Introduction
Hypertension, a complex trait determined by genetic, epigenetic, and environmental factors and their intricate interaction, is an important public health challenge worldwide because of its high prevalence and concomitant increase in the risk for cardiovascular disease. Unfortunately, the prevalence of hypertension is increasing in both developed and developing countries.1 As a consequence of the increase in global prevalence, the total number of adults with hypertension is predicted to increase to 1.56 billion in 2025.2 This prospect is daunting, given that, in 2010, high blood pressure was already the biggest single contributor to global mortality and disease burden.
The pathogenesis of essential hypertension is complex. Many organs and systems including kidneys, arteries, microcirculation, heart, immune system, nervous system, and endocrine factors are involved in the pathophysiology of hypertension. Among them, kidneys and arteries are major contributors to the development of hypertension.3, 4 Various agonists binding to plasma membrane receptors regulate renal sodium transport and fluid balance and maintain the equilibrium between vasoconstriction and vasodilation. Many of these agonists transmit their “information” via G protein–coupled receptors (GPCRs). GPCRs mediate cellular responses to diverse extracellular stimuli and play a vital role in the control of physiology and behavior.5 GPCR kinases (GRKs) interact with the agonist‐activated GPCRs to promote receptor phosphorylation and to initiate receptor desensitization.6 The wide variety of GPCRs that are responsible for optimal blood pressure control7 leaves no doubt that GRKs play a vital role in the regulation of blood pressure. A number of studies have shown that GRKs are associated with hypertension, blood pressure response to antihypertensive medicines, and adverse cardiovascular outcomes of antihypertensive treatment.8, 9, 10, 11 In this paper, we reviewed our evolving understanding of the role of GRKs in hypertension, summarized the current knowledge of GRK‐mediated regulatory mechanisms, and highlighted the potential for targeting GRKs in the treatment of hypertension. This information may advance our understanding of the role of GRKs in the control of blood pressure and provide novel insights into the field of translational medicine, especially regarding the design of new therapeutic approaches for the treatment of hypertension.
Abnormal GPCR Function and Hypertension
GPCRs, the largest and most functionally diverse superfamily of cell‐surface receptors, share a common architecture consisting of 7 transmembrane domains connected by extracellular and intracellular loops.5 Upon stimulation, GPCRs interact with heterotrimeric G proteins that in turn dissociate into 2 functional units, namely, Gα and Gβγ subunits, both of which stimulate the activation of downstream proteins (Figure 1). In the vasculature, some GPCRs mediate vasoconstriction and/or vascular remodeling, such as angiotensin II (Ang II) type 1 receptor (AT1R), α‐adrenergic receptor (α‐AR), endothelin A receptor, and neuropeptide Y receptor, whereas other GPCRs induce vasodilatation and/or inhibition of vascular remodeling, including the acetylcholine receptor, β‐AR, the endothelin B receptor, and the dopamine receptor, among others. Similar to some renal tubular receptors (e.g., dopamine receptor, atrial natriuretic peptide receptor, AT2R, Mas receptor, and endothelin B receptor) decrease renal sodium reabsorption, whereas others including the AT1R, insulin receptor, and mineralocorticoid receptor increase renal sodium reabsorption. The balance between pro‐ and antihypertensive receptor activity is important to keep the blood pressure in the normal range. Abnormal GPCR functions lead to increased blood pressure; for example, increased AT1R function and impaired dopamine receptor function are found in hypertensive patients and hypertensive animal models.12, 13 The causes of abnormal GPCR function are complex and may include perturbation of DNA modification, receptor expression, and phosphorylation.14, 15 Among these modifications, GPCR phosphorylation is important. In hypertensive states, for example, dopamine D1 receptor (D1R) is hyperphosphorylated, which leads to uncoupling of the dopamine receptor from its GαS/effector protein complex and impairment of dopamine‐mediated natriuresis and vasodilation.13, 15, 16 It is known that the state of phosphorylation of GPCRs is modified by 2 kinds of enzymes. Kinases (e.g., GRKs) increase GPCR phosphorylation, whereas phosphatases (e.g., protein phosphatase 2A) bring about GPCR dephosphorylation. Renal protein phosphatase 2A activity is decreased in adult spontaneously hypertensive rats (SHRs) but increased in young (aged 2 weeks) SHRs, whereas GRK4 activity is markedly increased in hypertension.16, 17, 18 Nevertheless, the GRKs have received, by far, the most attention in abnormal GPCR phosphorylation in renal tubules in hypertension.
Figure 1.
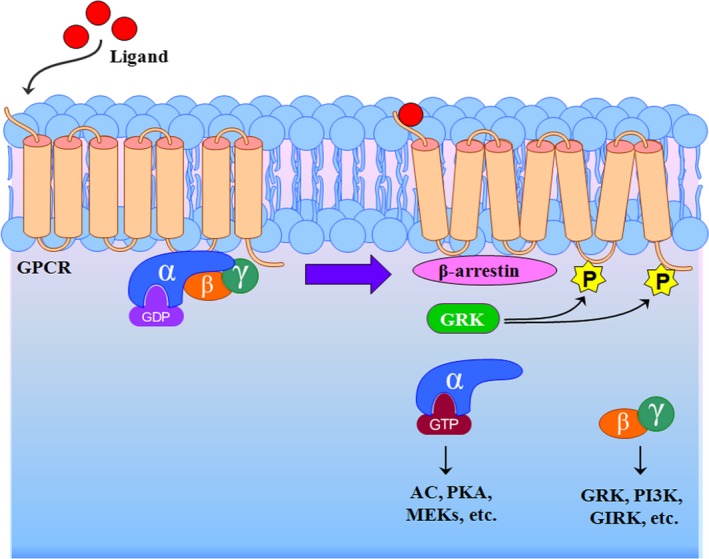
Schematic representation of the process of GPCR desensitization. On binding to their cognate ligands, GPCR activation initiates dissociation of cognate trimeric G protein, promoting GPCR phosphorylation by GRKs, leading to receptor association with members of the arrestin family, which inhibits further G protein activation. AC indicates adenylyl cyclase; GDP, Guanosine‐5′‐diphosphate; GIRK, G protein–gated inwardly rectifying potassium channel; GPCR, G protein–coupled receptor; GRK, G protein–coupled receptor kinase; GTP, Guanosine‐5′‐triphosphate; MEKs, mitogen‐activated protein/extracellular signal‐regulated protein kinase kinases; P, phosphorylation; PI3K, phosphatidylinositol‐3 kinase; PKA, protein kinase A.
Role of GRKs in the Regulation of Blood Pressure
GRK Family Members
Although there are >800 known GPCRs in the human genome, it is surprising that only 7 GRKs (GRK1–7) have been identified. The GRKs constitute a family of 7 serine/threonine protein kinases characterized by their ability to specifically recognize and phosphorylate agonist‐activated GPCRs. Based on divergent C‐terminal domain architecture and membrane‐targeting mechanisms, the GRKs are classified into 3 subfamilies: (1) the GRK1 subfamily, also known as the opsin kinase family, consisting of the rhodopsin kinase GRK1 and visual pigment kinase GRK7; (2) the GRK2‐like subfamily, also known as the β‐AR kinase family, consisting of GRK2 (β‐AR kinase 1) and GRK3 (β‐AR kinase 2); and (3) the GRK4‐like subfamily, consisting of GRK4, GRK5, and GRK6.6 All GRKs possess similar structural organization with an N‐terminal domain (≈185 amino acids), a catalytic domain (≈270 amino acids), and a C‐terminal domain (≈105–230 amino acids) (Figure 2). The carboxy tail region is GRK subtype‐specific; it is prenylated in the GRK1 subfamily, binds to Gβγ, contains a pleckstrin homology domain in the GRK2 subfamily, and has a C‐terminal helix/palmitoylation site in the GRK4 subfamily.6 The C‐terminal domain of GRKs is the most important determinant of subcellular localization and agonist‐dependent translocation.19 There is a nuclear localization sequence in all members of the GRK4 subfamily; the nuclear localization sequence in GRK5 and GRK6, but not GRK4, binds to DNA in vitro.20
Figure 2.
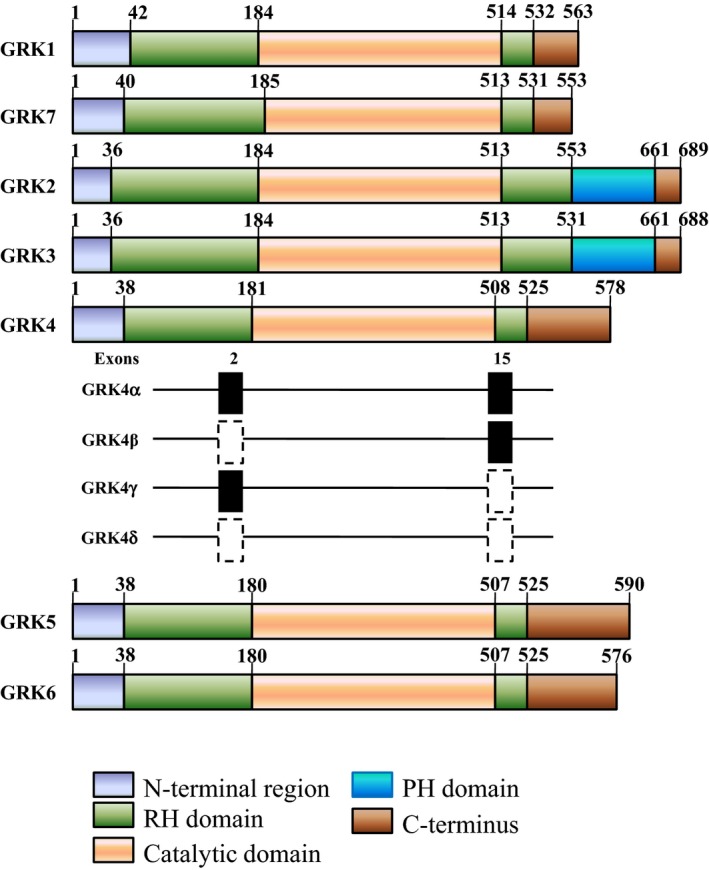
Structural domain distribution of GRKs. All GRKs possess an N‐terminal domain and a catalytic domain, both of which are followed by a Regulator of G protein signaling (RGS) homology domain, and a C‐terminal domain. GRK2 and GRK3 have another PH domain that interacts with G protein βγ subunits. The various isoforms of human GRK4 (GRK4α, GRK4β, GRK4γ, and GRK4δ) have differences in presence or in‐frame deletion of certain exons. The solid black square represents the presence of exon 2 and/or exon 15, whereas the dotted square represents the deletion of exon 2 and/or exon 15. GRK indicates G protein–coupled receptor kinase; PH, pleckstrin homology; RH, RGS homology.
The distribution of GRK subtype expression is different among subtypes. GRK1, GRK4, and GRK7 are expressed in limited numbers of tissues. GRK1 and GRK7 are found almost exclusively in the retina and regulate the opsins. GRK4 is expressed in testis, myometrium, kidney, artery, and intestine.13, 15, 16, 17, 18, 21 By contrast, other GRKs (GRK2, GRK3, GRK5, and GRK6) are expressed ubiquitously throughout the body. Accordingly, except for GRK1 and GRK7, other GRK members (GRK2–6) exert different physiological effects, specifically, the regulation of blood pressure by the cardiovascular system and the kidney.8, 9, 10, 11, 16, 17, 18, 20, 21, 22, 23, 24
G protein–coupled receptor kinase 2
The human GRK2 (official name ADRBK1) gene locus maps at the long arm of chromosome 11: 11q13.2 by Ensembl, 11q13.1 by Entrez Gene (National Center for Biotechnology Information), and 11q13 by the HUGO Gene Nomenclature Committee (HGNC). Human GRK2 cDNA encodes a protein of 689 amino acids (79.573 kDa) with an overall 98.0% amino acid and 92.5% nucleotide identity with bovine GRK2. The 23‐kb human GRK2 gene consists of 21 exons interrupted by 20 introns, with a predicted transcription start site ≈246 bases upstream of the start ATG. The human GRK2 gene has 12 highly conserved catalytic region subdomains in which 5 are encoded entirely within exons, specifically, exons 8, 9, 10, 11, and 12. Exons bounded by introns range in size from 52 (exon 7) to 163 bases (exon 18). The 2 largest exons represent the 5′‐flanking region (359 bases, including 113 bases of coding sequence) and the 3′‐coding plus noncoding region of the gene (>1200 bases). Sequence analysis of the 5′‐flanking/promoter region reveals many features characteristic of mammalian housekeeping genes, namely, the lack of a TATA box, an absent or nonstandardly positioned CAAT box, high GC content, and the presence of Sp1‐binding sites. The conserved region of the C‐terminal domain is important for enzyme–receptor interaction required for GRK2 to catalyze receptor phosphorylation.25 In addition, a Gβγ binding site of GRK2 is also localized in the C‐terminal pleckstrin homology domain. Phosphorylated Raf kinase inhibitory protein binds to the N terminus of GRK2, resulting in the inhibition of its function.26
Distribution of GRK2
GRK2 is ubiquitously expressed in mammals. In the cardiovascular system, GRK2 is expressed in the vascular endothelium, arterial smooth muscle, and myocardium.27, 28 GRK2 is also abundant in the kidney, especially in the renal proximal tubule.29 This renal expression indicates that GRK2 plays a vital role in the regulation of ion and fluid transport and, ultimately, blood pressure. GRK2 is expressed in both cytoplasm and the cell membrane. It shuttles between the cytosol and plasma membrane, anchoring to the latter through its pleckstrin homology and Gβγ binding domains at the C‐terminus.30 The subcellular localization of GRK2 at the mitochondrial outer membrane27, 31 may indicate a role of GRK2 in regulating mitochondrial biogenesis and cellular energy production. Indeed, GRK2 increases mitochondrial superoxide production and decreases oxygen consumption and ATP production.32
Regulation of GRK2 in the regulation of blood pressure
A role of GRK2 in the regulation of blood pressure has been shown in animal models with partial germline deletion, universal GRK2 knockdown, and targeted overexpression or knockdown in vascular smooth muscle and endothelial cells.22, 33, 34, 35, 36 GRK2 plays an important role in the regulation of blood pressure.7 Germline deletion of Grk2 is lethal.37 GRK2 deficiency in global adult hemizygous mice (Grk2 +/−) has no effect on basal blood pressure but increases the vasodilator response to acetylcholine or isoproterenol and protects against Ang II–induced hypertension and vascular remodeling that is partially caused by increased nitric oxide bioavailability.33 Cohn et al also found in mice that inhibition of vascular smooth muscle GRK2 by either overexpression of the C‐terminal portion of GRK2 or vascular smooth muscle–specific ablation of GRK2 protein expression has no effect on blood pressure.35 This method of GRK2 silencing also had no effect on the elevated blood pressure resulting from unilateral renal artery stenosis.35 In contrast, overexpression of GRK2 in vascular smooth muscle in mice increases resting blood pressure.34 This study would agree with the report that GRK2 inhibits adiponectin function; adiponectin may be antihypertensive.38, 39 Portal hypertension caused by common bile duct ligation is also associated with an increase in GRK2 expression in the mesenteric artery.40 Consequently, deletion of GRK2 should result in a decrease in blood pressure; However, global knockdown of Grk2 expression using small hairpin interfering RNA in male mice produces hypertension that is associated with vascular remodeling caused in part by increases in cell proliferation at age 6 months but not at 3 months.22 The causes of the differences are not known, but the differential effects of GRK2 on vasoconstriction and vasodilation may explain this apparently conflicting results. In small hairpin Grk2 knockdown mice, for example, both phenylephrine‐induced contractile responses and isoproterenol‐mediated vasodilation are increased; which one dominates would eventually determine the physiological phenotype.22 After inhibition of GRK2 by either peptide inhibition or gene ablation, downregulation of GRK2 does not only increase β‐AR–mediated vasodilation but also enhances α1D‐AR–stimulated vasoconstriction and could explain the lack of effect on blood pressure of a decrease in GRK2 expression or function.35 Whether or not the discrepant results could be related to sex differences were not determined, but the small hairpin Grk2 studies were performed only in male mice because the Grk2 small hairpin RNA transgene was incorporated into the Y chromosome22; the sexes of the mice in the other studies were not given. Nevertheless, the transgenic overexpression of GRK5 in vascular smooth muscle increases blood pressure to a greater extent in male than in female mice.23 The discrepant results may also be related to the extent of downregulation of GRK2 in different tissues.
GRK2 is expressed to a greater extent than GRK5 in endothelial cells.41 Increased GRK2 expression in injured endothelial cells in injured liver leads to intrahepatic portal hypertension, and knockdown of GRK2 in liver sinusoidal endothelial cells leads to an increase in portal pressure that is related to decreased endothelial nitric oxide synthase production of nitric oxide.36 Selective deletion of Grk2 in the endothelium affects the aorta's receptor‐dependent and ‐independent vasoconstriction and increases vascular inflammation and tissue degeneration by increasing mitochondrial reactive oxygen species production, which is also associated with hypertension.42 Exercise decreases blood pressure, improves insulin sensitivity, and decreases mesenteric arteriolar and myocardial GRK2 expression in SHRs.43 The effects in mesenteric arterioles were prevented by mesenteric arteriolar overexpression of GRK2. In contrast, downregulation of endothelial vascular GRK2 expression in SHRs that is initiated at the prehypertensive stage (age 4 weeks) subsequently improves vascular insulin sensitivity that helps to limit the progression of hypertension.43 GRK2 impairs insulin sensitivity by binding to the insulin receptor substrate 1 but not to the insulin receptor.44 GRK2 expression in renal preglomerular vessels increases with aging in male but not in female rats.45 GRK2 regulates the blood pressure by modulating other receptor‐mediated vascular responses, including endothelin A receptor, neurotensin receptor 1, and P2Y receptor.46, 47, 48 The reasons why knockdown of GRK2 in mice in different studies leads to different effects on blood pressure need to be elucidated.
GRK2 also plays an important role in the renal regulation of sodium excretion and blood pressure. GRK2 keeps amiloride‐sensitive epithelial Na+ channels in the active state.49, 50, 51 GRK2 upregulates epithelial Na+ channel activity by a mechanism that depends not on its kinase activity but rather on the ability of the RGS homology domain of GRK2 to interact with and inhibit the α subunit of Gq/11, a negative regulator of epithelial Na+ channels.50 GRK2‐mediated phosphorylation of the C‐terminus of β–epithelial Na+ channels and phosphorylation of Nedd4‐2 prevent its ability to inhibit epithelial Na+ channel activity.51, 52 GRK2 negatively regulates neurotensin receptor 1 function; there are more neurotensin binding sites in the renal cortex than in the renal medulla, and they decrease sodium excretion, but the mechanism is not known.47 GRK2 also regulates the phosphorylation of renal D1R and D1R‐mediated natriuresis.53 In the human kidney, antisense oligonucleotides against GRK2 and GRK4 blunt the later stages of D1R desensitization; heparin, a nonselective GRK inhibitor, decreases GRK2 and GRK4 expression and attenuates the desensitization of D1R.54 Both GRK2 and GRK4 are involved in the desensitization of renal D1R in obese Zucker rats.55 Oxidative stress is involved in the regulation of GRK2 of D1R.56 Both in vivo and in vitro studies show that oxidative stress activates nuclear factor κB, causing an increase in protein kinase c (PKC) activity, which leads to GRK2 translocation and subsequent D1R serine hyperphosphorylation.56 The functional consequence of this phenomenon is the inability of D1R to inhibit Na+,K+‐ATPase activity and promote sodium excretion, which could contribute to the increase in blood pressure.56 Interestingly, we recently found that prenatal lipopolysaccharide exposure results in increased GRK2 expression, increased D1R phosphorylation, and impaired D1R‐mediated natriuresis and diuresis in the offspring. These findings suggest that a dysfunction of the renal D1R induced by abnormal GRK2 expression is also involved in fetal‐programmed hypertension.57 GRK2 is also involved in desensitization of D2R; D2R dysfunction is involved in the pathogenesis of hypertension.58, 59
Role of GRK2 in spontaneous hypertension
GRK2 expression in several tissues is increased in several diseases, including spontaneous hypertension in humans and experimental animals and in animal models of diabetes and insulin resistance.28 Gros et al reported that in SHRs, GRK2 expression is increased in both lymphocytes and aortic vascular smooth muscle cells and is accompanied by impairment of β‐adrenergic–mediated stimulation of adenylyl cyclase activity and β‐AR–mediated vasodilation.60 The impairment in β‐adrenergic–mediated aortic vasodilation and increased vascular GRK2 expression are observed in SHRs aged 10 and 15 weeks but not 5 weeks. Increased aortic vascular GRK2 expression is also present in the Dahl salt‐sensitive hypertensive rats after 4 weeks of a high salt diet.60 Oliver et al also reported impaired aortic β1‐ and β2‐AR–mediated vasodilation, but not β3‐AR–mediated vasodilatation, and increased aortic expression of GRK2 in adult SHRs61; however, this group did not find such differences in the mesenteric artery of adult Wistar‐Kyoto (WKY) rats and SHRs.61 Moreover, in rats made hypertensive by L‐NAME, their aortas had increased β2‐AR–mediated vasodilation and decreased GRK2 expression; their mesenteric arteries had decreased β2‐AR‐mediated vasodilation, without changes in GRK2 expression—opposite to that found in SHRs.61 Whether or not inconsistencies are present in other models of hypertension remain to be determined.
GRK2 is expressed in peripheral blood mononuclear cells and lymphocytes.7, 62 GRK activity and GRK2 expression are increased in lymphocytes of hypertensive humans and experimental models of hypertension.60, 63, 64 Lymphocyte GRK2 mRNA expression directly correlates with systolic blood pressure and plasma norepinephrine levels.64 GRK2 in lymphocytes is elevated >30% among persons with systolic blood pressure >130 mm Hg. GRK2 protein expression in lymphocytes is also increased about 2‐fold, and its activity increased >40% in African Americans, a population at higher risk for hypertension and cardiovascular complications compared with other groups.64
G protein–coupled receptor kinase 3
The human GRK3 (official name ADRBK2) gene locus maps at the long arm of chromosome 22: 22q12.1 by Ensembl and Entrez Gene and 22q11 by HGNC. Similar to GRK2, the GRK3 gene also has 21 exons ranging in size from 52 to 163 bases. The amino acid sequence of human GRK3 is 84%, identical to that of human GRK2. Similarly, bovine GRK3 has 85% amino acid identity with GRK2. The most highly conserved region between GRK3 and GRK2 is the protein kinase catalytic domain, which has only 12 amino acid differences (95.0% identity), 4 of which are conservative substitutions (96.7% conservative). In contrast, the amino‐terminal domain (80.7% identity, 89.8% conservative) and carboxyl‐terminal domain (76.6% identity, 88.9% conservative) are less well conserved.
GRK3 belongs to the GRK2 subfamily and is ubiquitously expressed in the body; however, unlike GRK2 and GRK5, GRK3 is not expressed in endothelial cells.41 In contrast, in cardiac myocytes, the GRK2 subfamily expression is GRK5 to GRK3 to GRK2. Many studies have focused on the role of GRK2 and GRK3 on cardiac function.8, 65 GRK3 and GRK2, however, have distinct roles in receptor selectivity in cardiac myocytes and receptor‐mediated regulation of cardiac function; GRK3 has selectivity for the α1B‐ARs and for the thrombin receptor but exhibits less efficacy at β1‐ARs than GRK2.65 Their subcellular distribution in cardiac myocytes is also different. Consequently, GRK2 expression is increased in intercalated discs in rats with spontaneously hypertensive heart failure, whereas GRK3 expression is increased in cross‐striations in α‐actinin and Gα at Z‐lines.66
Unlike the prohypertensive action of GRK2,33, 34 GRK3 may play a protective role in the regulation of blood pressure. Cardiac myocytes of spontaneously hypertensive heart failure rats have increased expression of GRK3 and GRK6 and altered distribution, including that of GRK2.66 GRK3 expression in human lymphocytes significantly and inversely correlates with systolic and diastolic ambulatory blood pressure.9 The protective role for GRK3 in the regulation of blood pressure is supported by findings in transgenic mice in which cardiac myocyte–restricted inhibition of endogenous GRK3 causes hypertension because of increased cardiac output caused in part by cardiac myocyte α1‐AR hyperresponsiveness.67 GRK3 is important in α1B‐AR signaling; GRK5 has a partial effect, whereas GRK2 has no effect.68 Although α‐ARs are key regulators of vascular resistance and GRK3 is expressed in the vasculature, it remains unknown whether or not GRK3 can regulate the blood pressure by exerting some functions in vascular resistance. In addition, GRK3 reportedly regulates the phosphorylation of D1R53 and D2R; however, its physiological consequence is not clear.
G protein–coupled receptor kinase 4
The human GRK4 gene locus (4p16.3) is embedded in a gene cluster region on chromosome 4p16 that includes genes encoding dopamine receptor type 5 (4.p16.1) and α‐adducin (4p16.3), 2 variants of which (ADD1 and GRK4) are linked to hypertension.16, 69, 70 The human GRK4 gene is composed of 16 exons extending over 75 kb of DNA. Alternative splicing generates 4 isoforms of human GRK4 mRNA that differ in the presence or absence of exon 2 at the N‐terminal region and exon 15 in the C‐terminal region: GRK4α (578 amino acids, 66.5 kDa) is the full‐length isoform; GRK4β (546 amino acids, 62.9 kDa) lacks only the N‐terminal exon 2 (32‐codon deletion); GRK4γ (532 amino acids, 61.2 kDa) lacks only the C‐terminal exon 15 (46‐codon deletion); and the shortest splice variant is GRK4δ (500 amino acids, 57.6 kDa), missing both exons 2 and 15.71 In addition, 5 GRK4 splice variants (GRK4A–E) in rat and only 1 GRK4 splice in mouse have been reported. Only the GRKα isoform in humans, GRK4A in rats, and only GRK4 reported in mice are closely homologous (≈70%), whereas the mouse and rat GRK4 sequences retain 90% identity.
Distribution of GRK4
As noted previously, GRK2, GRK3, GRK5, and GRK6 are ubiquitously expressed, whereas GRK4 is expressed in a limited number of tissues. GRK4, for example, is abundantly expressed in the testes and human myometrium and, to a lesser extent, in a few other tissues, including the artery, brain, kidney, and intestine, but has minimal expression in the normal heart.16, 71, 72 The distinct distribution of GRK4 indicates its vital role in the regulation of blood pressure. In both WKY rats and SHRs, GRK4 expression is strongly expressed in subapical membranes of renal proximal tubules (S1 and S3 segments), thick ascending limbs of the loop of Henle, and the distal convoluted tubules and much less in glomeruli.16, 72, 73 GRK4 is also present in rat renal resistance vessels, but its physiological function remains unclear. Basal GRK4 expression in the renal cortex is much higher in SHRs than in WKY rats, whereas cardiac GRK4 expression is similar in the 2 rat strains, indicating that the increased GRK4 expression in hypertension has organ specificity.72
In our recent studies, we found that GRK4 is also expressed in the tunica media and adventitia of arteries from Sprague‐Dawley rats and C57BL/6J mice.21 GRK4 is expressed in both large and small vessels, including the thoracic aorta, superior mesenteric artery, carotid arteries, and renal artery, and there is no difference in GRK4 expression in these vessels. The physiological significance of GRK4 at the tunica adventitia, however, remains to be determined because GRK4 in this layer does not participate in the Ang II–mediated vasoconstriction.21 In addition, we found that GRK4 is expressed in the myocardium, which is involved in the regulation of myocardial ischemia. Overexpression of GRK4 or its variants in mice contributes to the aggravation of the ischemia induced by myocardial injury (L.P. Li, J. Yang, and C.Y. Zeng Ph.D., unpublished data, 2016).
Regulation by GRK4 of blood pressure
The dopaminergic system and the renin–angiotensin system are important regulators of sodium balance and blood pressure, which are relevant to the pathogenesis and/or maintenance of hypertension.13, 15, 16, 17, 18, 54, 55, 56, 57, 59, 71, 72, 73, 74, 75, 76, 77, 78 The dopaminergic system exerts a paracrine regulatory role on renal sodium transport in the proximal tubule via its 5 receptor subtypes. Dopamine receptors, pharmacologically grouped into D1‐like (D1 and D5) and D2‐like (D2, D3, and D4) receptors, as with the Ang II receptors (AT1R and AT2R), are expressed in brush border and basolateral membranes of renal proximal tubules. AT1R mediates the vast majority of renal actions of Ang II, including renal tubule sodium reabsorption. In contrast to the stimulatory effect of Ang II on sodium transport in renal proximal tubules, the major consequence of the activation of dopamine receptors is the inhibition of sodium transport.13, 15, 16, 17, 18, 54, 55, 56, 57, 59, 71, 72, 73, 74, 75, 76, 77, 78 Increasingly, studies show that GRK4 plays an important physiological role in the long‐term control of blood pressure and in sodium homoeostasis via the regulation of the renal D1R, D3R, and AT1R.
Studies have shown that increased GRK4 activity causes impaired renal D1R function in hypertension. GRK4 activity is increased in the kidneys of humans with essential hypertension, but the increased activity is caused not by increased renal GRK4 protein expression but rather by constitutively active variants of GRK4.16 In human renal proximal tubule cells, GRK4 constitutively phosphorylates the D1R in the absence of agonist activation; however, inhibition of GRK4 activity or depletion of GRK4 blunts the D1R desensitization.54 The abundance of basal GRK4 and serine‐phosphorylated D1R in renal cortical membranes are much higher in SHRs relative to WKY rats.72 Selective renal cortical inhibition of GRK4 expression decreases serine‐phosphorylated D1R to a greater extent in SHRs than in WKY rats; it also increases sodium excretion and attenuates the increase in arterial blood pressure with age in SHRs but not in WKY rats.72 Similar to GRK2, the dysfunction of the renal D1R induced by abnormal GRK4 expression is also involved in fetal‐programmed hypertension.57 In renal proximal tubules, however, GRK4 is more important than other GRKs in the desensitization of D1R and D3R.54, 73 These findings suggest the crucial role of renal GRK4 in the D1R‐ and D3R‐mediated control of sodium excretion and blood pressure.
The 3 human GRK4γ single nucleotide polymorphisms (SNPs; 65R>L, 142A>V, and 486A>V) markedly impair D1R‐mediated cAMP accumulation in the kidney, which is not due to differences in the quantity of the expression of either D1R or GRK4.16 Compared with GRK4γ wild‐type transgenic mice, GRK4γ142V transgenic mice are hypertensive and fail to increase urine flow and sodium excretion in response to the D1R agonist fenoldopam; the decreased ability of fenoldopam to inhibit renal sodium transport is also observed in vitro.16 The increase in blood pressure in GRK4γ142V transgenic mice is not related to chromosomal integration, copy number, or renal human GRK4 mRNA level but rather is mainly caused by the effect of the GRK4γ142V transgene acting via D1R.76 In vitro studies showed that in single‐variant (142A>V, 65R>L, or 486A>V) or double‐variant (65L/486V) GRK4γ‐transfected Chinese hamster ovary cells, there is an increase in basal D1R phosphorylation and impairment of D1R‐mediated cAMP production.16 We also found that the function of D3R is also impaired in the GRK4γ142V‐transfected human renal proximal tubule cells (J. Yang, MD, PhD, et al, unpublished data, 2016).
In addition to GRK4 regulation of D1R and D3R in the renal proximal tubule, there is also evidence that GRK4 regulates AT1R expression and activity in this nephron segment. The GRK4 gene variants that are associated with hypertension increase renal proximal tubule AT1R expression and activity. In GRK4γ142V transgenic mice, due to the inhibition of renal histone deacetylase type 1 (but not histone deacetylase type 2) activity, renal AT1R expression and activity are increased, which leads to increased blood pressure. In contrast, AT1R blockade or deletion of the AT1R gene normalizes the hypertension in GRK4γ142V transgenic mice.77 Our recent study also showed that, due to higher nuclear factor κB activity with more nuclear factor κB bound to the AT1R promoter, both AT1R expression and AT1R‐mediated vasoconstriction are higher in the aorta of GRK4γ142V than GRK4γ wild‐type transgenic mice.21 In GRK4γ142V transgenic mice. Ang II causes a greater increase in systolic blood pressure, whereas infusion of the AT1R antagonist candesartan causes a greater decrease in blood pressure in GRK4γ142V transgenic mice than their wild‐type counterparts.21, 77 Similarly, renal AT1R expression is also enhanced in GRK4γ486V transgenic mice fed a high salt diet, which may contribute to the salt‐sensitive phenotype of these mice; however, the GRK4γ wild‐type transgene prevents salt‐sensitive hypertension.77, 78
Role of GRK4 in human essential hypertension
The GRK4 locus on human chromosome 4p16.3 is linked to essential hypertension and salt sensitivity.79, 80, 81, 82, 83, 84, 85 Three missense SNPs ( ie, 65R>L, 142A>V, and 486A>V) in the coding region of GRK4γ are associated with increased blood pressure. Depending on the genetic background of the mouse, mice overexpressing the GRK4γ wild‐type transgene are normotensive and salt‐resistant, whereas GRK4γ142V transgenic mice have high blood pressure even with normal NaCl intake.16, 76, 77 In contrast, GRK4γ486V or GRK4γ65L transgenic mice become hypertensive only after an increase in sodium intake.77, 78
A number of studies have shown the genetic association of the 3 GRK4 SNPs with human essential hypertension in several ethnic groups. The association between GRK4486V and essential hypertension was found in Italian and Euro‐Australian populations.79, 80 In a study of northern Han Chinese participants, the GRK465L, GRK4142V, and GRK4A486 haplotypes are associated with a 6‐fold higher risk of systolic and diastolic hypertension.81 In these same Han Chinese participants, GRK4486V alone is associated with hypertension.82 In an African‐derived semi‐isolated Brazilian population, the combination of NOS3 rs1799983 and GRK4486V is associated with hypertension.83 GRK4486V is also associated with salt sensitivity in a Euro‐American population.84 In a Japanese cohort, the presence of all 3 GRK4 variants impaired the natriuretic effect of a dopaminergic agonist and correctly predicted the presence of salt‐sensitive hypertension in 94% of cases.85 The single‐locus model with only GRK4142V is 78.4% predictive, whereas a 2‐locus model of GRK4142V and aldosterone synthase CYP11B2 is 77.8% predictive of low‐renin hypertension.85 Some reports, however, do not show the association between GRK4 variants and hypertension that may be related to the failure to study all of the GRK4 variants or to the age of the participants.86, 87
G protein–coupled receptor kinase 5
The human GRK5 gene locus maps to the long arm of chromosome 10: 10q26.11 by Ensembl, Entrez Gene, and HGNC. GRK5, a 590‐amino acid protein kinase, has 34.8% and 47.2% amino acid identities with GRK2 and GRK1, respectively. GRK5 contains a centrally located protein kinase catalytic domain of 238 amino acid residues flanked by N‐terminal and C‐terminal regions of 193 and 159 amino acid residues, respectively. The atomic structure of GRK5 has been shown to be aligned in manner different from the other GRKs.88 GRK5 mRNA is found most abundantly in the lung, heart, retina, and lingual epithelium but is minimally expressed in brain, liver, kidney, and testis. Many studies of GRK5 have focused on its role in the exacerbation of pathological cardiac hypertrophy89; however, GRK5 is also involved in the pathogenesis of hypertension. GRK5, as with GRK2, for example, is also increased in lymphocytes from hypertensive humans and animal models of hypertension.90
The intracardiac injection of adenovirus encoding the amino‐terminal region of GRK5 increases the already elevated blood pressure of SHRs.91 GRK5 overexpression in vascular smooth muscle cells in mice increases blood pressure. The hypertension in male GRK5 transgenic mice is caused in part by a decrease in β1‐AR activity, whereas high blood pressure in female mice is caused by an increase in activity of AT1R.23 Grk5 knockout mice have impaired glucose tolerance and insulin sensitivity, indicating that GRK5 is a positive regulator of insulin sensitivity.92 Increased expression of GRK5 is associated with different animal models of hypertension, including Ang II‐, norepinephrine‐, and L‐NAME–induced hypertension.93, 94 There is a nuclear redistribution of GRK5 in hypertensive heart‐failure‐prone rats.95 The physical association of AT1R and GRK5 is increased in the heart in congestive heart failure but is reversed by exercise training.96 In vitro studies showed that both AT1R and D1R can be the substrates for GRK5. The agonist‐dependent phosphorylation of the AT1R is substantially increased in human embryonic kidney cells overexpressing GRK5, GRK2, or GRK3.97 GRK4 and GRK5 impair both the sensitivity and maximum response of D1R, whereas GRK2 and GRK3 impair only the sensitivity of D1R to agonist stimulation.16, 98 Whereas HDAC1 is involved in the increase in GRK4γ142A>V‐mediated increase in renal AT1R expression,77 HDAC5 is associated with the GRK5‐regulated gene transcription in heart failure.99
GRK5Leu41 is a nonsynonymous polymorphism of GRK5, common in African‐Americans, in which leucine is substituted for glutamine at position 41. A study showed that the GRK5Leu41 polymorphism decreased the risk for adverse cardiovascular response but not for the blood pressure response to antihypertensive medication.100 Another study showed a pharmacogenomic interaction between GRK5Leu41 and β‐AR‐blocker treatment in which the presence of the GRK5Leu41 polymorphism was associated with decreased mortality in African‐Americans with heart failure or cardiac ischemia.101
G protein–coupled receptor kinase 6
The human GRK6 gene locus maps to the long arm of chromosome 5: 5q35.3 by Ensembl, 5q35 by Entrez Gene, and 5q35 by HGNC. The crystal structure of GRK6 has been deciphered.102 GRK6 has higher homology with GRK5 (70.1% amino acid identity) compared with GRK2 (37.4%) and GRK1 (47.1%).The structure of GRK6 reveals a putative phospholipid binding site near the N‐terminus and structural elements within the kinase substrate channel that influence GPCR access and specificity.102 GRK6 is expressed ubiquitously throughout the body, including the brain, skeletal muscle, pancreas, and myometrium and at lower levels in the heart, lung, kidney, placenta, and liver.
As with the other GRKs, GRK6 also regulates the β‐AR and AT1R.103, 104, 105 GRK6 but not GRK2 or GRK5 is involved in the desensitization of calcitonin gene‐related peptide.106 GRK6 also regulates Na+/H+ exchanger regulatory factor107; Na+/H+ exchanger regulatory factor and Na+/H+ exchanger type 3 are involved in the regulation of renal sodium transport. Although D1‐like receptor‐mediated inhibition of renal sodium Na+/K+/ATPase activity requires Na+/H+ exchanger regulatory factor 1,108 and dopamine receptors are important in the regulation of renal sodium transport and blood pressure,59, 74 Na+/H+ exchanger regulatory factor 1 per se does not regulate blood pressure.109 Both D1‐ and D2‐like dopamine receptors are physiological targets of GRK6. Inhibition of GRK6 prevents agonist‐induced desensitization of intestinal D1‐like receptors in rat intestinal epithelial cells.107 Dopamine D2R hypersensitivity occurs with disruption of the GRK6 gene in mice.110 Consequently, abnormalities of the GRK6 gene can lead to D2R supersensitivity, which can result in dysfunction of D2R in the regulation of natriuresis and blood pressure.
The expression of GRK6 is affected by hypertensive status and associated with hypertension‐induced complications. Renal GRK6 levels are lower in hypertensive participants and SHRs than their normotensive controls111; however, GRK6 expression is increased in spontaneously hypertensive heart failure rats.66 Moreover, subcellular redistribution of GRK6 in spontaneously hypertensive heart failure rats is also involved in abnormal remodeling of cardiac myocytes in hypertensive hypertrophy and failure.66 GRK6 is also important in the negative regulation of inflammation,112 which is intimately involved in the regulation of blood pressure and development of hypertension.
GRK Gene Variants and Their Roles in Hypertension
Since the discovery of a linkage between GRKs and cardiovascular disease including hypertension and heart failure, GRKs—especially GRK2 and GRK4—have been considered pharmaceutical targets for the treatment of cardiovascular disease. Moreover, GRK gene variants are also important for guiding therapeutic antihypertensive strategies.100, 113
Current evidence shows that common variants of GRK4 are associated with human essential hypertension and predict the blood pressure response to antihypertensive medicines. Our recent study in hypertensive Japanese participants showed that carriers of GRK4142V had a greater decrease in systolic blood pressure in response to angiotensin receptor blockers than noncarrier hypertensive patients. In contrast, those with variants only at GRK4486V were less likely to achieve the blood pressure goal in response to angiotensin receptor blockers than those with no variants.10 Nevertheless, in a small cohort of Japanese hypertensive participants, those with GRK4486V has a good antihypertensive response to a low‐salt diet or diuretics.114 The association between GRK4 variants and the response to antihypertensive treatment has also been confirmed in American and European hypertensive participants. Results from the African American Study of Kidney Disease and Hypertension Study suggest a sex‐specific relationship between GRK4A142V and blood pressure response among African‐American men with early hypertensive nephrosclerosis. Men with GRK4A142 were less responsive to metoprolol if they also had a GRK4L65 variant, but the additive effect of A142 and L65 variants on blood pressure was not found in women.115 Another study from the Pharmacogenomic Evaluation of Antihypertensive Responses trial involving hypertensive African and Euro‐American participants found that GRK465L and GRK4142V variant alleles and increasing copies of the variant GRK465L and GRK4142V haplotypes are associated with reduced response to β‐blocker monotherapy. Moreover, all 3 GRK4 variants (65L, 142V, and 486V) are associated with increased risk for the primary outcome (first occurrence of all‐cause death, nonfatal myocardial infarction, or nonfatal stroke) in pooled white and Hispanic participants.11 European hypertensive patients who are homozygous for GRK465L and GRK4142V have been reported to need more antihypertensive treatment, especially diuretic therapy, to reach the same mean arterial blood pressure as homozygous carriers of only 1 variant or heterozygous/wild‐type carriers of R65L, A142V, and A486V alleles.116 These results suggest that the presence or absence of GRK4 gene variants may be important determinants in guiding therapeutic antihypertensive strategies.
GRK2 may also influence to response to some antihypertensive medicines. The SNPs in the GRK2 gene are more common in African‐Americans, who have a higher risk for increased blood pressure.100 Specifically, 2 GRK2 SNPs are associated with blood pressure response to antihypertensive medicines. Compared with African‐American patients with the rs4930416 homozygote, those with a heterozygote (rs4930416, A>C) have similar blood pressure at baseline but greater blood pressure reduction with hydrochlorothiazide. Diastolic blood pressure, but not systolic blood pressure, response to atenolol also differs by rs4930416 genotype.100 There are also trends toward different diastolic blood pressure and systolic blood pressure responses with another GRK2 gene SNP, rs1894111, in Euro‐American patients receiving hydrochlorothiazide; however, this SNP is neither associated with altered blood pressure response to atenolol nor response in African American patients.100
Conclusions
Overwhelming data demonstrate that GRKs (GRK2, GRK3, GRK4, GRK5, GRK6), via different mechanisms, play important roles in the regulation of blood pressure (Table).7, 8, 18, 59, 71, 74, 90, 113, 117 Aberrant GRKs in the cardiovascular system and kidney are involved in the pathogenesis of hypertension. Modulation of GRK activity has yielded promising results in the regulation of blood pressure, alleviating cardiovascular and renal dysfunction in a wide variety of animal models and cell culture systems. Genetic studies have found a strong association between GRK gene variants and hypertension. Identification of GRK variants is important in choosing antihypertensive medication and represents a valuable pharmaceutical target for novel therapeutic approaches in the treatment of hypertension. Increased understanding of GRKs in the regulation of blood pressure may give us a novel concept for the pathogenesis of hypertension and provide new therapeutic antihypertensive strategies in the future.
Table 1.
Summary of GRK Family and Hypertension
| GRK Isoform | Tissue Distribution | GRK Modification | Effects of GRK Modification on Blood Pressure and Related GPCRs | GRK Expression and Activity in Hypertension |
|---|---|---|---|---|
| GRK2 | Ubiquitous expression | VSM‐targeted overexpression | Impairs β‐AR induced vasodilation34; increases resting blood pressure34 | Increased GRK2 expression and GRK activity in lymphocytes and arteries in hypertensive patients and SHRs60, 63, 64; increased GRK2 expression in conductance and resistance vessels in SHRs61, 117; decreased GRK2 expression in conductance vessels and no change in GRK2 expression in resistance vessels in L‐NAME‐ induced hypertensive rats61; increased GRK2 expression and GRK activity in mesenteric artery of sedentary SHRs43; increased renal GRK2 expression in obese rats29; increased renal GRK2 expression in offspring of lipopolysaccharide‐treated dams57 |
| Hemizygous mice (GRK2+/−) | No effect on baseline blood pressure but protects against Ang II–induced hypertension and vascular remodeling33 | |||
| Global knockdown using a shRNA | Results in spontaneous hypertension22; increases both vasoconstriction in response to PE and vasodilatation in response to β‐AR stimulation22 | |||
| VSMC‐specific ablation of GRK2 | No effect on baseline blood pressure35; increases β‐AR–mediated vasodilation, but also enhances α1DAR‐stimulated vasoconstriction35 | |||
| Selective deletion of endothelial GRK2 | Blood pressure not measured; blunts vasoconstriction to different agonists42 | |||
|
Gene depletion Renal proximal tubule GRK2 gene depletion |
Blunts desensitization of arterial ETAR46; promotes insulin‐induced vasodilation of mesenteric arteries in SHRs43blunts desensitization of renal proximal tubule D1R54 | |||
| GRK3 | Ubiquitous expression | Cardiac‐restricted GRK3 inhibition | Increases blood pressure and cardiac output67; increased cardiac myocyte α1‐AR responsiveness67; attenuates cardiac dysfunction caused by pressure overload24 | No significant difference in GRK3 expression in the lymphocytes of hypertensive and normotensive patients9 |
| GRK4 | Testes, myometrium, brain, intestines, kidney, and artery | Overexpression of human GRK4142V | Increases blood pressure (normal salt diet)16, 77; impairs renal D1R function16; increases AT1R expression in the kidney and artery21, 77; increases in systolic blood pressure response to Ang II21 | Increased renal GRK4 expression in SHRs72; increased renal GRK activity in hypertensive subjects16; increased renal GRK4 expression and normalized by rosiglitazone (insulin sensitizer) in obese Zucker rats55; increased renal GRK4 expression in offspring of lipopolysaccharide‐treated dams57 |
| Overexpression of human GRK4486V | Increases blood pressure (on high salt diet) and renal AT1R expression78; increases in basal D1R phosphorylation and impairs the function of D1R16 | |||
| Overexpression of human GRK465L | Increase basal D1R phosphorylation and impairs D1R‐mediated cAMP production16 | |||
| GRK4 gene depletion | Increases sodium excretion, attenuates the increased blood pressure and renal serine‐phosphorylated D1R in SHRs72; blunts the D1R desensitization in human RPTCs54; blocks D1R phosphorylation and restores D1R‐ mediated cAMP accumulation in RPTCs from hypertensive participants16 | |||
| GRK5 | Ubiquitous expression | VSM‐specific overexpression | Increases blood pressure23 | Increased GRK5 expression in Ang II–treated VSMCs93; increased GRK5 expression in aortas of Ang II– and norepinephrine‐induced hypertension93; no significant difference in GRK5 expression in the lymphocytes of hypertensive and normotensive patients9 |
| Global knockout | Increases insulin resistance92 | |||
| Overexpression of GRK5 | Increases agonist‐dependent phosphorylation of the AT1R or D1R97, 98 | |||
| GRK6 | Ubiquitous expression | Global knockout | Causes striatum D2R supersensitivit110 | Decreased renal GRK6 expression in hypertensive participants and SHRs111; increased myocardial GRK6 expression in SHHF rats66 |
| Inhibition using antibody | Prevents intestinal D1R desensitization107 |
Ang II indicates angiotensin II; AR, adrenergic receptor; AT1R, angiotensin II type 1 receptor; D1R, dopamine D1 receptor; ETAR, endothelin A receptor; GPCR, G protein–coupled receptor; GRK, G protein–coupled receptor kinase; PE, phenylephrine; RPTC, renal proximal tubule cell; SHHF, spontaneously hypertensive heart failure; SHR, spontaneously hypertensive rat; shRNA, small hairpin RNA; VSM, vascular smooth muscle; VSMC, vascular smooth muscle cell.
Sources of Funding
These studies were supported in part by grants from National International Technology special grant (2014DFA31070), National Natural Science Foundation of China (81570379, 81100500), grants from National Institutes of Health, United States (R37HL023081, R01DK039308, R01HL092196, R01DK090908, P01HL068686 and P01HL074940), and by minigrants from the National Kidney Foundation of Maryland.
Disclosures
Dr Jose, who is the Scientific Director of Hypogen, Inc, owns US Patent Number 6 660 474 for G protein–related kinase mutants in essential hypertension. The other authors report no conflicts.
(J Am Heart Assoc. 2016;5:e003519 doi: 10.1161/JAHA.116.003519)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 3. Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol. 2012;2:2393–2442. [DOI] [PubMed] [Google Scholar]
- 4. Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing‐implications in hypertension. J Mol Cell Cardiol. 2015;83:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Curr Opin Cell Biol. 2014;27:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of G protein‐coupled receptor kinases in cardiac health and disease. Physiol Rev. 2015;95:377–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brinks HL, Eckhart AD. Regulation of GPCR signaling in hypertension. Biochim Biophys Acta. 2010;1802:1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santulli G, Trimarco B, Iaccarino G. G‐protein‐coupled receptor kinase 2 and hypertension: molecular insights and pathophysiological mechanisms. High Blood Press Cardiovasc Prev. 2013;20:5–12. [DOI] [PubMed] [Google Scholar]
- 9. Oliver E, Rovira E, Montó F, Valldecabres C, Julve R, Muedra V, Ruiz N, Barettino D, D'Ocon P. beta‐Adrenoceptor and GRK3 expression in human lymphocytes is related to blood pressure and urinary albumin excretion. J Hypertens. 2010;28:1281–1289. [DOI] [PubMed] [Google Scholar]
- 10. Sanada H, Yoneda M, Yatabe J, Williams SM, Bartlett J, White MJ, Gordon LN, Felder RA, Eisner GM, Armando I, Jose PA. Common variants of the G protein‐coupled receptor type 4 are associated with human essential hypertension and predict the blood pressure response to angiotensin receptor blockade. Pharmacogenomics J. 2016;16:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vandell AG, Lobmeyer MT, Gawronski BE, Langaee TY, Gong Y, Gums JG, Beitelshees AL, Turner ST, Chapman AB, Cooper‐DeHoff RM, Bailey KR, Boerwinkle E, Pepine CJ, Liggett SB, Johnson JA. G protein receptor kinase 4 polymorphisms: β‐blocker pharmacogenetics and treatment‐related outcomes in hypertension. Hypertension. 2012;60:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collett JA, Hart AK, Patterson E, Kretzer J, Osborn JL. Renal angiotensin II type 1 receptor expression and associated hypertension in rats with minimal SHR nuclear genome. Physiol Rep. 2013;1:e00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chugh G, Lokhandwala MF, Asghar M. Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension. 2012;59:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pei F, Wang X, Yue R, Chen C, Huang J, Huang J, Li X, Zeng C. Differential expression and DNA methylation of angiotensin type 1A receptors in vascular tissues during genetic hypertension development. Mol Cell Biochem. 2015;402:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, Felder RA, Jose PA. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int. 2006;70:1072–1079. [DOI] [PubMed] [Google Scholar]
- 16. Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen‐Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein‐coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA. 2002;99:3872–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Z, Yu P, Asico LD, Felder RA, Jose PA. Protein phosphatase 2A B56alpha during development in the spontaneously hypertensive rat. Clin Exp Hypertens. 2004;26:243–254. [DOI] [PubMed] [Google Scholar]
- 18. Harris RC. Abnormalities in renal dopamine signaling and hypertension: the role of GRK4. Curr Opin Nephrol Hypertens. 2012;21:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Small KM, Tanguay DA, Nandabalan K, Zhan P, Stephens JC, Liggett SB. Gene and protein domain‐specific patterns of genetic variability within the G‐protein coupled receptor superfamily. Am J Pharmacogenomics. 2003;3:65–71. [DOI] [PubMed] [Google Scholar]
- 20. Johnson LR, Robinson JD, Lester KN, Pitcher JA. Distinct structural features of G protein‐coupled receptor kinase 5 (GRK5) regulate its nuclear localization and DNA‐ binding ability. PLoS One. 2013;8:e62508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen K, Fu C, Chen C, Liu L, Ren H, Han Y, Yang J, He D, Zhou L, Yang Z, Zhang L, Jose PA, Zeng C. Role of GRK4 in the regulation of arterial AT1 receptor in hypertension. Hypertension. 2014;63:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tutunea‐Fatan E, Caetano FA, Gros R, Ferguson SS. GRK2 targeted knock‐down results in spontaneous hypertension, and altered vascular GPCR signaling. J Biol Chem. 2015;290:5141–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keys JR, Zhou RH, Harris DM, Druckman CA, Eckhart AD. Vascular smooth muscle overexpression of G protein‐coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on Gi‐mediated signaling. Circulation. 2005;112:1145–1153. [DOI] [PubMed] [Google Scholar]
- 24. von Lueder TG, Gravning J, How OJ, Vinge LE, Ahmed MS, Krobert KA, Levy FO, Larsen TS, Smiseth OA, Aasum E, Attramadal H. Cardiomyocyte‐restricted inhibition of G protein‐coupled receptor kinase‐3 attenuates cardiac dysfunction after chronic pressure overload. Am J Physiol Heart Circ Physiol. 2012;303:H66–H74. [DOI] [PubMed] [Google Scholar]
- 25. Sterne‐Marr R, Leahey PA, Bresee JE, Dickson HM, Ho W, Ragusa MJ, Donnelly RM, Amie SM, Krywy JA, Brookins‐Danz ED, Orakwue SC, Carr MJ, Yoshino‐Koh K, Li Q, Tesmer JJ. GRK2 activation by receptors: role of the kinase large lobe and carboxyl‐terminal tail. Biochemistry. 2009;48:4285–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmid E, Neef S, Berlin C, Tomasovic A, Kahlert K, Nordbeck P, Deiss K, Denzinger S, Herrmann S, Wettwer E, Weidendorfer M, Becker D, Schäfer F, Wagner N, Ergün S, Schmitt JP, Katus HA, Weidemann F, Ravens U, Maack C, Hein L, Ertl G, Müller OJ, Maier LS, Lohse MJ, Lorenz K. Cardiac RKIP induces a beneficial β‐adrenoceptor‐dependent positive inotropy. Nat Med. 2015;21:1298–1306. [DOI] [PubMed] [Google Scholar]
- 27. Chen M, Sato PY, Chuprun JK, Peroutka RJ, Otis NJ, Ibetti J, Pan S, Sheu SS, Gao E, Koch WJ. Prodeath signaling of G protein‐coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal‐regulated kinase‐dependent heat shock protein 90‐mediated mitochondrial targeting. Circ Res. 2013;112:1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taguchi K, Matsumoto T, Kobayashi T. G‐protein‐coupled receptor kinase 2 and endothelial dysfunction: molecular insights and pathophysiological mechanisms. J Smooth Muscle Res. 2015;51:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banday AA, Lokhandwala MF. Oxidative stress reduces renal dopamine D1 receptor‐Gq/11alpha G protein‐phospholipase C signaling involving G protein‐coupled receptor kinase 2. Am J Physiol Renal Physiol. 2007;293:F306–F315. [DOI] [PubMed] [Google Scholar]
- 30. Sorriento D, Ciccarelli M, Santulli G, Illario M, Trimarco B, Iaccarino G. Trafficking GRK2: cellular and metabolic consequences of GRK2 subcellular localization. Transl Med UniSa. 2014;10:3–7. [PMC free article] [PubMed] [Google Scholar]
- 31. Fusco A, Santulli G, Sorriento D, Cipolletta E, Garbi C, Dorn GW II, Trimarco B, Feliciello A, Iaccarino G. Mitochondrial localization unveils a novel role for GRK2 in organelle biogenesis. Cell Signal. 2012;24:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sato PY, Chuprun JK, Ibetti J, Cannavo A, Drosatos K, Elrod JW, Koch WJ. GRK2 compromises cardiomyocyte mitochondrial function by diminishing fatty acid‐mediated oxygen consumption and increasing superoxide levels. J Mol Cell Cardiol. 2015;89:360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Avendaño MS, Lucas E, Jurado‐Pueyo M, Martínez‐Revelles S, Vila‐Bedmar R, Mayor F Jr, Salaices M, Briones AM, Murga C. Increased nitric oxide bioavailability in adult GRK2 hemizygous mice protects against angiotensin II‐induced hypertension. Hypertension. 2014;63:369–375. [DOI] [PubMed] [Google Scholar]
- 34. Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. Vascular‐targeted overexpression of G protein‐coupled receptor kinase‐2 in transgenic mice attenuates beta‐adrenergic receptor signaling and increases resting blood pressure. Mol Pharmacol. 2002;61:749–758. [DOI] [PubMed] [Google Scholar]
- 35. Cohn HI, Harris DM, Pesant S, Pfeiffer M, Zhou RH, Koch WJ, Dorn GW II, Eckhart AD. Inhibition of vascular smooth muscle G protein‐coupled receptor kinase 2 enhances alpha1D‐adrenergic receptor constriction. Am J Physiol Heart Circ Physiol. 2008;295:H1695–H1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952–958. [DOI] [PubMed] [Google Scholar]
- 37. Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of beta‐adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci USA. 1996;93:12974–12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Gao E, Lau WB, Wang Y, Liu G, Li JJ, Wang X, Yuan Y, Koch WJ, Ma XL. G‐protein‐coupled receptor kinase 2‐mediated desensitization of adiponectin receptor 1 in failing heart. Circulation. 2015;131:1392–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim DH, Kim C, Ding EL, Townsend MK, Lipsitz LA. Adiponectin levels and the risk of hypertension: a systematic review and meta‐analysis. Hypertension. 2013;62:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Du QH, Han L, Jiang JJ, Li PT, Wang XY, Jia X. Increased endothelin receptor B and G protein coupled kinase‐2 in the mesentery of portal hypertensive rats. World J Gastroenterol. 2013;19:2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vinge LE, Øie E, Andersson Y, Grøgaard HK, Andersen G, Attramadal H. Myocardial distribution and regulation of GRK and beta‐arrestin isoforms in congestive heart failure in rats. Am J Physiol Heart Circ Physiol. 2001;281:H2490–H2499. [DOI] [PubMed] [Google Scholar]
- 42. Ciccarelli M, Sorriento D, Franco A, Fusco A, Del Giudice C, Annunziata R, Cipolletta E, Monti MG, Dorn GW II, Trimarco B, Iaccarino G. Endothelial G protein‐coupled receptor kinase 2 regulates vascular homeostasis through the control of free radical oxygen species. Arterioscler Thromb Vasc Biol. 2013;33:2415–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xing W, Li Y, Zhang H, Mi C, Hou Z, Quon MJ, Gao F. Improvement of vascular insulin sensitivity by downregulation of GRK2 mediates exercise‐induced alleviation of hypertension in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2013;305:H1111–H1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cipolletta E, Campanile A, Santulli G, Sanzari E, Leosco D, Campiglia P, Trimarco B, Iaccarino G. The G protein coupled receptor kinase 2 plays an essential role in beta‐adrenergic receptor‐induced insulin resistance. Cardiovasc Res. 2009;84:407–415. [DOI] [PubMed] [Google Scholar]
- 45. Passmore JC, Joshua IG, Rowell PP, Tyagi SC, Falcone JC. Reduced alpha adrenergic mediated contraction of renal preglomerular blood vessels as a function of gender and aging. J Cell Biochem. 2005;96:672–681. [DOI] [PubMed] [Google Scholar]
- 46. Morris GE, Nelson CP, Standen NB, Challiss RA, Willets JM. Endothelin signalling in arterial smooth muscle is tightly regulated by G protein‐coupled receptor kinase 2. Cardiovasc Res. 2010;85:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inagaki S, Ghirlando R, Vishnivetskiy SA, Homan KT, White JF, Tesmer JJ, Gurevich VV, Grisshammer R. G protein‐coupled receptor kinase 2 (GRK2) and 5 (GRK5) exhibit selective phosphorylation of the neurotensin receptor in vitro. Biochemistry. 2015;54:4320–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morris GE, Nelson CP, Everitt D, Brighton PJ, Standen NB, Challiss RA, Willets JM. G protein‐coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y‐purinoceptor signalling. Cardiovasc Res. 2011;89:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baines D. Kinases as targets for ENaC regulation. Curr Mol Pharmacol. 2013;6:50–64. [DOI] [PubMed] [Google Scholar]
- 50. Lee IH, Song SH, Campbell CR, Kumar S, Cook DI, Dinudom A. Regulation of the epithelial Na+ channel by the RH domain of G protein‐coupled receptor kinase, GRK2, and Galphaq/11. J Biol Chem. 2011;286:19259–19269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanchez‐Perez A, Kumar S, Cook DI. GRK2 interacts with and phosphorylates Nedd4 and Nedd4‐2. Biochem Biophys Res Commun. 2007;359:611–615. [DOI] [PubMed] [Google Scholar]
- 52. Dinudom A, Fotia AB, Lefkowitz RJ, Young JA, Kumar S, Cook DI. The kinase Grk2 regulates Nedd4/Nedd4‐2‐dependent control of epithelial Na+ channels. Proc Natl Acad Sci USA. 2004;101:11886–11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sedaghat K, Tiberi M. Cytoplasmic tail of D1 dopaminergic receptor differentially regulates desensitization and phosphorylation by G protein‐coupled receptor kinase 2 and 3. Cell Signal. 2011;23:180–192. [DOI] [PubMed] [Google Scholar]
- 54. Watanabe H, Xu J, Bengra C, Jose PA, Felder RA. Desensitization of human renal D1 dopamine receptors by G protein‐coupled receptor kinase 4. Kidney Int. 2002;62:790–798. [DOI] [PubMed] [Google Scholar]
- 55. Trivedi M, Lokhandwala MF. Rosiglitazone restores renal D1A receptor‐Gs protein coupling by reducing receptor hyperphosphorylation in obese rats. Am J Physiol Renal Physiol. 2005;289:F298–F304. [DOI] [PubMed] [Google Scholar]
- 56. Fardoun RZ, Asghar M, Lokhandwala M. Role of nuclear factor kappa B (NF‐kappaB) in oxidative stress‐induced defective dopamine D1 receptor signaling in the renal proximal tubules of Sprague‐Dawley rats. Free Radic Biol Med. 2007;42:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang X, Luo H, Chen C, Chen K, Wang J, Cai Y, Zheng S, Yang X, Zhou L, Jose PA, Zeng C. Prenatal lipopolysaccharide exposure results in dysfunction of the renal dopamine D1 receptor in offspring. Free Radic Biol Med. 2014;76:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ito K, Haga T, Lameh J, Sadée W. Sequestration of dopamine D2 receptors depends on coexpression of G‐protein‐coupled receptor kinases 2 or 5. Eur J Biochem. 1999;260:112–119. [DOI] [PubMed] [Google Scholar]
- 59. Cuevas S, Villar VA, Jose PA, Armando I. Renal dopamine receptors, oxidative stress, and hypertension. Int J Mol Sci. 2013;14:17553–17572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gros R, Chorazyczewski J, Meek MD, Benovic JL, Ferguson SS, Feldman RD. G‐Protein‐coupled receptor kinase activity in hypertension: increased vascular and lymphocyte G‐protein receptor kinase‐2 protein expression. Hypertension. 2000;35:38–42. [DOI] [PubMed] [Google Scholar]
- 61. Oliver E, Flacco N, Arce C, Ivorra MD, D'Ocon MP, Noguera MA. Changes in adrenoceptors and G‐protein‐coupled receptor kinase 2 in L‐NAME‐induced hypertension compared to spontaneous hypertension in rats. J Vasc Res. 2014;51:209–220. [DOI] [PubMed] [Google Scholar]
- 62. Huang CJ, Slusher AL, Whitehurst M, Wells M, Mock JT, Maharaj A, Shibata Y. Acute aerobic exercise mediates G protein‐coupled receptor kinase 2 expression in human PBMCs. Life Sci. 2015;135:87–91. [DOI] [PubMed] [Google Scholar]
- 63. Izzo R, Cipolletta E, Ciccarelli M, Campanile A, Santulli G, Palumbo G, Vasta A, Formisano S, Trimarco B, Iaccarino G. Enhanced GRK2 expression and desensitization of betaAR vasodilatation in hypertensive patients. Clin Transl Sci. 2008;1:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cohn HI, Xi Y, Pesant S, Harris DM, Hyslop T, Falkner B, Eckhart AD. G protein‐coupled receptor kinase 2 expression and activity are associated with blood pressure in black Americans. Hypertension. 2009;54:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vinge LE, Andressen KW, Attramadal T, Andersen GØ, Ahmed MS, Peppel K, Koch WJ, Freedman NJ, Levy FO, Skomedal T, Osnes JB, Attramadal H. Substrate specificities of G protein‐coupled receptor kinase‐2 and ‐3 at cardiac myocyte receptors provide basis for distinct roles in regulation of myocardial function. Mol Pharmacol. 2007;72:582–591. [DOI] [PubMed] [Google Scholar]
- 66. Yi XP, Zhou J, Baker J, Wang X, Gerdes AM, Li F. Myocardial expression and redistribution of GRKs in hypertensive hypertrophy and failure. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:13–23. [DOI] [PubMed] [Google Scholar]
- 67. Vinge LE, von Lueder TG, Aasum E, Qvigstad E, Gravning JA, How OJ, Edvardsen T, Bjørnerheim R, Ahmed MS, Mikkelsen BW, Oie E, Attramadal T, Skomedal T, Smiseth OA, Koch WJ, Larsen TS, Attramadal H. Cardiac‐restricted expression of the carboxyl‐terminal fragment of GRK3 uncovers distinct functions of GRK3 in regulation of cardiac contractility and growth: GRK3 controls cardiac alpha1‐adrenergic receptor responsiveness. J Biol Chem. 2008;283:10601–10610. [DOI] [PubMed] [Google Scholar]
- 68. Eckhart AD, Duncan SJ, Penn RB, Benovic JL, Lefkowitz RJ, Koch WJ. Hybrid transgenic mice reveal in vivo specificity of G protein‐coupled receptor kinases in the heart. Circ Res. 2000;86:43–50. [DOI] [PubMed] [Google Scholar]
- 69. Manunta P, Ferrandi M, Cusi D, Ferrari P, Staessen J, Bianchi G. Personalized therapy of hypertension: the past and the future. Curr Hypertens Rep. 2016;18:24. [DOI] [PubMed] [Google Scholar]
- 70. Hasenkamp S, Telgmann R, Staessen JA, Hagedorn C, Dördelmann C, Bek M, Brand‐Herrmann SM, Brand E. Characterization and functional analyses of the human G protein‐coupled receptor kinase 4 gene promoter. Hypertension. 2008;52:737–746. [DOI] [PubMed] [Google Scholar]
- 71. Yang J, Villar VA, Jones JE, Jose PA, Zeng C. G protein‐coupled receptor kinase 4: role in hypertension. Hypertension. 2015;65:1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of genetic hypertension by suppression of renal G protein‐coupled receptor kinase type 4 expression. Hypertension. 2006;47:1131–1139. [DOI] [PubMed] [Google Scholar]
- 73. Villar VA, Jones JE, Armando I, Palmes‐Saloma C, Yu P, Pascua AM, Keever L, Arnaldo FB, Wang Z, Luo Y, Felder RA, Jose PA. G protein‐coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor. J Biol Chem. 2009;284:21425–21434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang MZ, Harris RC. Antihypertensive mechanisms of intra‐renal dopamine. Curr Opin Nephrol Hypertens. 2015;24:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Natarajan AR, Eisner GM, Armando I, Browning S, Pezzullo JC, Rhee L, Dajani M, Carey RM, Jose PA. The renin‐angiotensin and renal dopaminergic systems interact in normotensive humans. J Am Soc Nephrol. 2016;27:265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang Z, Armando I, Asico LD, Escano C, Wang X, Lu Q, Felder RA, Schnackenberg CG, Sibley DR, Eisner GM, Jose PA. The elevated blood pressure of human GRK4γA142V transgenic mice is not associated with increased ROS production. Am J Physiol Heart Circ Physiol. 2007;292:H2083–H2092. [DOI] [PubMed] [Google Scholar]
- 77. Wang Z, Zeng C, Villar VA, Chen SY, Konkalmatt P, Wang X, Asico LD, Jones JE, Yang Y, Sanada H, Felder RA, Eisner GM, Weir MR, Armando I, Jose PA. Human GRK4γ142V variant promotes angiotensin II type I receptor‐mediated hypertension via renal histone deacetylase type 1 inhibition. Hypertension. 2016;67:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang Z, Asico L, Wang X, Escano C, Jose P. Human G protein‐coupled receptor kinase type 4γ (GRK4γ) 486V‐promoted salt sensitivity in transgenic mice is related with increased AT1 receptor (AT1R) [abstract]. J Am Soc Nephrol. 2007;18:148A. [Google Scholar]
- 79. Bengra C, Mifflin TE, Khripin Y, Manunta P, Williams SM, Jose PA, Felder RA. Genotyping essential hypertension SNPs using a homogenous PCR method with universal energy transfer primers. Clin Chem. 2002;48:2131–2140. [PubMed] [Google Scholar]
- 80. Speirs HJ, Katyk K, Kumar NN, Benjafield AV, Wang WY, Morris BJ. Association of G‐protein‐coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. J Hypertens. 2004;22:931–936. [DOI] [PubMed] [Google Scholar]
- 81. Wang Y, Li B, Zhao W, Liu P, Zhao Q, Chen S, Li H, Gu D. Association study of G protein‐coupled receptor kinase 4 gene variants with essential hypertension in northern Han Chinese. Ann Hum Genet. 2006;70:778–783. [DOI] [PubMed] [Google Scholar]
- 82. Gu D, Su S, Ge D, Chen S, Huang J, Li B, Chen R, Qiang B. Association study with 33 single‐nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47:1147–1154. [DOI] [PubMed] [Google Scholar]
- 83. Kimura L, Angeli CB, Auricchio MT, Fernandes GR, Pereira AC, Vicente JP, Pereira TV, Mingroni‐Netto RC. Multilocus family‐based association analysis of seven candidate polymorphisms with essential hypertension in an African‐derived semi‐isolated Brazilian population. Int J Hypertens. 2012;2012:859219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, Park MJ, Sobota RS, Underwood PC, Williams J, Sun B, Raby B, Lasky‐Su J, Hopkins PN, Adler GK, Williams SM, Jose PA, Felder RA. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium‐bicarbonate cotransporter. Hypertension. 2012;60:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Watanabe T, Moore JH, Ritchie MD, Williams SM, Pezzullo JC, Sasaki M, Eisner GM, Jose PA, Felder RA. Single‐nucleotide polymorphisms for diagnosis of salt sensitive hypertension. Clin Chem. 2006;52:352–360. [DOI] [PubMed] [Google Scholar]
- 86. Staessen JA, Kuznetsova T, Zhang H, Maillard M, Bochud M, Hasenkamp S, Westerkamp J, Richart T, Thijs L, Li X, Brand‐Herrmann SM, Burnier M, Brand E. Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension. 2008;51:1643–1650. [DOI] [PubMed] [Google Scholar]
- 87. Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, Schork NJ, O'Connor DT. Population‐based sample reveals gene‐gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. [DOI] [PubMed] [Google Scholar]
- 88. Komolov KE, Bhardwaj A, Benovic JL. Atomic structure of GRK5 reveals distinct structural features novel for G protein‐coupled receptor kinases. J Biol Chem. 2015;290:20629–20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hullmann JE, Grisanti LA, Makarewich CA, Gao E, Gold JI, Chuprun JK, Tilley DG, Houser SR, Koch WJ. GRK5‐mediated exacerbation of pathological cardiac hypertrophy involves facilitation of nuclear NFAT activity. Circ Res. 2014;115:976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Harris DM, Cohn HI, Pesant S, Eckhart AD. GPCR signalling in hypertension: role of GRKs. Clin Sci (Lond). 2008;115:79–89. [DOI] [PubMed] [Google Scholar]
- 91. Sorriento D, Santulli G, Fusco A, Anastasio A, Trimarco B, Iaccarino G. Intracardiac injection of AdGRK5‐NT reduces left ventricular hypertrophy by inhibiting NF‐kappaB‐dependent hypertrophic gene expression. Hypertension. 2010;56:696–704. [DOI] [PubMed] [Google Scholar]
- 92. Wang L, Shen M, Wang F, Ma L. GRK5 ablation contributes to insulin resistance. Biochem Biophys Res Commun. 2012;429:99–104. [DOI] [PubMed] [Google Scholar]
- 93. Ishizaka N, Alexander RW, Laursen JB, Kai H, Fukui T, Oppermann M, Lefkowitz RJ, Lyons PR, Griendling KK. G protein‐coupled receptor kinase 5 in cultured vascular smooth muscle cells and rat aorta. Regulation by angiotensin II and hypertension. J Biol Chem. 1997;272:32482–32488. [DOI] [PubMed] [Google Scholar]
- 94. Montó F, Oliver E, Vicente D, Buendía F, Rueda J, Agüero J, Almenar L, Valldecabres C, Rovira E, Muedra V, Noguera MA, Ivorra MD, D'Ocon P. β2‐ and β1‐adrenoceptor expression exhibits a common regulatory pattern with GRK2 and GRK5 in human and animal models of cardiovascular diseases. J Cardiovasc Pharmacol. 2015;66:478–486. [DOI] [PubMed] [Google Scholar]
- 95. Yi XP, Gerdes AM, Li F. Myocyte redistribution of GRK2 and GRK5 in hypertensive, heart‐failure‐prone rats. Hypertension. 2002;39:1058–1063. [DOI] [PubMed] [Google Scholar]
- 96. Haack KK, Engler CW, Papoutsi E, Pipinos II, Patel KP, Zucker IH. Parallel changes in neuronal AT1R and GRK5 expression following exercise training in heart failure. Hypertension. 2012;60:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Oppermann M, Freedman NJ, Alexander RW, Lefkowitz RJ. Phosphorylation of the type 1A angiotensin II receptor by G protein‐coupled receptor kinases and protein kinase C. J Biol Chem. 1996;271:13266–13272. [DOI] [PubMed] [Google Scholar]
- 98. Tiberi M, Nash SR, Bertrand L, Lefkowitz RJ, Caron MG. Differential regulation of dopamine D1A receptor responsiveness by various G protein‐coupled receptor kinases. J Biol Chem. 1996;271:3771–3778. [DOI] [PubMed] [Google Scholar]
- 99. Zhang Y, Matkovich SJ, Duan X, Gold JI, Koch WJ, Dorn GW II. Nuclear effects of G‐protein receptor kinase 5 on histone deacetylase 5‐regulated gene transcription in heart failure. Circ Heart Fail. 2011;4:659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lobmeyer MT, Wang L, Zineh I, Turner ST, Gums JG, Chapman AB, Cooper‐DeHoff RM, Beitelshees AL, Bailey KR, Boerwinkle E, Pepine CJ, Johnson JA. Polymorphisms in genes coding for GRK2 and GRK5 and response differences in antihypertensive‐ treated patients. Pharmacogenet Genomics. 2011;21:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, Diwan A, Martini JS, Sparks L, Parekh RR, Spertus JA, Koch WJ, Kardia SL, Dorn GW II. A GRK5 polymorphism that inhibits beta‐adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lodowski DT, Tesmer VM, Benovic JL, Tesmer JJ. The structure of G protein‐coupled receptor kinase (GRK)‐6 defines a second lineage of GRKs. J Biol Chem. 2006;281:16785–16793. [DOI] [PubMed] [Google Scholar]
- 103. Simon V, Robin MT, Legrand C, Cohen‐Tannoudji J. Endogenous G protein‐coupled receptor kinase 6 triggers homologous beta‐adrenergic receptor desensitization in primary uterine smooth muscle cells. Endocrinology. 2003;144:3058–3066. [DOI] [PubMed] [Google Scholar]
- 104. Baameur F, Morgan DH, Yao H, Tran TM, Hammitt RA, Sabui S, McMurray JS, Lichtarge O, Clark RB. Role for the regulator of G‐protein signaling homology domain of G protein‐coupled receptor kinases 5 and 6 in beta 2‐adrenergic receptor and rhodopsin phosphorylation. Mol Pharmacol. 2010;77:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. Beta‐arrestin2‐mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA. 2006;103:16284–16289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Aiyar N, Disa J, Dang K, Pronin AN, Benovic JL, Nambi P. Involvement of G protein‐coupled receptor kinase‐6 in desensitization of CGRP receptors. Eur J Pharmacol. 2000;403:1–7. [DOI] [PubMed] [Google Scholar]
- 107. Fraga S, Luo Y, Jose P, Zandi‐Nejad K, Mount DB, Soares‐da‐Silva P. Dopamine D1‐like receptor‐mediated inhibition of Cl/HCO3‐ exchanger activity in rat intestinal epithelial IEC‐6 cells is regulated by G protein‐coupled receptor kinase 6 (GRK 6). Cell Physiol Biochem. 2006;18:347–360. [DOI] [PubMed] [Google Scholar]
- 108. Salyer S, Lesousky N, Weinman EJ, Clark BJ, Lederer ED, Khundmiri SJ. Dopamine regulation of Na+‐K+‐ATPase requires the PDZ‐2 domain of sodium hydrogen regulatory factor‐1 (NHERF‐1) in opossum kidney cells. Am J Physiol Cell Physiol. 2011;300:C425–C434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ. Targeted disruption of the mouse NHERF‐1 gene promotes internalization of proximal tubule sodium‐phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci USA. 2002;99:11470–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, Premont RT. Dopaminergic supersensitivity in G protein‐coupled receptor kinase 6‐deficient mice. Neuron. 2003;38:291–303. [DOI] [PubMed] [Google Scholar]
- 111. Xu J, Watanabe H, Felder RA, Jose PA. GRK6 in the kidney in human and rat genetic hypertension [abstract]. FASEB J. 2001;15:A774. [Google Scholar]
- 112. Kavelaars A, Vroon A, Raatgever RP, Fong AM, Premont RT, Patel DD, Lefkowitz RJ, Heijnen CJ. Increased acute inflammation, leukotriene B4‐induced chemotaxis, and signaling in mice deficient for G protein‐coupled receptor kinase 6. J Immunol. 2003;171:6128–6134. [DOI] [PubMed] [Google Scholar]
- 113. Rayner B, Ramesar R. The importance of G protein‐coupled receptor kinase 4 (GRK4) in pathogenesis of salt sensitivity, salt sensitive hypertension and response to antihypertensive treatment. Int J Mol Sci. 2015;16:5741–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sanada H, Yatabe J, Yatabe MS, Yokokawa H, Williams S, Bartlett J, Wang Z, Felder R, Jose PA. G Protein‐coupled receptor type 4 gene variants and response to antihypertensive medication [abstract]. Circulation. 2009;120:S1087. [Google Scholar]
- 115. Bhatnagar V, O'Connor DT, Brophy VH, Schork NJ, Richard E, Salem RM, Nievergelt CM, Bakris GL, Middleton JP, Norris KC, Wright J, Hiremath L, Contreras G, Appel LJ, Lipkowitz MS; AASK Study Investigators . G‐protein‐coupled receptor kinase 4 polymorphisms and blood pressure response to metoprolol among African Americans: sex‐specificity and interactions. Am J Hypertens. 2009;22:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Muskalla AM, Suter PM, Saur M, Nowak A, Hersberger M, Krayenbuehl PA. G‐protein receptor kinase 4 polymorphism and response to antihypertensive therapy. Clin Chem. 2014;60:1543–1548. [DOI] [PubMed] [Google Scholar]
- 117. Oliver E, Martí D, Montó F, Flacco N, Moreno L, Barettino D, Ivorra MD, D'Ocon P. The impact of alpha1‐adrenoceptors up‐regulation accompanied by the impairment of beta‐adrenergic vasodilatation in hypertension. J Pharmacol Exp Ther. 2009;328:982–990. [DOI] [PubMed] [Google Scholar]


