Abstract
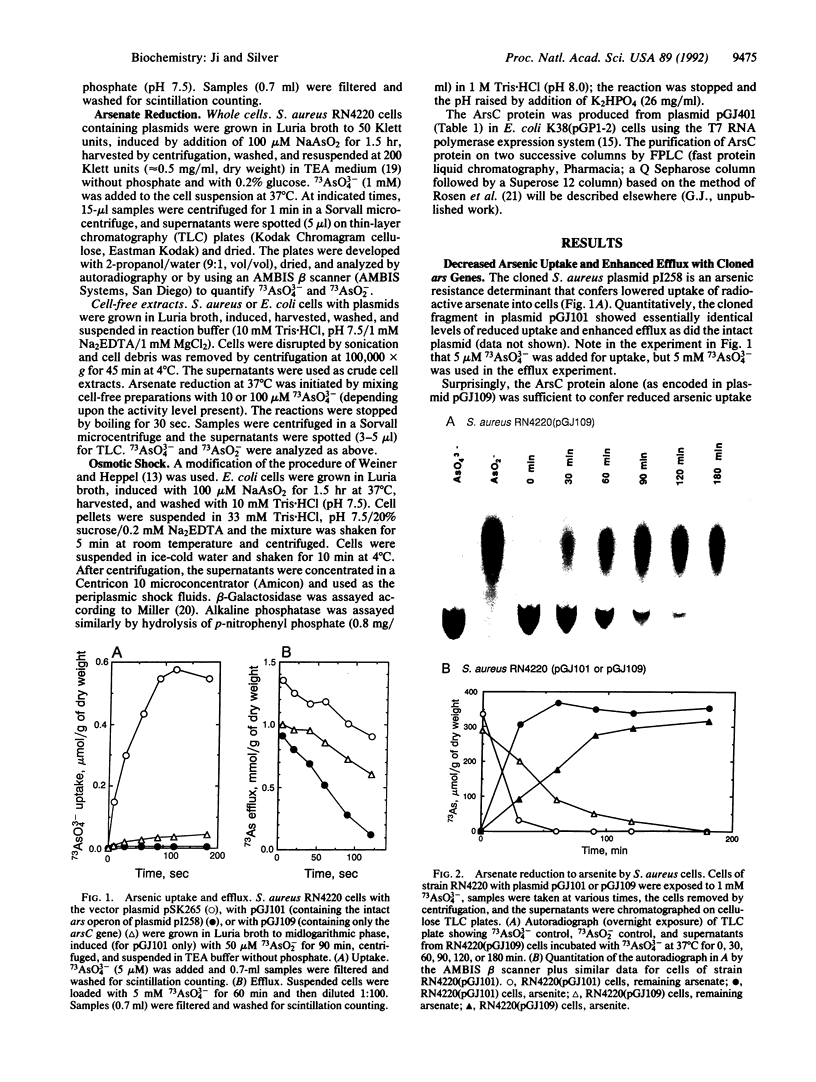
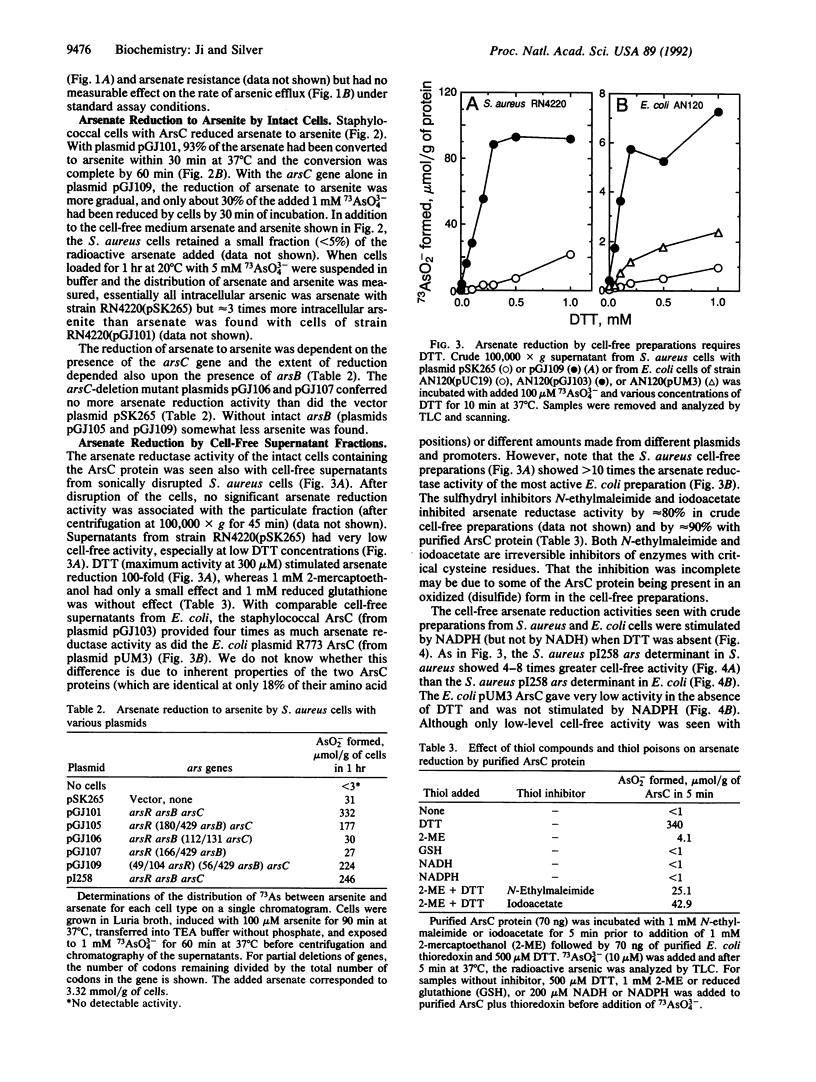
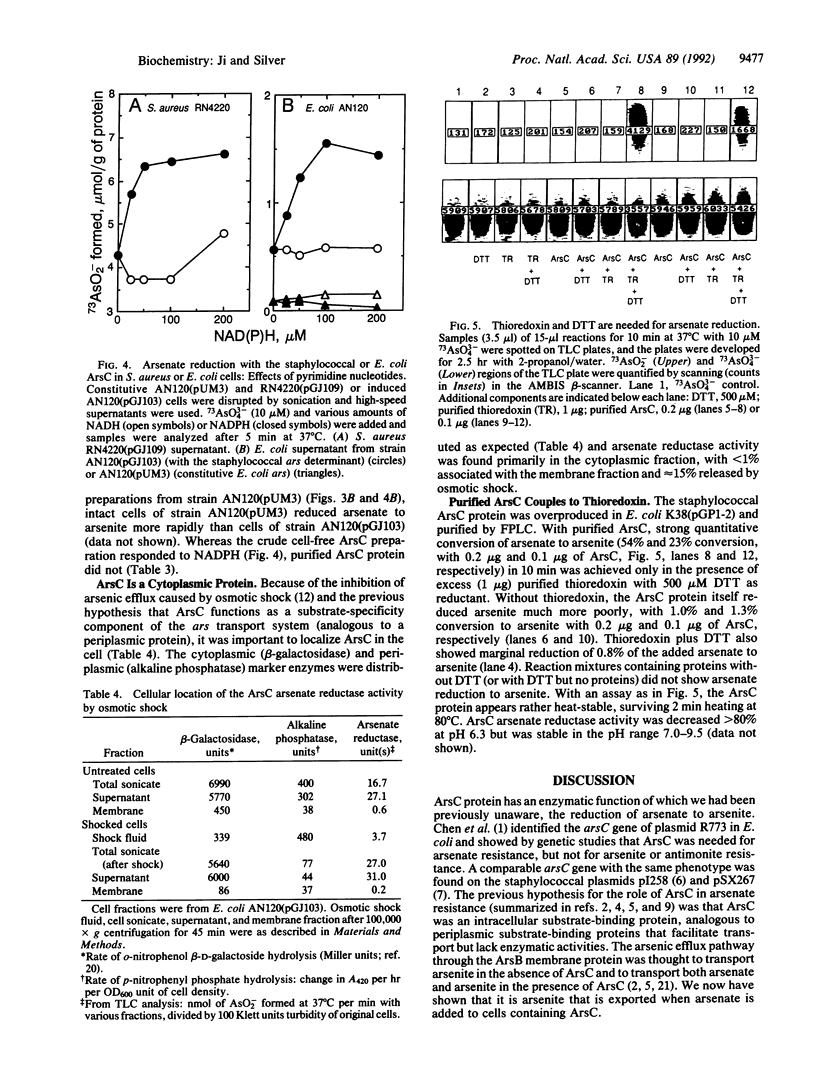
The arsenic resistance operon of Staphylococcus aureus plasmid pI258 consists of three genes, arsR (encoding the repressor regulatory protein), arsB (the determinant of the membrane efflux protein that confers resistance by pumping arsenic from the cells), and arsC (the small gene whose protein product is required for arsenate resistance only, not for arsenite resistance). ArsC has now been shown to be an arsenate reductase, converting intracellular arsenate [As(V)] to arsenite [As(III)], which is then exported from the cells by an energy-dependent efflux process. The arsenate reductase activity was found in the soluble cytoplasmic fraction in Escherichia coli (and not associated with the periplasmic fraction or the sedimentable cell envelope). Purified ArsC protein coupled in vitro with thioredoxin plus dithiothreitol (but not 2-mercaptoethanol or reduced glutathione) to reduce arsenate to arsenite.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Misra T. K., Silver S., Rosen B. P. Nucleotide sequence of the structural genes for an anion pump. The plasmid-encoded arsenical resistance operon. J Biol Chem. 1986 Nov 15;261(32):15030–15038. [PubMed] [Google Scholar]
- Gleason F. K., Lim C. J., Gerami-Nejad M., Fuchs J. A. Characterization of Escherichia coli thioredoxins with altered active site residues. Biochemistry. 1990 Apr 17;29(15):3701–3709. doi: 10.1021/bi00467a016. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Hsu C. M., Rosen B. P. Characterization of the catalytic subunit of an anion pump. J Biol Chem. 1989 Oct 15;264(29):17349–17354. [PubMed] [Google Scholar]
- Ji G., Silver S. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J Bacteriol. 1992 Jun;174(11):3684–3694. doi: 10.1128/jb.174.11.3684-3694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., Rosen B. P. Plasmid-encoded resistance to arsenic and antimony. Plasmid. 1992 Jan;27(1):29–40. doi: 10.1016/0147-619x(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Kren B., Parsell D., Fuchs J. A. Isolation and characterization of an Escherichia coli K-12 mutant deficient in glutaredoxin. J Bacteriol. 1988 Jan;170(1):308–315. doi: 10.1128/jb.170.1.308-315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Rosen B. P. Energetics of plasmid-mediated arsenate resistance in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6119–6122. doi: 10.1073/pnas.79.20.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., Weigel U., Karkaria C., Gangola P. Molecular characterization of an anion pump. The arsA gene product is an arsenite(antimonate)-stimulated ATPase. J Biol Chem. 1988 Mar 5;263(7):3067–3070. [PubMed] [Google Scholar]
- Rosen B. P., Weigel U., Monticello R. A., Edwards B. P. Molecular analysis of an anion pump: purification of the ArsC protein. Arch Biochem Biophys. 1991 Feb 1;284(2):381–385. doi: 10.1016/0003-9861(91)90312-7. [DOI] [PubMed] [Google Scholar]
- Rosenstein R., Peschel A., Wieland B., Götz F. Expression and regulation of the antimonite, arsenite, and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J Bacteriol. 1992 Jun;174(11):3676–3683. doi: 10.1128/jb.174.11.3676-3683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Francisco M. J., Hope C. L., Owolabi J. B., Tisa L. S., Rosen B. P. Identification of the metalloregulatory element of the plasmid-encoded arsenical resistance operon. Nucleic Acids Res. 1990 Feb 11;18(3):619–624. doi: 10.1093/nar/18.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Keach D. Energy-dependent arsenate efflux: the mechanism of plasmid-mediated resistance. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6114–6118. doi: 10.1073/pnas.79.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisa L. S., Rosen B. P. Molecular characterization of an anion pump. The ArsB protein is the membrane anchor for the ArsA protein. J Biol Chem. 1990 Jan 5;265(1):190–194. [PubMed] [Google Scholar]
- Tisa L. S., Rosen B. P. Transport systems encoded by bacterial plasmids. J Bioenerg Biomembr. 1990 Aug;22(4):493–507. doi: 10.1007/BF00762959. [DOI] [PubMed] [Google Scholar]
- Wu J., Rosen B. P. The ArsR protein is a trans-acting regulatory protein. Mol Microbiol. 1991 Jun;5(6):1331–1336. doi: 10.1111/j.1365-2958.1991.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Zitomer R. S., Lowry C. V. Regulation of gene expression by oxygen in Saccharomyces cerevisiae. Microbiol Rev. 1992 Mar;56(1):1–11. doi: 10.1128/mr.56.1.1-11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]