Abstract
Large-conductance, calcium-activated, and voltage-gated K+ (BK) channels are expressed in many tissues of the human body, where they play important roles in signaling not only in excitable but also in nonexcitable cells. Because BK channel properties are rendered in part by their association with four β and four γ subunits, their channel function can differ drastically, depending on in which cellular system they are expressed. Recent studies verify the importance of apically expressed BK channels for airway surface liquid homeostasis and therefore of their significant role in mucociliary clearance. Here, we review evidence that inflammatory cytokines, which contribute to airway diseases, can lead to reduced BK activity via a functional down-regulation of the γ regulatory subunit LRRC26. Therefore, manipulation of LRRC26 and pharmacological opening of BK channels represent two novel concepts of targeting epithelial dysfunction in inflammatory airway diseases.
Keywords: BK channels, airway surface liquid, mucociliary clearance
The large conductance, calcium-activated, and voltage-gated K+ channel is a member of the voltage-gated K+ (Kv) channel superfamily (1). Even though usually called BK channels, they are also known as MaxiK, Slo1, or KCa1.1 and are found in many different tissues in the human body (2, 3). These channels are unique not only because of their large single-channel conductance, but also because of their activation pattern: they can be opened by either a rise in cytoplasmic Ca2+ ([Ca2+]i), by membrane depolarization, or by a combination of both, acting in a synergistic manner (4). On opening, K+ follows its electrochemical gradient, thereby leading to K+ efflux and subsequent hyperpolarization of the membrane.
This review briefly describes BK channel structure but focuses mainly on BK’s recently discovered role in airway surface liquid (ASL) homeostasis and therefore mucociliary clearance (MCC), an important innate host defense mechanism (5, 6). The function of the BK channel in the airway epithelium expands some of the previously recognized basic principles of ASL volume regulation.
Structure of BK Channels
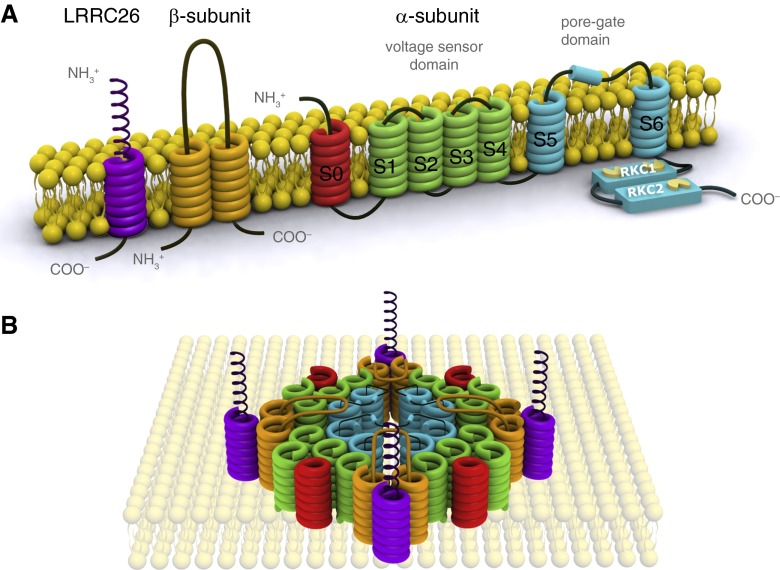
BK channels are located on various cell membranes, including the plasma membrane as well as the mitochondrial and the nuclear membranes (3). The KCNMA1 or SLO1 gene encodes the pore-forming α subunit of the BK channel, which also senses Ca2+− and voltage changes and therefore plays a key role in mediating pore opening (2, 3). The α subunit assembles as a homotetramer and contains four major domains: the voltage-sensing domain (VSD), the cytosolic domain, the extracellular domain, and the pore-gate domain (PGD) (7) (Figure 1). Each α subunit has seven transmembrane segments (S0–S6) and an extracellular N-terminus. The S1–S4 segments contain charged residues, especially in the S4 region, that sense the transmembrane voltage and make up the VSD. The pore is lined by the S5–S6 segments, which are part of the PGD. Although the S1–S6 segments are similar to the ones found in other Kv channels, the S0 is unique in that it gives rise to an extracellular N-terminus. Depolarization of the membrane potential initiates a multistep process. The activation of the VSD leads to interactions between segments of the VSD and the PGD, which results in conformational changes of the pore domain. However, PGD opening can occur by Ca2+ binding at the cytosolic domain, even when the VSD is in its resting state (8).
Figure 1.
(A) Schematic of the structure of the BK channel (adapted from structural depictions of the BK channel). The α subunit consists of seven transmembrane segments, which form the voltage sensor domain (VSD) (S1–4, green) and the pore-gate domain (S5–6, blue). The two “regulators of K+ conductance” domains (also known as cytosolic domains) have Ca2+-binding regions (yellow) and play a role in the channel’s Ca2+ ion-sensing properties. The S0 segment (red) affects VSD activation. β subunits have two transmembrane segments (orange) and a long extracellular loop. The leucine-rich repeat-containing (LRRC) proteins (purple), also known as γ subunits, contain a single transmembrane segment with an extracellular leucine-rich repeat motif. We have shown that LRRC26 modulates BK channel activation in airway epithelial cells (21). (B) Hypothetical spatial representation of the BK channel. The four α subunits form a homotetramer with a central pore. The auxiliary β- and γ subunits are found in the cleft of two adjacent α subunits and regulate BK channel opening.
The extracellular domain is important for interactions with the β1 subunit (1) and possibly the γ subunits. In fact, there are four β subunits (β1–4) and four γ subunits composed of proteins of the leucine-rich repeat-containing (LRRC) proteins. They are LRRC26, 38, 52, and 55 (9, 10). The conformation of the four β subunits is similar. They have two transmembrane segments, a large extracellular loop, and a short cytosolic terminus. Recent studies suggest that the two transmembrane segments are located in the cleft between two α subunits, close to the S0 and S1 segments, whereas the extracellular loop extends over the pore (8). The localization of the γ subunits is not well understood, although studies suggest them to be positioned in the cleft between the VSDs of two α subunits. These subunits can have regulatory and modulatory functions and coassemble with the tetrameric α subunit complex.
β subunits can influence the BK channel kinetics and gating behavior. Because they are expressed in a tissue-specific manner, BK channels can depict particular characteristics in different tissues. The β1, 2, and 4 subunits can enhance channel activity by stabilizing the VSD in an active conformational state (3), whereas some of them can also have inhibitory effects depending on their assembly with other specific subunits. The β2 (dual effects; see Reference 3) and β3 subunits can inhibit K+ flux via membrane hyperpolarization and a physical interaction with the pore (3). The β4 subunit can have different effects depending on [Ca2+]i: β4 appears to decrease channel activation when [Ca2+]i is low and increase it when [Ca2+]i is high (3).
To make matters even more complex, four γ subunits have now been identified. These are all LRRC proteins that have similar molecular weights of around 35 kD. They shift the voltage dependence of BK channel activation toward membrane potentials found in nonexcitatory cells. The largest shift is conferred by LRRC26 (2). They can also shift the [Ca2+]i requirement for BK opening to lower levels. LRRC proteins contain a single transmembrane domain, an N-terminal extracellular leucine-rich repeat domain, and a short C-terminal tail. For proper localization to the membrane, especially of the N-terminal extracellular domain, LRRC26 requires cleavage of its plasma membrane targeting signal peptide. Mutations in the sequence of the signaling peptide result in the loss of the regulatory function of the γ subunit (8), indicating that the extracellular domain is important for LRRC proteins’ function. However, LRRC proteins’ exact interactions with the α subunit and the mechanisms of channel regulation are still poorly understood (2).
In addition, there are at least 10 different splice sites in the KCNMA1 gene that result in variants with diverse functions as recognized by changes in Ca2+ sensitivity and/or voltage, responses to phosphorylation, and membrane expression (3).
Regulation of BK Channel Activity
Modulation of BK channel activity occurs via direct interactions with endogenous and exogenous factors or molecules, usually with the α subunit or less commonly by changing the expression of β subunits (11). BK channel inhibitors are commonly from scorpion venom: peptides such as charybdotoxin, iberiotoxin, slotoxin, and martentoxin can modulate BK channel properties and have been used extensively to identify and characterize the channel (11). On the other hand, arachidonic acids; nitric oxide; high intracellular H+; zinc; protein kinase A, C, and G; and Ca2+ MKII are endogenous activators (12).
BK mutations, commonly associated with loss of function, have been associated with diseases such as asthma, epilepsy, hypertension, cardiac hypertrophy, urinary incontinence, and erectile dysfunction (1, 12, 13). Therefore, using BK channel activation as an antiepileptic strategy and as a protection against cardiac reperfusion injury was a logical development made on the basis of promising preclinical findings. Unfortunately, only a few studies were moved to human trials, partially because of the poor selectivity of compounds and extensive off-target effects (reviewed in Reference 12).
Importance of BK Channels for Mucociliary Function in the Airways
MCC plays a key role in the defense against destructive agents or microbes in the airways. The epithelium maintains an approximately 7-μm–high periciliary layer that allows effective ciliary beating to remove mucus from the airways (14–16). ASL maintains this layer and is also required to hydrate mucus. ASL depletion leads to mucociliary dysfunction, as exemplified by cystic fibrosis (CF) as a severe case and chronic bronchitis as a less severe case. Water availability in the airways is regulated by transepithelial ion transport. Apical Cl− secretion and Na+ absorption play critical roles in ASL volume homeostasis (17). Although Na+ absorption occurs through the epithelial sodium channel, thereby leading to water absorption, both cystic fibrosis transmembrane conductance regulator (CFTR) and calcium-activated chloride channels (CaCC) secrete chloride to make more water available if an appropriate electrochemical gradient for Cl− exit exists. Basolaterally expressed cAMP-dependent potassium channels participate in creating this gradient for CFTR (18), but whether other K+ channels contribute as well, especially at the apical side and particularly to create an apical gradient for Cl− exit through CaCC, has been debated.
Apical potassium channels have been identified previously, but these belong mainly to the cAMP-activated group (19). We recently described the presence of BK channels that are functional at the apical membrane of airway epithelial cells and play important roles in creating the electrochemical gradient necessary for Cl− secretion, at least through CaCC and maybe even through CFTR (20, 21) (Figure 2).
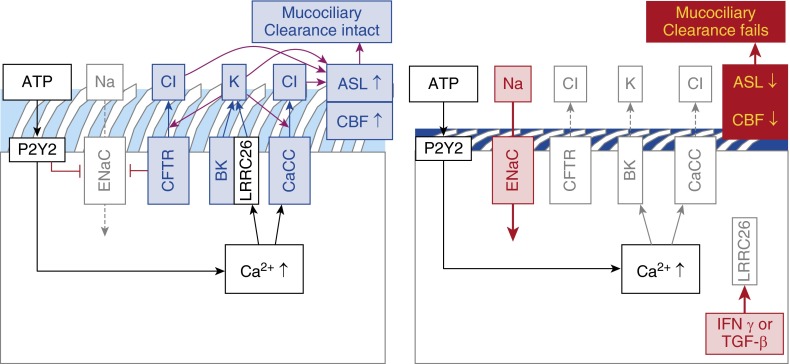
Figure 2.
Apical regulation of ion fluxes to control airway surface liquid (ASL) volume. (Left panel) Normal situation with ATP stimulation of P2Y2 receptors. [Ca2+]i increases stimulate the opening of calcium-activated chloride channels (CaCC) and BK channels. BK channels can only open in these cells because of the presence of the γ regulatory subunit LRCC26. Potassium secretion creates an apical loop current that facilitates Cl− secretion through both CFTR and CaCC channels. The ENaC current is low. (Right panel) When inflammation reduces apical availability of LRRC26, BK channels can no longer open. Cl− secretion is reduced, ENaC currents are now increasing and consequently, ASL volume decreases. CBF = ciliary beat frequency; CFTR = cystic fibrosis transmembrane conductance regulator; ENaC = epithelial sodium channel; LRRC = leucine-rich repeat-containing; TGF = transforming growth factor
We found that BK channels were expressed in freshly isolated cells as well as in air– liquid interface cultured normal human bronchial epithelial (NHBE) cells by quantitative polymerase chain reaction (qPCR). The α subunit and the β2 and β4 regulatory subunits were expressed abundantly, whereas the β3 subunit levels were low, and the β1 subunit expression was low to undetectable. We also described single-channel currents in inside-out patches of trypsinized NHBE cells, with voltage-dependent, high K+ conductance in agreement with the properties expected for BK channels. These currents were sensitive to the BK channel inhibitor paxilline. Ussing chamber experiments using basolaterally permeabilized NHBE cells exposed to a serosal to mucosal K+ gradient supported the presence of BK channels at the apical, but not the basolateral, membrane (20).
The exact cell type expressing BK in the airway epithelium remains unknown. However, the loop current created by them (see discussion below) suggests that the most probable cell type is the ciliated cell. This is supported by electrophysiological data showing that patch clamp currents from ciliated cells were consistent with BK channels (20). In addition, LRRC26 and KCNMA1 messenger RNA (mRNA) expressions during differentiation at the air–liquid interface sharply increase at the time when ciliation occurs (our unpublished results). Thus, basal cells likely do not express functional BK channels, and their expression in goblet cells, if present, is lower than in ciliated cells (Figure 2).
There are several lines of evidence that apical BK channels are important for ASL homeostasis. First, modeling of apical K+ secretion shows that the exit of K+ facilitates Cl− efflux up to threefold (20). K+ exit hyperpolarizes the apical membrane and increases the driving force for Cl− secretion by acting as its counter ion. This creates a short circuit loop favoring airway surface hydration. Interestingly, the modeled [K+] in the ASL reached about 25–30 mM, which is in the range measured in the ASL in a mouse model (19). The latter may not be precisely representative of human tissues, because BK activity in the murine airway seems to be regulated differently compared with human airways, as outlined below.
Because BK channels are activated by increases in [Ca2+]i, one would assume that these channels mainly create an electrochemical gradient for CaCC channels. However, the long-term inhibition of BK channels with antagonists (paxillin for 48 h) or the knockdown of the BK α-subunit KCNMA1, lead to airway surface dehydration and thereby periciliary fluid collapse, as revealed by low ciliary beat frequency (20). Thus, BK channels seem critical for adequate ASL volume maintenance and thus MCC (20, 21), possibly for both CaCC and CFTR.
Except for LRRC26, other γ subunits were not expressed at relevant levels in airway epithelial cells by qPCR (Reference 21 and our unpublished data). We could demonstrate that IFNγ and transforming growth factor (TGF)-β1 down-regulated LRRC26 without affecting the surface availability of the BK α subunits (21, 22). Not surprisingly, overall BK function was therefore decreased, which was associated with ASL volume loss (21, 22). These results suggest that LRRC26 is indirectly an important regulator of ASL homeostasis via modulating BK’s ability to regulate ASL volume.
Interestingly, expression of LRRC26 seems to follow a different pattern in the mouse: there, it is expressed in club cells early in development and disappears from the peripheral airways during development. Furthermore, it is expressed in a Notch-dependent fashion favoring the development of goblet cells (23). In the adult mouse, LRRC26 is present only proximally and not in the peripheral airways (23). In the human airway, LRRC26 is expressed in the entire tracheobronchial system, as shown by qPCR. In Ussing chamber experiments with primary human airway epithelial cells, BK function was invariably and directly related to LRRC26 expression, as measured by qPCR. Furthermore, human airways contain few club cells. Therefore and except for the trachea, the murine airway may not be a good model for human airways for studying ASL volume homeostasis that depends on BK activity, specifically concerning LRRC26. Possibly, additional γ subunits play a role there. Other murine models show similar conflicting results when compared with human airways. These include the CFTR knock-out mouse, which does not develop lung disease (24–26), likely because of high expression of CaCC. Another example is the primary ciliary dyskinesia mouse, which reveals sinus but no lung disease (27).
Changes in BK Activity in Response to IFNγ
IFNγ is an important cytokine for immune defense but also contributes to inflammation and has been associated with airway diseases such as asthma (28–30) and chronic obstructive pulmonary disease (31, 32). Because BK plays a major role in mucociliary dysfunction, we examined the effect of IFNγ on BK activity. Indeed, IFNγ suppressed apical BK activity and caused a decrease in ciliary beat frequency that was consistent with ASL volume depletion (21, 33).
Although real-time quantification showed a statistically significant decrease in KCNMA1 mRNA levels in IFNγ-treated samples, total protein expression, normalized to β-actin, was not significantly changed in Western blots. The β3 subunit did not show a statistically significant difference on IFNγ treatment. On the other hand, β2 was increased, and β4 decreased, by qPCR. However, these modulations do not explain the BK dysfunction after IFNγ exposure. Therefore, surface expression of the pore-forming subunit was assessed, and the results suggested that surface availability of the BK α subunit was not changed (21).
Finally, the function of LRRC26 was evaluated. We used the property of mallotoxin, a special BK opener (34), because mallotoxin only opens BK in the absence of LRRC26 (35). In fact, IFNγ reduced LRRC26 mRNA expression and increased the sensitivity to mallotoxin in our experiments. This is consistent with the hypothesis that IFNγ induces a decrease in LRRC26’s association with BK, thereby contributing to decreased BK activity in airway epithelial cells.
Effect of TGF-β1 on BK Channel Activity and Airway Surface Liquid in CF Cells
TGF-β has been identified as a disease modifier in CF (36). Increased production of TGF-β1 is common in CF airways, especially during exacerbations, and has been associated with worse pulmonary outcome (37, 38). We therefore tested the effects of TGF-β1 on ASL volume in CF cells from patients homozygous for ΔF508 (22). We found that, as with IFNγ, TGF-β1 indeed caused ASL depletion by inhibiting BK activity (22). ASL volume reduction could also be achieved in these CF cells by LRRC26 knockdown. On the other hand, LRRC26 overexpression rescued TGF-β1–induced ASL volume reduction. Finally, TGF-β1–mediated ASL hyperabsorption could be reversed by the BK opener mallotoxin. Perhaps clinically most important, the TGF-β signaling inhibitor pirfenidone, used in practice for slowing down lung function deterioration in patients with interstitial fibrosis, increased BK activity via rescue of LRRC26 and also rescued ASL volume despite the presence of TGF-β1 (22).
Clinical Implications
Currently, there are no in vivo studies assessing the role of BK in regulating MCC in health and disease. On the other hand, a good correlation between preclinical data on ASL volume regulation using human air–liquid interface cultures and clinical outcome was reported in patients with CF (39, 40). In cells from patients with the G551D mutation, ASL volume was reduced in vitro but could be rescued with ivacaftor to an extent similar to that seen with in vivo therapy (10% FEV1 improvement). The same was true for inhaled hypertonic saline (3% FEV1 improvement). These data suggest that BK channel dysfunction could have major implications for in vivo ASL volume homeostasis.
Conclusions
BK channels are expressed ubiquitously and play multiple roles in different cellular systems. One of the more novel findings is that BK channels are important regulators of ASL volume homeostasis. When inflammatory states in the airway are simulated, for instance with IFNγ or TGF-β1, BK activity is reduced and with it, ASL volume. The mechanism seems to be related to a functional down-regulation of the γ regulatory subunit LRRC26 (Figure 2). Further examinations are needed to assess how LRRC26 is down-regulated and whether other inflammatory cytokines have the same effect. Finally, the BK channel and LRRC26 may be amenable to therapeutic interventions. There is at least one BK activator that is still in clinical trials for asthma. In addition, the carbon anhydrase inhibitor acetazolamide has been shown to open BK channels with beneficial effects in animal models (41–43). There has been at least one study with inhaled acetazolamide, showing that it could be inhaled safely as well (44). Therefore, exploring acetazolamide may be an interesting avenue, unless systemic side effects will be prohibitive. In addition, antiinflammatory therapy may rescue LRRC26 function in inflammatory states. One example is the use of pirfenidone in states with elevated TGF-β 1. Furthermore, molecules could be screened for their ability to increase LRRC26’s interaction with BK if LRRC26 association with BK channels is confirmed to be critical for the maintenance of ASL volume. This concept would represent a novel therapeutic strategy to rescue ASL volume in disease states in which expression levels of LRRC26 are reduced.
Acknowledgments
Acknowledgment
The authors thank Gusztav Istvan Velicsek for three-dimensional rendering of Figure 1.
Footnotes
Supported by the Cystic Fibrosis Foundation, the Flight Attendant Medical Research Institute, and the National Institutes of Health.
The views expressed in this article are the views of the authors and do not communicate an official position of funding sources.
Author Contributions: writing of first draft and conception of Figure 1, A.K.; revision of manuscript, S.K.; collection of original data cited in the manuscript, revision of manuscript, and conception of Figure 2, N.B.; finalizing manuscript and figures, and conception of original data cited in manuscript, M.S.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rothberg BS. The BK channel: a vital link between cellular calcium and electrical signaling. Protein Cell. 2012;3:883–892. doi: 10.1007/s13238-012-2076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Yan J. Regulation of BK channels by auxiliary γ subunits. Front Physiol. 2014;5:401. doi: 10.3389/fphys.2014.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle BD, Braun AP. The regulation of BK channel activity by pre- and post-translational modifications. Front Physiol. 2014;5:316. doi: 10.3389/fphys.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magleby KL. Gating mechanism of BK (Slo1) channels: so near, yet so far. J Gen Physiol. 2003;121:81–96. doi: 10.1085/jgp.20028721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 7.Lee US, Cui J. BK channel activation: structural and functional insights. Trends Neurosci. 2010;33:415–423. doi: 10.1016/j.tins.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun X, Zaydman MA, Cui J. Regulation of voltage-activated K(+) channel gating by transmembrane β subunits. Front Pharmacol. 2012;3:63. doi: 10.3389/fphar.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan J, Aldrich RW. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc Natl Acad Sci USA. 2012;109:7917–7922. doi: 10.1073/pnas.1205435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 2010;466:513–516. doi: 10.1038/nature09162. [DOI] [PubMed] [Google Scholar]
- 11.Torres YP, Granados ST, Latorre R. Pharmacological consequences of the coexpression of BK channel α and auxiliary β subunits. Front Physiol. 2014;5:383. doi: 10.3389/fphys.2014.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentzen BH, Olesen SP, Rønn LC, Grunnet M. BK channel activators and their therapeutic perspectives. Front Physiol. 2014;5:389. doi: 10.3389/fphys.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semenov I, Wang B, Herlihy JT, Brenner R. BK channel β1 subunits regulate airway contraction secondary to M2 muscarinic acetylcholine receptor mediated depolarization. J Physiol. 2011;589:1803–1817. doi: 10.1113/jphysiol.2010.204347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 15.Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54:1359–1371. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 16.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarran R. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc Am Thorac Soc. 2004;1:42–46. doi: 10.1513/pats.2306014. [DOI] [PubMed] [Google Scholar]
- 18.Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med. 2012;2:a009563. doi: 10.1101/cshperspect.a009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namkung W, Song Y, Mills AD, Padmawar P, Finkbeiner WE, Verkman AS. In situ measurement of airway surface liquid [K+] using a ratioable K+-sensitive fluorescent dye. J Biol Chem. 2009;284:15916–15926. doi: 10.1074/jbc.M808021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, Larsson HP, Salathe M. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem. 2011;286:19830–19839. doi: 10.1074/jbc.M110.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manzanares D, Srinivasan M, Salathe ST, Ivonnet P, Baumlin N, Dennis JS, Conner GE, Salathe M. IFN-γ-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. Am J Physiol Lung Cell Mol Physiol. 2014;306:L453–L462. doi: 10.1152/ajplung.00247.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manzanares D, Krick S, Baumlin N, Dennis JS, Tyrrell J, Tarran R, Salathe M. Airway surface dehydration by growth factor TGF-β in cystic fibrosis is due to decreased function of a voltage-dependent potassium channel and can be rescued by the drug pirfenidone. J Biol Chem. 2015;290:25710–25716. doi: 10.1074/jbc.M115.670885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guha A, Vasconcelos M, Zhao R, Gower AC, Rajagopal J, Cardoso WV. Analysis of Notch signaling-dependent gene expression in developing airways reveals diversity of Clara cells. PLoS One. 2014;9:e88848. doi: 10.1371/journal.pone.0088848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kent G, Iles R, Bear CE, Huan LJ, Griesenbach U, McKerlie C, Frndova H, Ackerley C, Gosselin D, Radzioch D, et al. Lung disease in mice with cystic fibrosis. J Clin Invest. 1997;100:3060–3069. doi: 10.1172/JCI119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 26.Clarke LL, Grubb BR, Gabriel SE, Smithies O, Koller BH, Boucher RC. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 1992;257:1125–1128. doi: 10.1126/science.257.5073.1125. [DOI] [PubMed] [Google Scholar]
- 27.Ostrowski LE, Yin W, Rogers TD, Busalacchi KB, Chua M, O’Neal WK, Grubb BR. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am J Respir Cell Mol Biol. 2010;43:55–63. doi: 10.1165/rcmb.2009-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar RK, Yang M, Herbert C, Foster PS. Interferon-γ, pulmonary macrophages and airway responsiveness in asthma. Inflamm Allergy Drug Targets. 2012;11:292–297. doi: 10.2174/187152812800958951. [DOI] [PubMed] [Google Scholar]
- 29.Kumar RK, Webb DC, Herbert C, Foster PS. Interferon-gamma as a possible target in chronic asthma. Inflamm Allergy Drug Targets. 2006;5:253–256. doi: 10.2174/187152806779010909. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi M, Ashino S, Shiohama Y, Wakita D, Kitamura H, Nishimura T. IFN-γ elevates airway hyper-responsiveness via up-regulation of neurokinin A/neurokinin-2 receptor signaling in a severe asthma model. Eur J Immunol. 2012;42:393–402. doi: 10.1002/eji.201141845. [DOI] [PubMed] [Google Scholar]
- 31.Panzner P, Lafitte JJ, Tsicopoulos A, Hamid Q, Tulic MK. Marked up-regulation of T lymphocytes and expression of interleukin-9 in bronchial biopsies from patients with chronic bronchitis with obstruction. Chest. 2003;124:1909–1915. doi: 10.1378/chest.124.5.1909. [DOI] [PubMed] [Google Scholar]
- 32.Tzanakis N, Chrysofakis G, Tsoumakidou M, Kyriakou D, Tsiligianni J, Bouros D, Siafakas NM. Induced sputum CD8+ T-lymphocyte subpopulations in chronic obstructive pulmonary disease. Respir Med. 2004;98:57–65. doi: 10.1016/j.rmed.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Harvey PR, Tarran R, Garoff S, Myerburg MM. Measurement of the airway surface liquid volume with simple light refraction microscopy. Am J Respir Cell Mol Biol. 2011;45:592–599. doi: 10.1165/rcmb.2010-0484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zakharov SI, Morrow JP, Liu G, Yang L, Marx SO. Activation of the BK (SLO1) potassium channel by mallotoxin. J Biol Chem. 2005;280:30882–30887. doi: 10.1074/jbc.M505302200. [DOI] [PubMed] [Google Scholar]
- 35.Almassy J, Begenisich T. The LRRC26 protein selectively alters the efficacy of BK channel activators. Mol Pharmacol. 2012;81:21–30. doi: 10.1124/mol.111.075234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, Zariwala M, Fargo D, Xu A, Dunn JM, et al. Gene Modifier Study Group. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med. 2005;353:1443–1453. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 37.Arkwright PD, Pravica V, Geraghty PJ, Super M, Webb AK, Schwarz M, Hutchinson IV. End-organ dysfunction in cystic fibrosis: association with angiotensin I converting enzyme and cytokine gene polymorphisms. Am J Respir Crit Care Med. 2003;167:384–389. doi: 10.1164/rccm.200204-364OC. [DOI] [PubMed] [Google Scholar]
- 38.Arkwright PD, Laurie S, Super M, Pravica V, Schwarz MJ, Webb AK, Hutchinson IV. TGF-beta(1) genotype and accelerated decline in lung function of patients with cystic fibrosis. Thorax. 2000;55:459–462. doi: 10.1136/thorax.55.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tricarico D, Mele A, Calzolaro S, Cannone G, Camerino GM, Dinardo MM, Latorre R, Conte Camerino D. Emerging role of calcium-activated potassium channel in the regulation of cell viability following potassium ions challenge in HEK293 cells and pharmacological modulation. PLoS One. 2013;8:e69551. doi: 10.1371/journal.pone.0069551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinardo MM, Camerino G, Mele A, Latorre R, Conte Camerino D, Tricarico D. Splicing of the rSlo gene affects the molecular composition and drug response of Ca2+-activated K+ channels in skeletal muscle. PLoS One. 2012;7:e40235. doi: 10.1371/journal.pone.0040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tricarico D, Barbieri M, Mele A, Carbonara G, Camerino DC. Carbonic anhydrase inhibitors are specific openers of skeletal muscle BK channel of K+-deficient rats. FASEB J. 2004;18:760–761. doi: 10.1096/fj.03-0722fje. [DOI] [PubMed] [Google Scholar]
- 44.Foresi A, Cavigioli G, Pelucchi A, Mastropasqua B, Marazzini L. Effect of acetazolamide on cough induced by low-chloride-ion solutions in normal subjects: comparison with furosemide. J Allergy Clin Immunol. 1996;97:1093–1099. doi: 10.1016/s0091-6749(96)70263-4. [DOI] [PubMed] [Google Scholar]