measurements were compared with 150 controls. In KD patients, the mean cIMT was increased compared with controls (0.375 mm [95% CI 0.372–0.378 mm] versus 0.363 mm [95% CI 0.358–0.368 mm]; P<0.001). Furthermore, mean cIMT of CAA‐negative patients was 0.373 mm (P<0.01 compared with controls), of patients with small–medium CAA was 0.374 mm (P<0.05 compared with controls), and of patients with giant CAA was 0.381 mm (P<0.01 compared with controls). Compared with controls, CAA‐negative participants started with an increased cIMT (+0.0193±0.0053 mm, P<0.001) but showed slower progression (−0.0014±0.0006 mm/year, P=0.012). Patients with giant CAA showed a trend toward increased cIMT progression (0.0013±0.0007 mm/year, P=0.058).
Conclusions
We observed a positive correlation between cIMT and KD severity of coronary arteritis at the acute stage. Although initially increased, the cIMT in CAA‐negative patients normalized at a later age. In contrast, patients with a history of KD complicated by giant CAA showed a trend toward persistently increased cIMT. These patients may need cardiovascular counseling and follow‐up beyond the heart.
Keywords: coronary aneurysm, intima‐media thickness, Kawasaki disease
Subject Categories: Peripheral Vascular Disease, Coronary Artery Disease, Pediatrics, Cardiovascular Disease
Introduction
Kawasaki disease (KD) is an acute systemic vasculitis occurring predominantly in children aged <5 years.1 The main complication of this disease is the development of coronary artery aneurysm (CAA). CAA develops in 15% to 25% of untreated patients.2
KD is the leading cause of acquired heart disease in developed countries.3 Standard treatment consists of a single administration of high‐dose intravenous immunoglobulins (IVIG) and oral aspirin for 6 to 8 weeks. This has been shown to reduce the risk of CAA to <10% when treated within 10 days.4 Both the etiology and pathophysiology of KD as well as the working mechanism of IVIG have remained unclear to date.
It has been hypothesized that KD represents a systemic vasculitis that, apart from the absence or presence of CAA, results in increased cardiovascular disease risk at a later age. Although controversial, this hypothesis was supported by abnormal myocardial perfusion, as shown by nuclear scintigraphy, even when echocardiography of the arteries was unremarkable.5 Dysfunctional vasculature, however, may not be limited to the heart, and cardiovascular disease may be more widespread than just to the coronary arteries, as shown by increased flow‐mediated dilatation of the brachial artery after KD.6 In addition, a study by Kato et al followed 594 patients from the acute KD phase up to 20 years afterward.7 In 2.2% of the patients, they found more widespread disease with extracardiac vascular lesions, although this study was performed at a time when IVIG infusions were not routine.
Carotid intima‐media thickness (cIMT) is a well‐validated noninvasive surrogate marker for cardiovascular disease risk in multiple populations.8, 9, 10
In several studies, cIMT was compared between participants with a history of KD and unaffected controls.11, 12, 13 Some found increased cIMT in all KD patients or in CAA‐positive patients compared with controls, whereas others did not find any difference; therefore, results were conflicting, and often the studies showed methodological limitations.6 Furthermore, the included studies had a cross‐sectional design or were cohort studies with short follow‐up (<6 months) and were not able to show the course of the cIMT change over time. In our own cross‐sectional study, we observed that children with KD had significantly increased cIMT following early disease compared with siblings.14 In a large number of patients, ≥2 cIMT measurements were obtained. Having collected these cIMT data over a period of almost 15 years, we investigated whether KD patients had increased cIMT in a data set with long‐term follow‐up and whether these data supported our previous conclusions from our initial cross‐sectional study data.
Methods
Participants
The study was conducted between October 2001 and December 2014 at the Emma Children's Hospital, a tertiary referral center. Participants with a history of KD, based on criteria of the American Heart Association, were recruited consecutively during follow‐up as outpatients.3 Patients in the acute or subacute (specified as within 6 months after the disease) phase of KD were excluded to minimize the potential confounding influence of acute or subacute inflammation.
Unaffected siblings of children with KD and other unaffected persons (family of staff members and staff of our hospital) without a history of KD were eligible as controls if they did not take any cardiovascular medication. All participants and/or their parents gave informed consent, as approved by the institution's research ethics board.
Study Protocol
Blood pressure, body height, and weight were measured in participants. Using data from the fifth Dutch growth study performed in 2009 in 20 867 children in the Netherlands, standard deviation scores for body mass index (BMI) were calculated based on the age and sex of each participant (http://groeiweb.pgdata.nl/calculator.asp [in Dutch]).
The medical records of the KD patients were retrospectively reviewed to collect clinical details: age at onset of disease, treatment with IVIG, and presence of CAA. The coronary arteries had been evaluated by 2‐dimensional echocardiography. CAAs were specified by worst‐ever z scores: z scores adjusted for basal surface area.15, 16 We defined the CAAs by their worst‐ever scores because arteries may be damaged even when the lumen of a previously affected coronary artery has returned to its normal size, indicating more severe initial systemic vasculitis compared with children who never had enlargement. CAA was defined as a coronary z score ≥2.5, and a giant aneurysm was defined as a z score ≥10 or a diameter of ≥8 mm.
Following KD, after an overnight fast, a venous blood sample was taken to measure total cholesterol, high‐ and low‐density lipoprotein cholesterol, and triglycerides.
We measured cIMT in the participants with a history of KD when they visited the outpatient clinic from the age of 5 years onward. Siblings who were aged ≥7 years were invited for a cIMT measurement once.
Measurements of cIMT
All participants were scanned by 2 experienced and certified sonographers. Over the course of 14 years, 3 ultrasound machines were used: an Acuson 128XP (October 2001 to June 2006), an Acuson Aspen (June 2006 to January 2008), and an Acuson Sequoia 512 (January 2008 to January 2015). L7 linear array vascular transducers were used on the Acuson 128 XP and Aspen, and an 8L5 transducer was used on the Acuson Sequoia (Siemens AG).
All scans were performed according to a validated and standardized scanning and image analysis protocol. Briefly, in all participants, the right and left common carotid, carotid bulb, and internal carotid arterial wall segments were visualized. For each segment, a 5‐second cine‐loop of 25 full frames per second was temporarily saved in the memory of the ultrasound equipment. Because cIMT is known to change slightly (≈6–8%) during the cardiac cycle, cIMT measurement at a fixed point in the arterial cycle is preferred.17
All ultrasound instruments were equipped with a dedicated carotid scan protocol in which a high‐persistence scan setting was used. Because of rapid arterial wall movement, this high‐persistence setting provides a slight movement artifact on the averaged image when the artery is in systole, at which, at the relative resting phase of the diastole, the averaged image of the arterial wall is crisp. From the cine‐loop, the sonographer was trained and certified to select and save the highest quality and crispest image frame of the segment as a 2×2‐cm high‐resolution DICOM still. The scan protocol is described in full elsewhere.18
The image analyses were done offline. The mean combined cIMT per participant was calculated as follows: ([mean of the left and right common carotid arteries]+[mean of the left and right carotid bulb]+[mean of the left and right internal carotid arteries])/3. For participants in whom 1 of the segments failed, the cIMT of the same segment of the opposite carotid artery was taken as the mean of both carotid arteries. When both sides failed, the segment was considered missing.
One image analyst performed all cIMT measurements manually and was blinded to the patients' case and CAA status. Twenty images of the Acuson Sequoia were analyzed twice to assess intrarater reliability. The intraclass correlation coefficient was 0.92 (95% CI 0.75–0.97) for the mean cIMT.
The Acuson Sequioa, compared with the less advanced and technically similar XP and Aspen, has improved hardware, software, and transducer properties regarding signal‐to‐noise ratio, image display/pixel density, and image file size and format. To allow for comparison of cIMT data of different machines, normalization of measurements is required; therefore, we created a correction factor for the scans provided by the different instruments. In 10 volunteers on the same day, the most reliable artery segment—the common carotid artery far walls—was scanned on the Acuson Aspen and Sequoia. This comparison revealed systematic differences in cIMT between instruments. Subsequently, we evaluated cIMT data for comparable age groups of all KD study participants by ultrasound instruments. This statistical evaluation of cIMT participant data revealed the same and systematic differences in cIMT between instruments as those of the volunteer scans. Based on both calculations (Δ mean cIMT and measurement differences within the cohort), a correction factor was applied with the most advanced ultrasound instrument, the Acuson Sequoia, as the reference.
Statistics
We evaluated differences in age and sex between patients and controls at the time of the first cIMT measurement by using Mann–Whitney U and chi‐square tests, respectively. Differences in the remaining demographics (length, weight, mean arterial pressure, and BMI standard deviation score) between patients with KD and controls were assessed by linear regression analysis, taking family bonds into account by creating a random term. Nonnormal variables were log‐transformed before analysis. Differences in demographics between KD subgroups were evaluated by ANOVA for parameters with normal distribution, by Kruskal–Wallis test for parameters with a nonnormal distribution, and by chi‐square test for binary parameters.
A multilevel, repeated‐measures, linear mixed‐effects model was used to evaluate the association between KD and cIMT. In the first model, all KD patients were compared with controls; in the second model, 4 groups were compared: controls, CAA‐negative patients, patients with small–medium aneurysms, and patients with giant aneurysms. The analyses were adjusted for potentially confounding variables (age, sex, mean arterial pressure, and BMI standard deviation score), which were entered as fixed effects. In addition, family relations were taken into account and were adjusted for by creating a random term. Because measurements started at age 5 in patients, we calculated the intercept at this age.
To evaluate whether IVIG treatment, total cholesterol, low‐density lipoprotein cholesterol, and triglycerides were of significant influence on cIMT, we also performed—in patients only—a linear mixed‐effect analysis including these variables.
Multiple imputation was performed for missing blood pressure (30%) and missing segment (4.7%) values. For each missing value, 5 imputations were performed based on age, weight, height, BMI standard deviation score, and the remaining segments that did not fail. These were subsequently combined into 1 effect estimate.
A P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 22.0 software (IBM Corp). A figure was created using R statistics version 3.0.1 (R Foundation for Statistical Computing).
Results
A total of 319 participants with a history of KD were included, with a median age of 8.1 years during their first cIMT measurement (range 5.0–43.3 years). In these patients, 528 cIMT measurement were performed. Of these cases, 171 KD patients had a single measurement and 148 had ≥2 cIMT data sets. A total of 150 controls (130 siblings and 20 unrelated participants) were included with a median age of 12.5 years (range 7.0–31.1 years). The demographic characteristics of patients and controls during the first cIMT measurement are shown in Table 1. The number of cIMT measurements per subgroup are listed in Table 2.
Table 1.
Demographics of Controls and Kawasaki Disease Subgroups at the Time of Their First cIMT Measurement
| Controls (n=150) | Kawasaki Disease Patients (n=319) | P Value | CAA Negative (n=241) | Small–Medium Coronary Artery Aneurysm (n=51) | Giant Coronary Artery Aneurysm (n=27) | P Value | |
|---|---|---|---|---|---|---|---|
| Age, ya | 12.5 (9.6–15.6) | 8.1 (6.4–12.0) | <0.001b | 7.9 (6.3–12.0) | 7.7 (6.3–11.4) | 11.9 (8.5–16.4) | 0.001 |
| Sex (% male) | 53.3 | 60.5 | 0.160b | 54.8 | 72.5 | 88.9 | <0.001 |
| Length, cm | 157±18 | 139±22 | <0.001 | 138±22 | 136±21 | 154±20 | 0.002 |
| Weight, kga | 46.5 (31.5–62.0) | 29.0 (23.0–45.0) | <0.001 | 28.2 (22.6–43.6) | 27.9 (21.4–44.5) | 42.9 (29.4–67.8) | 0.004 |
| BMI standard deviation score | 0.19±1.08 | 0.46±1.16 | 0.081b | 0.43±1.12 | 0.59±1.27 | 0.42±1.29 | 0.083 |
| Mean arterial pressure, mm Hg | 77.2±8.1 | 76.2±9.2 | 0.261b | 76.1±8.8 | 76.5±9.5 | 76.5±11.3 | 0.957 |
| Ethnicity (%) | |||||||
| White | 136 (91) | 246 (77) | 190 (79) | 37 (73) | 19 (70) | ||
| Black | 0 (0) | 9 (3) | 7 (3) | 2 (4) | 0 (0) | ||
| Mediterranean | 0 (0) | 18 (6) | 13 (5) | 3 (6) | 2 (7) | ||
| Mixed | 9 (6) | 27 (8) | 17 (7) | 6 (12) | 4 (15) | ||
| Otherc | 5 (3) | 13 (4) | 9 (4) | 2 (4) | 2 (7) | ||
| Unknown | 0 (0) | 6 (2) | 5 (2) | 1 (2) | 0 (0) | ||
BMI indicates body mass index; CAA, coronary artery aneurysm; cIMT, carotid intima‐media thickness.
Median (interquartile range).
These variables were adjusted for in the model.
Mainly consisting of individuals of indo‐Surinamese and Asian descent.
Table 2.
Number of cIMT Measurements Per Subgroup
| Controls (n=150) | Kawasaki Disease Patients (n=319) | CAA Negative (n=241) | Small–Medium Coronary Artery Aneurysm (n=51) | Giant Coronary Artery Aneurysm (n=27) | |
|---|---|---|---|---|---|
| 1 cIMT measurement | 150 | 171 | 143 | 20 | 8 |
| 2 cIMT measurements | — | 98 | 64 | 19 | 15 |
| 3 cIMT measurements | — | 40 | 28 | 8 | 4 |
| 4 cIMT measurements | — | 9 | 5 | 4 | 0 |
| 5 cIMT measurements | — | 1 | 1 | 0 | 0 |
| Total | 150 | 528 | 380 | 98 | 50 |
CAA indicates coronary artery aneurysm; cIMT, carotid intima‐media thickness.
Of the 319 participants with a history of KD, 241 (75.5%) had a worst‐ever coronary artery z score <2.5. The other participants had CAA, of whom 51 (16.0%) had CAA with a z score of 2.5 to 10 and 27 (8.5%) had giant aneurysms, with a z score of ≥10 and/or a diameter of ≥8 mm.
There was no significant difference in sex between patients and controls, but there were significantly more male participants in the small–medium CAA group (P=0.02) and in the giant CAA group (P<0.001) compared with the CAA‐negative patients.
The clinical characteristics of KD patients and the KD subgroups are shown in Table 3. There was no significant difference in percentage of IVIG treatment between the groups. Patients with small–medium CAA were significantly younger during their disease compared with patients without CAA. There was no significant difference in age at KD between patients with giant aneurysms and CAA‐negative patients.
Table 3.
Clinical Characteristics of the Kawasaki Disease Patients
| Kawasaki Disease Patients (n=319) | CAA Negative (n=241) | Small–Medium CAA (n=51) | Giant CAA (n=27) | P Value | |
|---|---|---|---|---|---|
| Age at disease onset, ya | 3.3 (1.2–5.3) | 3.5 (1.7–5.4) | 1.2 (0.4–4.7) | 2.7 (0.4–5.7) | 0.003 |
| Time since Kawasaki disease at first ultrasound, yearsa | 5.0 (2.2–8.0) | 4.6 (1.8–7.7) | 5.5 (3.1–7.1) | 8.6 (5.5–13.2) | <0.001 |
| Intravenous immunoglobulin–treated patients, % | 89.7 | 90.5 | 90.2 | 81.5 | 0.345 |
| Median follow‐up time, yearsa, b | 4.1 (3.0–5.6) | 4.2 (3.0–5.9) | 4.3 (3.0–6.2) | 3.6 (2.2–4.6) | 0.193 |
CAA indicates coronary artery aneurysm.
Median (interquartile range).
Median follow‐up time between first and last carotid intima‐media thickness measurement of patients with >1 measurement (total n=148; CAA‐negative, n=98; small–medium CAA, n=31; giant CAA, n=19).
Carotid Intima‐Media Thickness
Patients with a history of KD had a significantly higher estimated marginal mean compared with controls (0.375 mm [95% CI 0.372–0.378 mm] versus 0.363 mm [95% CI 0.358–0.368 mm]; P=0<0.001). The model for the longitudinal cIMT data analysis over time shows that patients with a history of KD started with a higher cIMT (intercept +0.0145 mm [95% CI: 0.0042–0.0248 mm; P=0.006] at age 5 compared with controls). There was no difference in increase per age‐year (−0.0004 mm [95% CI −0.0014 to 0.0007 mm; P=0.490] increase per year compared with controls).
In comparing the different groups based on CAA status in our KD cohort, we found that the estimated marginal mean of CAA‐negative patients was 0.373 mm (95% CI 0.369–0.376 mm; P<0.01 compared with controls), of patients with small–medium CAA was 0.374 mm (95% CI 0.367–0.382 mm; P<0.05 compared with controls), and of patients with giant aneurysms of 0.381 mm (95% CI 0.370–0.392 mm; P<0.01 compared with controls).
The complete model shown in Table 4 indicates that, compared with controls, CAA‐negative patients started with a significantly higher cIMT at the age of 5 years (+0.0193 mm [95% CI 0.0089–0.0297 mm]; P<0.001), but this difference decreased per year (−0.0014 mm per year [95% CI −0.0025 to −0.0003 mm]; P=0.012). There was no significant difference between controls and patients with small–medium CAA either in cIMT at the age of 5 years or in cIMT progression per year. Compared with controls, patients with giant CAA had a higher but nonsignificant cIMT level at age 5 years and a trend toward increased cIMT progression per year (0.0013 mm per year, [95% CI −0.0000 to 0.0027 mm]; P=0.058).
Table 4.
Association Between cIMT and Kawasaki Disease Subgroups and Controls
| Estimated Intercept at Age 5 Years ±SE (mm) | P Value | Estimated β ±SE (mm) | P Value | |
|---|---|---|---|---|
| CAA status; controls as reference | ||||
| No enlargement | 0.0193±0.0053 | <0.001 | −0.0014±0.0006a | 0.012 |
| Small or medium CAA | 0.0105±0.0072 | 0.144 | 0.0001±0.0008a | 0.869 |
| Giant CAA | 0.0087±0.0090 | 0.335 | 0.0013±0.0007a | 0.058 |
| CAA status; patients with no enlargement as reference | ||||
| Small or medium CAA | −0.0088±0.0060 | 0.140 | 0.0015±0.0007a | 0.038 |
| Giant CAA | −0.0106±0.0082 | 0.192 | 0.0027±0.0006a | <0.001 |
| Age, per 1‐year increaseb | 0.0030±0.0005 | <0.001 | ||
| Sex (reference: women) | −0.0116±0.0027 | <0.001 | ||
| BMI, per 1‐SD score increase | 0.0017±0.0011 | 0.123 | ||
| Mean arterial pressure, per 1‐mm Hg increase | 0.0001±0.0002 | 0.716 | ||
BMI indicates body mass index; CAA, coronary artery aneurysm; cIMT, carotid intima‐media thickness.
Increase per year.
The estimated β in millimeters per year was based on the model in which controls were reference, and the P value of age and the estimated β of the other covariates including the P values were identical in both models.
In comparing CAA‐negative patients with patients with small–medium and giant CAA, both groups had comparable intercepts at age 5 years but had significantly increased progression (0.0015 mm per year [95% CI 0.0001–0.0030 mm]; P=0.038; and 0.0027 mm per year [95% CI 0.0015–0.0039 mm]; P<0.001, respectively).
Figures 1 and 2 show the regression lines (95% CI) for cIMT against age, corrected for sex, BMI z score, mean arterial pressure, and family relations for controls and patients and for controls and the different patient groups based on CAA worst‐ever z score.
Figure 1.
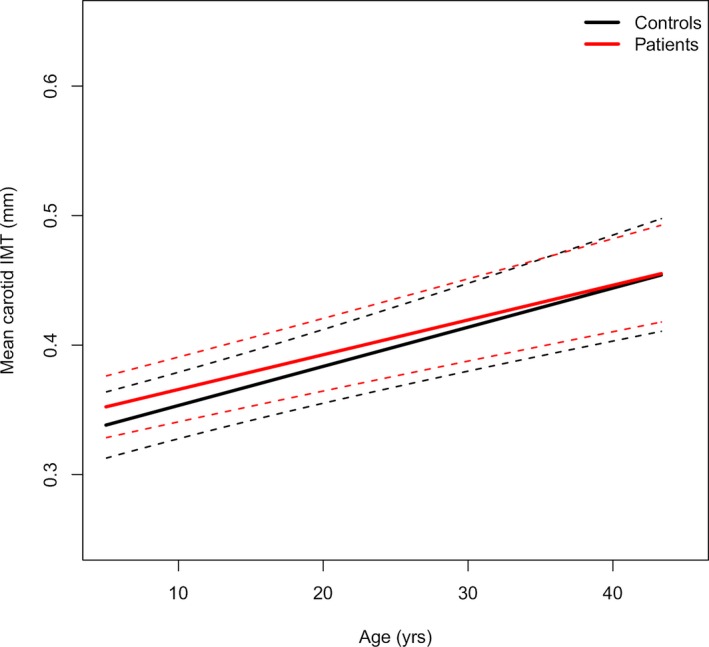
The mean carotid IMT regression line (95% CI) of patients and controls against age. The mean regression line is represented by the continuous line, and the 95% CIs are indicated by dashed lines, after adjusting for sex, body mass index z score, mean arterial pressure, and family relations. IMT indicates intima‐media thickness.
Figure 2.
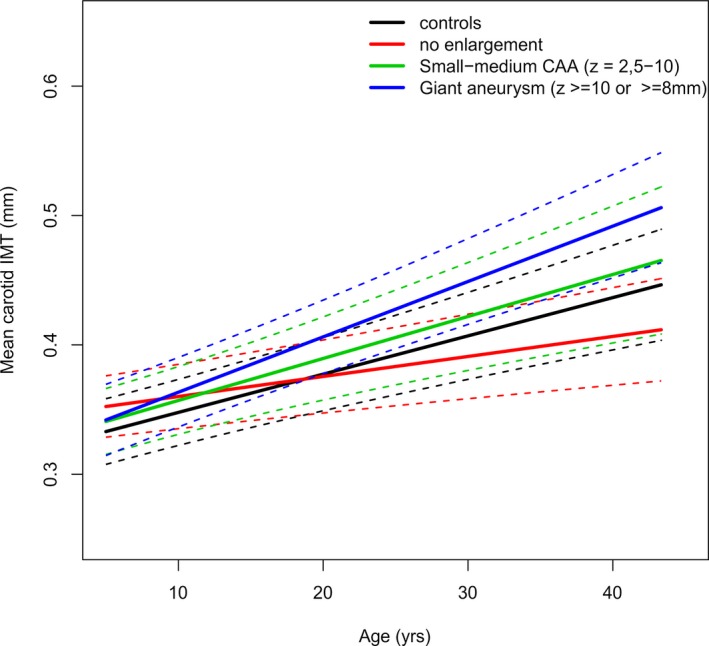
The mean carotid IMT regression line (95% CIs) of the different patient groups based on CAA worst‐ever z score and controls against age. The mean regression line is represented by the continuous line, and the 95% CIs are indicated by dashed lines after adjusting for sex, body mass index z score, mean arterial pressure, and family relations. CAA indicates coronary artery aneurysm; IMT, intima‐media thickness.
IVIG, total cholesterol, low‐density lipoprotein cholesterol, and triglycerides were not significantly associated with cIMT in the multivariable model.
Additional and Post Hoc Analyses
Because not all of our patients obtained multiple measurements, we also analyzed the data including only the measurements of patients with follow‐up data. This analysis showed results similar to the analyses including all patients. Patients with small–medium and giant CAA had comparable intercepts at age 5 compared with CAA‐negative patients (P=0.131 and P=0.071, respectively), but progression was significantly increased in patients with giant CAA (0.0035 mm [95% CI 0.0019–0.0051 mm]; P<0.001); progression was not significantly increased in patients with small–medium CAA (0.0019 mm [−0.0002 to 0.0039 mm]; P=0.081).
After obtaining the results of the increased progression in patients with giant aneurysms but not in patients with small–medium aneurysms (z scores 2.5–10), we performed a post hoc analysis to evaluate whether the participants with giant aneurysms composed a different group as such or represented the more extreme phenotype of a spectrum of the disease.
Children with medium‐sized aneurysms (z scores 5–10, n=15, 29 cIMT measurements) had an estimated marginal mean of 0.381 mm (95% CI 0.368–0.394). In evaluating the model, these patients showed a trend toward an increased intercept (0.0192 mm, [−0.0008 to 0.0392 mm]; P=0.060) with comparable progression in cIMT parallel to the control curves (P=0.833), indicating that normalization toward the healthy siblings in participants without CAA was absent in those with medium CAA.
Discussion
Our longitudinal cohort study in KD demonstrated that the severity of the coronary arteritis at the acute stage of the disease is associated with cIMT of the extracardiac vasculature. The estimated marginal means of the cIMT of the carotid artery increased with increasing severity of the original vasculitis, as reflected by the extent and diameter of CAA. The cIMT of CAA‐negative patients was observed to be initially increased but normalized over time. Patients with the most severe CAA at the initial stage of the disease showed a trend toward significantly increased cIMT progression over time compared with controls. In comparing patients with small–medium and giant CAA with patients without CAA, both groups had significantly increased progression.
Together, these data suggest a spectrum of disease, not only of the coronary arteries but also of the peripheral vasculature.
Multiple studies have evaluated cIMT after KD in a cross‐sectional manner. Some studies found significantly increased cIMT in KD patients compared with controls,19 whereas others did not.12 In comparing CAA‐positive patients with controls, some studies found significantly increased cIMT,11, 20 and again others did not report any difference13, 21; however, most of these studies were small, used variable study designs, or lacked a sufficient number of CAA‐positive patients, and none of the studies had a follow‐up of >6 months.6
Our study is the first longitudinal cIMT study in KD and demonstrates an apparently gradual increase in cIMT in KD patients with larger CAA. The cIMT means gradually increased from controls to patients with giant aneurysms. The cIMT of CAA‐negative patients showed an increased cIMT following complete convalescence of the acute disease, which normalized over time. Patients with small CAA showed increased (but nonsignificant) initial cIMT but comparable cIMT progression parallel to the curves of controls. Patients with medium‐sized CAA showed a trend toward increased initial cIMT with cIMT progression comparable to that of controls. Patients with giant CAA showed a trend toward increased cIMT progression over time. In evaluating this model, patients with giant CAA and, to a much lesser degree, patients with medium‐sized CAA showed a trend toward continuously increasing cIMT compared with controls. Patients with giant CAA are most severely affected by the original vasculitis of the coronary vasculature. Our study suggests that these patients should be followed up for broader cardiovascular assessment beyond the heart. Although these findings need to be confirmed in additional prospective cohort studies, our study also suggests that patients without any enlargement at any point of the disease may not need lifelong follow‐up.
Several factors such as age, sex, BMI, blood pressure, lipids, and lifestyle influence cIMT.22 For the latter, a suitable control group is vital; therefore, we included siblings as controls, having the same environmental and genetic factors. Sex differed between controls and patients and among the different KD subgroups. There were significantly more male participants in the small–medium and giant CAA group compared with the CAA‐negative patients. This was expected because male sex is a known risk factor for aneurysms. It should be emphasized that in the model used, sex, BMI, age, and blood pressure were adjusted for, and lipids were not significantly associated with cIMT in our patient group.
cIMT is strongly correlated with cardiovascular events.9 A systematic review by Lorenz et al calculated a relative risk of 1.15 (95% CI 1.12–1.17) per 0.10‐mm cIMT difference for myocardial infarction and a relative risk of 1.18 (95% CI 1.16–1.21) per 0.10 mm cIMT difference for stroke from studies mainly investigating older populations. Eikendal et al found a hazard ratio of 1.4 per standard deviation increase in cIMT for myocardial infarction or stroke in adults aged <45 years.10
Increased cIMT is seen in children and adolescents with known cardiovascular risk factors such as familial hypercholesterolemia or obesity.23, 24, 25
Increased cIMT progression was found to be significantly related to the incidence of stroke by Polak et al.26 In contrast, a meta‐analysis of individual patient data from longitudinal studies in 2012 showed no significant association between cIMT progression and cardiovascular events in mainly middle‐aged to older adults.27 This could be explained by the different methods of measuring cIMT at the different institutes. Another systematic review of randomized controlled trials measuring cIMT change over time found a statistically significant association between mean change in cIMT over time and the likelihood of developing nonfatal myocardial infarction (P=0.018) and the combined end point of myocardial infarction and death (P=0.021).28
In younger participants, the Bogalusa Heart Study found a significant association between some cIMT segment progression and multiple cardiovascular risk factors such as waist circumference, waist/height ratio, mean arterial pressure, cholesterol, and smoking.29 The Young Finns study showed that young adult cIMT progression was associated with risk factors in childhood such as BMI, physical activity, and fruit consumption.30 Although the exact risk prediction of (increased) cIMT in children and young adults is still unknown, cIMT is clearly correlated with cardiovascular risk factors in a younger population.
Although cIMT is often considered a surrogate marker for clinical or subclinical atherosclerosis, it is unlikely that this is also the case in KD patients. First, the cIMT course in CAA‐negative patients does not seem to be concordant with atherosclerosis because one would expect the cIMT values to worsen over time instead of normalize. This suggests that the increased cIMT in KD patients originates from a different type of vasculopathy and is supported by earlier postmortem (histology) reports that show no accumulation of lipid in the intima or other features consistent with atherosclerosis in coronary arteries.31, 32 The etiology and consequences of this KD vasculopathy have yet to be determined. Most cIMT studies in adults relate increased cIMT to the extent of atherosclerosis; therefore, the cardiovascular risks derived from these studies cannot be directly adopted for the KD population.
Moreover, Lorenz et al found a relative risk of myocardial infarction of 1.15 per 0.10‐mm cIMT increase, whereas our study shows a difference of 0.012 mm between patients and controls.9 Although much smaller than in adult atherosclerosis, in patients with giant CAA, this difference might increase each year, potentially leading to relevant peripheral vasculature changes over time.
The increase in cIMT at the extreme end of the spectrum is not completely unexpected. Suda et al described 76 patients with giant aneurysms (>8 mm) in a retrospective cohort with a median follow‐up of 19 years and found that 7 patients died during follow‐up.33 They calculated 10‐, 20‐, and 30‐year survival rates of 95%, 88%, and 88%, respectively, and 5‐, 15‐, and 25‐year cumulative coronary intervention rates of 28%, 43%, and 59%, respectively, indicating that the coronary arteries are still remodeling years after acute disease.
Study Limitations
Our study has some limitations. First, although the cIMT protocol did not change throughout the years, different ultrasound machines were used. We solved this issue by calculating a correction factor between the different machines. Second, blood pressure data at the time of cIMT was missing in ≈30% of children. By imputing the missing data based on many of the known variables, we were able to correct for mean arterial pressure in our model. Because blood pressure did not seem to influence the cIMT (almost all of the children were normotensive), the missing data were unlikely to have influenced the results. Third, patients were stratified based on their worst‐ever z score. Because the study was conducted in a tertiary referral center, most of the echocardiograms in the acute phase were not performed at our own center but rather by pediatric cardiologists at other centers. This may have led to misclassification of some patients in the CAA subgroups. Moreover, our study was conducted at a tertiary referral center, with inevitable referral bias. In our case, the relatively large number of patients with CAA, and especially with giant CAA, helped identify that these patients had the most abnormal response at the peripheral noncardiac vasculature.
Finally, our controls and a proportion of our patients did not undergo >1 cIMT measurement; however, by creating a multilevel, repeated‐measures, linear mixed‐effects model, we could use all cIMT measurements to create a large study group.
Even though this is the largest study of cIMT in patients after KD, there is a possible lack of power in the subgroups. The arterial walls of the young encompass ≈0.4 mm, whereas cIMT as measured by B‐mode ultrasound has an axial resolution of ≈0.04 to 0.05 mm; therefore, to detect submillimeter differences, large group sizes are required. We observed a trend toward significance in both intercept and progression in different subgroups; however, because significance is dependent on the sample size, and because in both groups the mean was indeed significantly increased, it is likely that a significant finding will be present in larger groups. Consequently, our data need confirmation in a (very) large number of participants with longitudinal follow‐up, in particular in the smaller subgroups of CAA‐positive patients.
Conclusion
The cIMT of KD patients is increased compared with healthy controls. Although the cIMT of CAA‐negative children is initially increased, the values normalize at a later age, suggesting vascular repair of a generalized vasculopathy distinctive from atherosclerosis. Patients with a history of KD complicated by giant and, to a lesser degree, medium‐sized CAA have a trend toward continued increased cIMT, suggesting more severe impact on the arterial wall. Until more data become available, these patients need cardiovascular counseling and follow‐up beyond the heart.
Appendix: Dutch Kawasaki Study Group
Maartje ten Berge MD PhD, St Antonius hospital, Nieuwegein, The Netherlands. Maarten H. Biezeveld MD PhD, OLVG Oost, Amsterdam, The Netherlands. Martijn Bruijn MD PhD, Noordwest Ziekenhuisgroep, Alkmaar, The Netherlands. Luçan C. Delemarre MD, Hospital Amstelland, Amstelveen, The Netherlands. Koert M. Dolman MD PhD, OLVG West, Amsterdam, The Netherlands. Luc H.P.M. Filippini MD, Juliana Children's Hospital, The Hague, The Netherlands. Tom Hendriks MD, Catharina Hospital Eindhoven, Eindhoven, The Netherlands. Dianne A.P.G.F. Maingay‐Visser, Flevo Hospital, Almere, The Netherlands. Jeroen G. Noordzij MD PhD, Reinier de Graaf Hospital, Delft, The Netherlands. Roos Nuboer MD, Meander Medical Center, Amersfoort, The Netherlands. Frans B. Plötz MD PhD, Tergooi Hospital, Blaricum, The Netherlands. Lieke Rozendaal MD, Leids University Medical Center, Willem Alexander Children's Hospital, Leiden, The Netherlands. Gavin W. ten Tusscher MD PhD, Westfriesgasthuis, Hoorn, The Netherlands. Sander Starreveld MD PhD, Groene Hart Hospital, Gouda, The Netherlands. Jennifer J. Verhoeven MD PhD, Maasstad Hospital, Rotterdam, The Netherlands. Nielske M. Weggelaar, MD, Waterland Hospital, Purmerend, The Netherlands. Olivier Weijer MD, Slotervaart Hospital, Amsterdam, The Netherlands. Peter de Winter MD PhD, Spaarne Hospital, Hoofddorp, The Netherlands.
Sources of Funding
This work was supported by the Stinafo Foundation (The Hague, The Netherlands). The sponsor had no role in the study design, the data collection and analysis, the writing of the report, or the decision to submit the manuscript for publication.
Disclosures
None.
Acknowledgments
We thank Linda Landman and Johan Gort of the department of Vascular Medicine, AMC for performing all ultrasonographies, and Michel Hof of the department of Clinical Epidemiology, Biostatistics and Bioinformatics, AMC for his help with the figure.
(J Am Heart Assoc. 2016;5:e003414 doi: 10.1161/JAHA.116.003414)
References
- 1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2. Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997;131:888–893. [DOI] [PubMed] [Google Scholar]
- 3. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA; Committee on Rheumatic Fever E, Kawasaki Disease CoCDitYAHA . Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. [DOI] [PubMed] [Google Scholar]
- 4. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. [DOI] [PubMed] [Google Scholar]
- 5. Paridon SM, Galioto FM, Vincent JA, Tomassoni TL, Sullivan NM, Bricker JT. Exercise capacity and incidence of myocardial perfusion defects after Kawasaki disease in children and adolescents. J Am Coll Cardiol. 1995;25:1420–1424. [DOI] [PubMed] [Google Scholar]
- 6. Dietz SM, Tacke CE, Hutten BA, Kuijpers TW. Peripheral endothelial (dys)function, arterial stiffness and carotid intima‐media thickness in patients after Kawasaki disease: a systematic review and meta‐analyses. PLoS One. 2015;10:e0130913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long‐term consequences of Kawasaki disease. A 10‐ to 21‐year follow‐up study of 594 patients. Circulation. 1996;94:1379–1385. [DOI] [PubMed] [Google Scholar]
- 8. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima‐media thickness and plaque consensus (2004‐2006‐2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation. 2007;115:459–467. [DOI] [PubMed] [Google Scholar]
- 10. Eikendal AL, Groenewegen KA, Anderson TJ, Britton AR, Engstrom G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Holewijn S, Ikeda A, Kitagawa K, Kitamura A, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Dekker JM, Okazaki S, O'Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Hoefer IE, Peters SA, Bots ML, den Ruijter HM; Group U‐IP . Common carotid intima‐media thickness relates to cardiovascular events in adults aged <45 years. Hypertension. 2015;65:707–713. [DOI] [PubMed] [Google Scholar]
- 11. Noto N, Okada T, Yamasuge M, Taniguchi K, Karasawa K, Ayusawa M, Sumitomo N, Harada K. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesions. Pediatrics. 2001;107:1095–1099. [DOI] [PubMed] [Google Scholar]
- 12. Selamet Tierney ES, Gal D, Gauvreau K, Baker AL, Trevey S, O'Neill SR, Jaff MR, de Ferranti S, Fulton DR, Colan SD, Newburger JW. Vascular health in Kawasaki disease. J Am Coll Cardiol. 2013;62:1114–1121. [DOI] [PubMed] [Google Scholar]
- 13. Ikemoto Y, Ogino H, Teraguchi M, Kobayashi Y. Evaluation of preclinical atherosclerosis by flow‐mediated dilatation of the brachial artery and carotid artery analysis in patients with a history of Kawasaki disease. Pediatr Cardiol. 2005;26:782–786. [DOI] [PubMed] [Google Scholar]
- 14. Dietz SM, Tacke CE, Gort J, Kuipers IM, de Groot E, Wiegman A, Hutten BA, Kuijpers TW. Carotid intima‐media thickness in patients with a history of Kawasaki disease. Circ J. 2015;79:2682–2687. [DOI] [PubMed] [Google Scholar]
- 15. Manlhiot C, Millar K, Golding F, McCrindle BW. Improved classification of coronary artery abnormalities based only on coronary artery z‐scores after Kawasaki disease. Pediatr Cardiol. 2010;31:242–249. [DOI] [PubMed] [Google Scholar]
- 16. McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, Vetter VL, Gersony WM, Mitchell PD, Newburger JW; Pediatric Heart Network I . Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116:174–179. [DOI] [PubMed] [Google Scholar]
- 17. Polak JF, Johnson C, Harrington A, Wong Q, O'Leary DH, Burke G, Yanez ND. Changes in carotid intima‐media thickness during the cardiac cycle: the Multi‐Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2012;1:e001420 doi: 10.1161/JAHA.112.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, Kastelein JJ. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109:III33–III38. [DOI] [PubMed] [Google Scholar]
- 19. Meena RS, Rohit M, Gupta A, Singh S. Carotid intima‐media thickness in children with Kawasaki disease. Rheumatol Int. 2014;34:1117–1121. [DOI] [PubMed] [Google Scholar]
- 20. Cheung YF, Wong SJ, Ho MH. Relationship between carotid intima‐media thickness and arterial stiffness in children after Kawasaki disease. Arch Dis Child. 2007;92:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishikawa T, Iwashima S. Endothelial dysfunction in children within 5 years after onset of Kawasaki disease. J Pediatr. 2013;163:1117–1121. [DOI] [PubMed] [Google Scholar]
- 22. Dawson JD, Sonka M, Blecha MB, Lin W, Davis PH. Risk factors associated with aortic and carotid intima‐media thickness in adolescents and young adults: the Muscatine Offspring Study. J Am Coll Cardiol. 2009;53:2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wiegman A, de Groot E, Hutten BA, Rodenburg J, Gort J, Bakker HD, Sijbrands EJ, Kastelein JJ. Arterial intima‐media thickness in children heterozygous for familial hypercholesterolaemia. Lancet. 2004;363:369–370. [DOI] [PubMed] [Google Scholar]
- 24. Narverud I, Retterstol K, Iversen PO, Halvorsen B, Ueland T, Ulven SM, Ose L, Aukrust P, Veierod MB, Holven KB. Markers of atherosclerotic development in children with familial hypercholesterolemia: a literature review. Atherosclerosis. 2014;235:299–309. [DOI] [PubMed] [Google Scholar]
- 25. Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62:1309–1319. [DOI] [PubMed] [Google Scholar]
- 26. Polak JF, Pencina MJ, O'Leary DH, D'Agostino RB. Common carotid artery intima‐media thickness progression as a predictor of stroke in Multi‐Ethnic Study of Atherosclerosis. Stroke. 2011;42:3017–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM, Kiechl S, Rundek T, Desvarieux M, Lind L, Schmid C, DasMahapatra P, Gao L, Ziegelbauer K, Bots ML, Thompson SG; Group P‐IS . Carotid intima‐media thickness progression to predict cardiovascular events in the general population (the PROG‐IMT collaborative project): a meta‐analysis of individual participant data. Lancet. 2012;379:2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldberger ZD, Valle JA, Dandekar VK, Chan PS, Ko DT, Nallamothu BK. Are changes in carotid intima‐media thickness related to risk of nonfatal myocardial infarction? A critical review and meta‐regression analysis. Am Heart J. 2010;160:701–714. [DOI] [PubMed] [Google Scholar]
- 29. Nguyen QM, Toprak A, Xu JH, Srinivasan SR, Chen W, Berenson GS. Progression of segment‐specific carotid artery intima‐media thickness in young adults (from the Bogalusa Heart Study). Am J Cardiol. 2011;107:114–119. [DOI] [PubMed] [Google Scholar]
- 30. Juonala M, Viikari JS, Kahonen M, Taittonen L, Laitinen T, Hutri‐Kahonen N, Lehtimaki T, Jula A, Pietikainen M, Jokinen E, Telama R, Rasanen L, Mikkila V, Helenius H, Kivimaki M, Raitakari OT. Life‐time risk factors and progression of carotid atherosclerosis in young adults: the Cardiovascular Risk in Young Finns study. Eur Heart J. 2010;31:1745–1751. [DOI] [PubMed] [Google Scholar]
- 31. Suzuki A, Miyagawa‐Tomita S, Komatsu K, Nishikawa T, Sakomura Y, Horie T, Nakazawa M. Active remodeling of the coronary arterial lesions in the late phase of Kawasaki disease: immunohistochemical study. Circulation. 2000;101:2935–2941. [DOI] [PubMed] [Google Scholar]
- 32. Gordon JB, Kahn AM, Burns JC. When children with Kawasaki disease grow up: myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54:1911–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suda K, Iemura M, Nishiono H, Teramachi Y, Koteda Y, Kishimoto S, Kudo Y, Itoh S, Ishii H, Ueno T, Tashiro T, Nobuyoshi M, Kato H, Matsuishi T. Long‐term prognosis of patients with Kawasaki disease complicated by giant coronary aneurysms: a single‐institution experience. Circulation. 2011;123:1836–1842. [DOI] [PubMed] [Google Scholar]


