MR imaging–detected multicentric cancers that represent ductal carcinoma in situ (DCIS) or invasive cancer smaller than 1 cm could arguably result in overtreatment of patients and more aggressive surgical treatment in approximately three-quarters of patients in this series, whereas 3% (two of 73; 95% confidence interval [CI]: 0.3, 10) of patients had unsuspected invasive cancer in the setting of DCIS, which resulted in underdiagnosis and possible undertreatment, and 3% (two of 73; 95% CI: 0.3, 10) of patients had additional cancer with a higher histologic grade.
Abstract
Purpose
To review the magnetic resonance (MR) imaging and pathologic features of multicentric cancer detected only at MR imaging and to evaluate its potential biologic value.
Materials and Methods
This retrospective study was institutional review board approved and HIPAA compliant; informed consent was waived. A review of records from 2001 to 2011 yielded 2021 patients with newly diagnosed breast cancer who underwent biopsy after preoperative MR imaging, 285 (14%) of whom had additional cancer detected at MR imaging that was occult at mammography. In 73 patients (3.6%), MR imaging identified 87 cancers in different quadrants than the known index cancer, constituting the basis of this report. In 62 of 73 patients (85%; 95% confidence interval [CI]: 75, 92), one additional cancer was found, and in 11 of 73 (15%; 95% CI: 8, 25), multiple additional cancers were found. A χ2 test with adjustment for multiple lesions was used to examine whether MR imaging and pathologic features differ between the index lesion and additional multicentric lesions seen only at MR imaging.
Results
Known index cancers were more likely to be invasive than MR imaging–detected multicentric cancers (88% vs 76%, P = .023). Ductal carcinoma in situ (21 of 87 lesions [24%]; 95% CI: 15, 36) represented a minority of additional MR imaging–detected multicentric cancers. Overall, the size of MR imaging–detected multicentric invasive cancers (median, 0.6 cm; range, 0.1–6.3 cm) was smaller than that of the index cancer (median, 1.2 cm; range, 0.05–7.0 cm; P = .023), although 17 of 73 (23%) (95% CI: 14, 35) patients had larger MR imaging–detected multicentric cancers than the known index lesion, and 18 of 73 (25%) (95% CI: 15, 36) had MR imaging–detected multicentric cancers larger than 1 cm. MR imaging–detected multicentric cancers and index cancers differed in histologic characteristics, invasiveness, and grade in 27 of 73 (37%) patients (95% CI: 26, 49). In four of 73 (5%) patients (95% CI: 2, 13), MR imaging–detected multicentric cancers were potentially more biologically relevant because of the presence of unsuspected invasion or a higher grade.
Conclusion
Multicentric cancer detected only at MR imaging was invasive in 66 of 87 patients (76%), larger than 1 cm in 18 of 73 patients (25%), larger than the known index cancer in 17 of 73 patients (23%), and more biologically important in four of 73 women (5%). An unsuspected additional multicentric cancer seen only at MR imaging is likely clinically relevant disease.
© RSNA, 2015
Introduction
Breast magnetic resonance (MR) imaging is the most sensitive technique for depicting breast cancer, with widespread applications in screening of high-risk patients and preoperative planning (1–3). In younger women and those with dense breasts, MR imaging depicts additional disease that is occult at mammography and ultrasonography (US), both in the same and contralateral breast, and is considered more accurate for evaluating the extent of tumor (4,5). The reported incidence of additional MR imaging–detected disease in the ipsilateral breast is 3%–34%, and it is 3%–24% in the contralateral breast (6,7). However, the clinical importance of additional disease detected at MR imaging is debated.
Recent trials suggest that the addition of MR imaging to traditional imaging makes no difference in reoperation rates (8). In addition, preoperative MR imaging may not change short-term patient outcomes in terms of reduction rates of local recurrence after breast-conserving surgery (9). This may signify that additional MR imaging–detected disease that is occult at mammography is not biologically important or may potentially be treated with radiation and/or chemotherapy (9,10). Additional disease may not be biologically relevant. In general, it is accepted that radiation therapy can likely treat invasive cancer of less than 1 cm, although trials that assess primary radiation therapy for treatment of breast cancer are scarce. Additional controversy surrounds the detection of additional ductal carcinoma in situ (DCIS), as many DCIS lesions may not progress to invasion. Less disputed is the need to treat invasive cancer. The purpose of this study was to review the MR imaging and pathologic features of multicentric cancer detected only at MR imaging and to evaluate its potential biologic value.
Materials and Methods
This study was institutional review board approved and Health Insurance Portability and Accountability Act compliant, and informed consent was waived. The hospital database was searched for women who underwent breast MR imaging between January 2001 and December 2011 where at least one additional suspicious lesion was detected and percutaneous biopsy that yielded cancer was performed within 6 weeks of MR imaging. A total of 2021 patients fit these criteria. Of these, 285 of 2021 (14%) were found to have mammographically occult additional cancer that was only depicted at MR imaging. A breast imaging radiologist (C.I., with 7 years of experience in breast imaging) reviewed these 285 MR imaging examinations and determined that 73 (25%) had cancer located in a separate quadrant or more than 4 cm away from the index lesion. All mammograms were reviewed by the same radiologist (C.I.) to confirm that the cancers were mammographically occult and classify the index cancers according to the Breast Imaging and Reporting Data System. According to the Breast Imaging and Reporting Data System, breast density was retrospectively and qualitatively assessed (11).
The 73 patients with multicentric cancer that was only depicted at MR imaging constitute the basis of this report. All patients underwent mammography less than 6 months before or 2 weeks after breast MR imaging. Our group of patients did not undergo breast US before MR imaging.
US was not routinely performed before MR imaging, but it was used to evaluate patients after MR imaging and guide biopsy if findings were visible. All patients underwent mastectomy. Exclusion criteria included breast MR imaging performed for positive margins (18 of 285 patients [6%]), neoadjuvant chemotherapy [four of 285 patients, 1.5%], no mammogram for evaluation [three of 285 patients, 1%], mammographic evidence of additional disease in a location similar to that seen at MR imaging [54 of 285 patients, 19%], additional disease in the same quadrant or less than 4 cm from the index cancer [124 of 285 patients, 43.5%], and an inability to correlate additional MR imaging–detected disease with the histopathologic report [nine of 285 patients, 3%]).
Breast MR Imaging Methods
Breast MR imaging was performed with patients in the prone position and with a 1.5-T (n = 62) or 3-T (n = 11) imager and a dedicated breast coil. In our institution, patients were randomly assigned to a 1.5- or 3-T machine for both diagnostic and MR imaging–guided biopsy. The standard imaging protocol included a localizing sequence followed by sagittal T2-weighted fat-suppressed, sagittal T1-weighted without fat suppression, and bilateral simultaneous sagittal T1-weighted fat-suppressed sequences performed before and three times after intravenous administration of a bolus of 0.1 mmol/L of gadopentetate dimeglumine (Magnevist; Bayer, Wayne, NJ) per kilogram of body weight. Imager types and sequence acquisition parameters were previously reported (1,12). Section thickness was 3 mm.
MR Imaging Analysis
Breast MR imaging findings were described as having mass or nonmass enhancement, and morphologic and kinetic characteristics were evaluated according to the Breast Imaging and Reporting Data System (12). Kinetic evaluation was performed with Sentinelle Aegis 2.0.1 (Sentinelle Medical, Toronto, Canada) and a segmentation threshold of 80%.
Twenty-two of 87 (25%) additional multicentric MR imaging–detected cancers were biopsied with US guidance, 31 of 87 (36%) were biopsied or localized with MR imaging guidance, and 34 or 87 (39%) additional lesions were reviewed by a pathologist and a radiologist, who correlated MR imaging findings with pathologic sections and the pathologic report for women who were undergoing planned mastectomy without preoperative biopsy or localization. In these patients, anatomic landmarks and MR imaging mutiplanar reconstruction were used for revision.
Pathologic Analysis
All available hematoxylin-eosin and immunostained slides from surgical resection were reviewed (L.G., with 1 year of experience in breast and anatomic pathology). Lesions were assessed for size, tubule formation, nuclear pleomorphism, mitotic count in 10 high-power fields, and necrosis of invasive carcinoma. The modified Scarff-Bloom-Richardson grade was calculated, and the presence of lymphovascular invasion was recorded (13). Invasive carcinoma was classified as having ductal, lobular, or mixed features on the basis of morphologic characteristics. Nuclear grade (low, intermediate, or high), architectural pattern, and the presence of necrosis in DCIS and carcinoma in situ with mixed ductal and lobular features were also evaluated. Size was only evaluated in patients with invasive cancer.
The status of estrogen receptor (ER), progesterone receptor, and human epidermal growth factor receptor type 2 (HER2) was recorded. Invasive carcinomas that were positive for ER and progesterone receptor staining in at least 1% of cells were regarded as being positive for these markers according to the American Society of Clinical Oncology/College of American Pathologists guidelines (14). HER2 staining and HER2 fluorescence in situ hybridization results were recorded as negative, equivocal, or positive according to the American Society of Clinical Oncology/College of American Pathologists guidelines (15). If multiple foci of invasive carcinoma were present in a breast, staining was performed in all foci of invasive carcinoma that appeared morphologically dissimilar. ER staining was performed in DCIS if it was the worst lesion in a mastectomy or lumpectomy specimen without associated microinvasive carcinoma. When foci of DCIS were found in a mastectomy specimen in a patient with an established diagnosis of invasive carcinoma in the ipsilateral breast, ER staining was not performed.
Statistical Methods
The χ2 test was used to examine whether MR imaging features are different between additional lesions and the index lesion, with correction proposed by Rao and Scott (16) that accounts for multiple lesions in patients. The pathologic lesion sizes of the index lesion and additional lesions were compared with the general estimating equations method, a robust covariance matrix, and an independent correlation structure assuming binomial distribution and using the logit link function to take into account multiple lesions per patient. Ninety-five percent exact binomial confidence intervals were calculated for percentages in patients. P < .05 was considered to indicate a significant difference. All statistical analyses were performed with SAS 9.2 (SAS Institute, Cary, NC) and STATA/IC 13.0 (StataCorp, College Station, Tex) software packages.
Results
Of 285 patients with additional mammographically occult cancer detected at MR imaging, 73 (26%; 95% confidence interval [CI]: 21, 31) were found to have 87 additional lesions that were proved to represent cancer in a separate quadrant from that of the index cancer. In 58 of 73 patients (79.5%; 95% CI: 68, 88), the clinical indication for MR imaging was preoperative evaluation, and in 15 of 73 patients (20.5%; 95% CI: 12, 32), it was results of high-risk screening. The median age was 53 years (range, 31–79 years). Sixty-two of 73 patients (85%; 95% CI: 75, 92) had one additional cancer that represented multicentric disease, and 11 of 73 (15%; 95% CI: 8, 25) had multiple additional cancers. Nine patients had two additional cancer sites, and two had three additional disease sites in separate quadrants.
Additional cancers were found in heterogeneously or extremely dense breasts in 59 of 73 patients (81%; 95% CI: 70, 89), and in 14 of 73 patients (19%; 95% CI: 11, 30), they were found in breasts that were classified as predominantly fatty or having scattered fibroglandular tissue. At mammography, breasts were predominantly fatty in two of 73 patients (3%; 95% CI: 0.3, 10), contained scattered fibroglandular tissue in 12 of 73 patients (16%; 95% CI: 9, 27), were heterogeneously dense in 47 of 73 patients (64%; 95% CI: 52, 75), and were extremely dense in 12 of 73 patients (16%; 95% CI: 9, 27).
Index cancers were identified at mammography in 70 of 73 patients (96%; 95% CI: 88, 99) and were mammographically occult in three of 73 patients (4%; 95% CI: 1, 12), manifesting as palpable abnormalities. Of the 70 index cancers that were visible at mammography, 39 (56%; 95% CI: 43, 68) were masses, 11 (16%; 95% CI: 8, 26) were distortion, 10 (14%; 95% CI: 7, 25) were calcifications, seven (10%; 95% CI: 4, 20) were masses and calcifications, four (6%; 95% CI: 2, 14) were asymmetries, and two (3%; 95% CI: 0.3, 10) were masses and distortion.
Index cancers were more frequently invasive than the MR imaging–detected multicentric cancers (88% vs 76%, respectively; P = .023), although the majority of additional MR imaging–detected multicentric cancers were invasive (Table 1). MR imaging–detected multicentric cancers were more likely to be purely DCIS without invasion than were index cancers (24% vs 12%, respectively) (Fig 1). The size of additional MR imaging–detected invasive disease measured at pathologic analysis was smaller than that of the index cancer (median, 0.6 cm; range, 0.1–6.3 cm vs median, 1.2 cm; range, 0.05–7.0 cm, respectively; P = .023) (Table 2). Index cancers were more likely to be a mass at breast MR imaging than were MR imaging–detected multicentric cancers (72% vs 48%, respectively; P = .002) and were more often described as heterogeneous or rim enhancing than were MR imaging–detected multicentric cancers (P = .004).
Table 1.
MR Imaging Features of Index Lesions and Dominant Additional Lesions
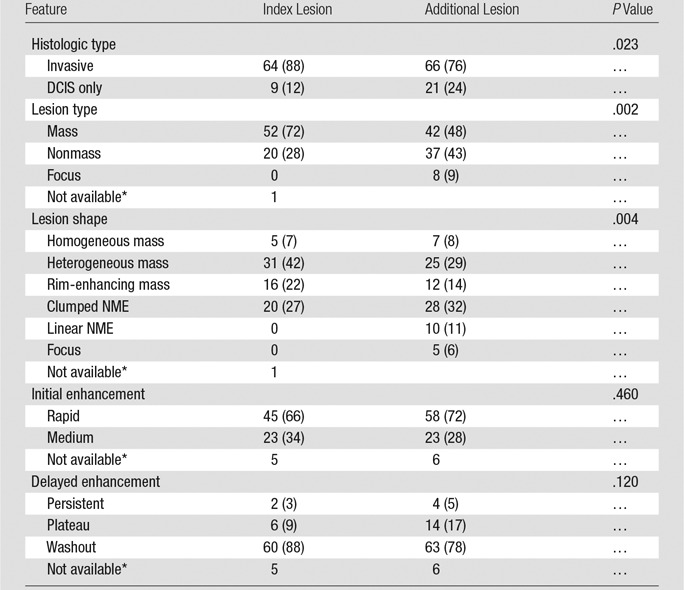
Note.—Unless otherwise indicated, data are numbers of lesions, and data in parentheses are percentages. NME = nonmass enhancement.
*Not included in the test. No residual enhancement was seen after biopsy of the index lesion, and only the clip was visible at MR imaging.
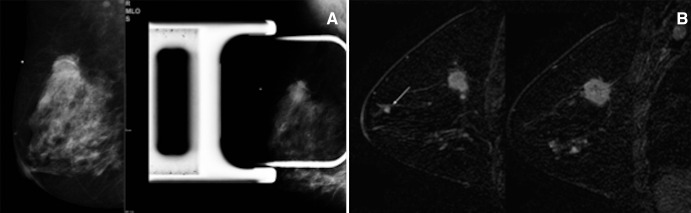
Figure 1:
Mammography and MR imaging in a 46-year-old woman with a palpable mass in the right breast. A, Mediolateral oblique (left) and spot compression (right) mammograms in the right breast show a spiculated mass in the upper-outer quadrant. B, Sagittal dynamic breast MR images in the right breast show an enhancing mass with spiculated margins (the index lesion, IDC) in the right upper outer quadrant, an additional focus near the nipple (DCIS), and nonmass enhancement in the inferior quadrants (DCIS). The additional lesions were not visible at US and were biopsied with MR imaging guidance.
Table 2.
Characteristics of Index Cancers versus MR Imaging–detected Multicentric Cancers
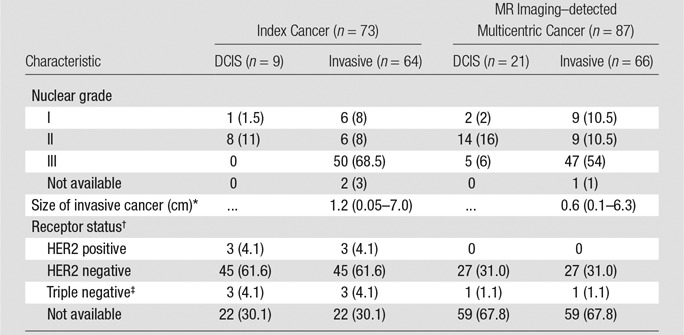
Note.—Unless otherwise indicated, data are numbers of lesions, and data in parentheses are percentages.
*Data are the median, and data in parentheses are the range.
†Receptor status refers to invasive cancers.
‡Negative at ER, progestin receptor, and HER2 staining.
MR imaging–detected multicentric cancers that were located in a separate quadrant represented invasive cancer in the majority of patients (56 of 73 [77%]; 95% CI: 65, 86) and were the following sizes: less than 1 cm in 55 of 73 patients (75%; 95% CI: 64, 85), 1.1–2.0 cm in 12 of 73 patients (16%; 95% CI: 9, 27), and more than 2 cm in six of 73 patients (8%; 95% CI: 3, 17). Purely DCIS was identified in 21 of 87 lesions (24%; 95% CI: 15, 36) and represented a minority in nine of 73 patients (12%; 95% CI: 6, 22).
In 17 of 73 patients (23%; 95% CI: 14, 35), MR imaging–detected multicentric cancer had the same histologic type and grade but was larger than the index cancer. In these patients, the median difference in size between the index cancer and additional MR imaging–detected cancer was 3 cm (range, 0.2–5.0 cm). In two of 73 patients (3%, 95% CI: 0.3, 10), MR imaging–detected multicentric cancer was grade III invasive cancer (size, 0.4 cm and 0.7 cm), whereas the index cancer was grade II DCIS. In two of 73 patients (3%), MR imaging–detected multicentric cancer was a higher grade than the invasive index cancer. In one patient, the index invasive ductal cancer (IDC) and DCIS were grade II (2.9 cm), with an additional grade III index IDC and DCIS lesion (0.3 cm), and in the other patient, the index lesion was grade 1 IDC and grade II DCIS (1.3 cm), with an additional grade II invasive lobular cancer and grade III DCIS (1 cm) (Fig 2). In seven of 73 patients (10%; 95% CI: 4, 19), the grade of additional MR imaging–detected invasive cancers was lower than that of the index lesion.
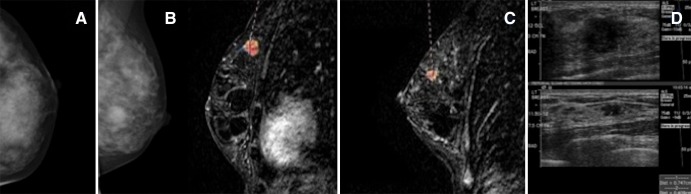
Figure 2:
Mammography and MR imaging in a 48-year-old woman with high risk for breast cancer. A, Screening mammogram shows a craniocaudal view of the left breast. B, Mediolateral oblique screening mammogram (left) shows the left breast, and dynamic sagittal MR image (right) of the left breast shows a 1.3-cm index lesion (grade I CDI and grade II DCIS). C, dynamic sagittal MR image shows an additional 1-cm lesion (grade II CLI and grade III DCIS). D, US image shows both lesions (the index lesion, located at the 12 o’clock position, 3 cm from the nipple, and an additional lesion, located at the 11:30–12 o’clock position, 7.5 cm from the nipple) and was used to guide biopsy.
Overall, there was no statistical difference in histologic grade between index invasive cancers and MR imaging–detected multicentric invasive cancer (P = .297) and between index cancers with both DCIS and invasion and MR imaging–detected multicentric cancers composed of DCIS (P = .245). Finally, no difference was noted in the receptor status between the index cancer and additional MR imaging–detected invasive cancers (P = .380) (Table 2).
For the 28 patients in whom receptor status data for all cases were known, there was no difference between the index cancer and additional MR imaging–detected cancers (P = .381) (Table 2). Among index cancers, there was no difference in kinetics between invasive and DCIS-only cancers.
Additional MR imaging–detected cancers were found across all breast densities, and it appears that breast density is not associated with the size of additional lesions (P = .214). Four of six MR imaging–detected multicentric cancers larger than 2 cm were found in women with extremely dense breasts, and 55 of 71 (77%) MR imaging–detected multicentric cancers smaller than 2 cm were found in women with heterogeneously dense breasts.
Discussion
Breast MR imaging can depict more disease than mammography in both the ipsilateral and contralateral breasts (3,5,17). In 1985, Holland et al (18) showed that 20% of patients with unifocal clinical or mammographic cancer smaller than 2 cm who underwent mastectomy had additional cancer that was mammographically occult and clinically unsuspected, a figure that increased to 43% among patients with primary tumors larger than 2 cm. Although additional disease was found at histologic analysis in 63% of mastectomy specimens, this finding poorly correlates with the low incidence of local treatment failure in women who undergo breast-conserving treatment because additional nonsurgical treatment of the breast is designed to sterilize any residual disease (19).
In our study, although MR imaging–detected multicentric cancers were more commonly discovered in heterogeneously or extremely dense breasts (80%), these cancers occurred in many women with fatty breasts or with scattered fibroglandular densities (20%). Additional disease detected only at MR imaging was more frequently represented by small subcentimeter invasive cancers and DCIS. However, in 76% of cases, it represented invasive cancer, and in approximately one-quarter of cases, it was larger than 1 cm.
In 17 of 73 patients (23%; 95% CI: 14, 35), MR imaging–detected multicentric cancers were larger than the index cancer, possibly changing the disease stage. In 27 of 73 patients (37%; 95% CI: 26, 49), an MR imaging–detected multicentric cancer differed from the index cancer with respect to invasion, grade, and/or receptor status. In two of 73 patients (3%; 95% CI: 0.3, 10), the grade of the additional MR imaging–detected invasive cancer was higher than that of the index cancer, and in two of 73 patients (3%; 95% CI: 0.3, 10), the additional MR imaging–detected cancer was smaller than 1 cm and invasive, while the index cancer was DCIS, possibly changing the patient’s treatment options.
MR imaging played an important role in the assessment of disease extent, depicting more extensive disease in 17 of 73 patients (23%; 95% CI: 14, 35). A more accurate preoperative assessment of disease, especially for additional MR imaging–detected disease larger than 1 cm (median, 3 cm) may improve surgical planning and patient outcomes (20).
The reported treatment failure rate for breast conservation at 10 years is approximately 5%–10%, a figure that supports the argument that most of the additional disease detected at MR imaging is successfully treated with nonsurgical methods (a combination of whole-breast radiation therapy, chemotherapy, and/or hormone therapy) (19). Our findings support that current therapy likely treats small amounts of additional disease, as the majority of additional invasive disease was less than 1 cm and DCIS, which is generally treated with radiation therapy. Confounding the known treatment failure rate of breast carcinoma was the incidence of larger, invasive tumors at sites beyond that of the index cancer in our series. In our analysis, additional invasive cancers that were 1.1–2.0 cm were found in 16% of women (12 of 73; 95% CI: 9, 27), and invasive cancers larger than 2 cm were found in 8% of women (six of 73; 95% CI: 3, 17). Considering local recurrence rates, it may be that the larger, invasive cancers are incompletely treated and result in local treatment failure.
A limitation of our study is the retrospective nature of the data. In addition, US was not routinely performed before preoperative MR imaging and may have depicted some of the multicentric lesions. Furthermore, many (39%) MR imaging–detected multicentric cancers were not biopsied before mastectomy because many high-risk patients opted to undergo mastectomy rather than breast-conserving therapy, limiting our histopathologic validation of lesions. Finally, MR imaging was performed on both 1.5- and 3-T machines, and magnet differences may have affected our results.
The majority of additional cancers seen only at MR imaging represent small-volume disease and may possibly be treated with current treatment algorithms. However, beyond the index cancer, additional MR imaging–detected cancers that are larger than 1 cm and, especially, invasive may not be reliably treated with breast-conserving therapy; such lesions were found in 23% (17 of 73; 95% CI: 14, 35) of our patients. We believe that invasive cancer larger than 1 cm is clinically relevant. In approximately three-quarters of the patients in this series, MR imaging–detected multicentric cancers that represent DCIS or invasive cancer smaller than 1 cm could, arguably, result in overtreatment and more aggressive surgical treatment, whereas 3% (two of 73; 95% CI: 0.3, 10) of patients had unsuspected invasive cancer in the setting of DCIS, resulting in underdiagnosis and, possibly, undertreatment, and 3% (two of 73; 95% CI: 0.3, 10) had additional cancer with a higher histologic grade.
In summary, having a majority of patients undergo potential overtreatment versus a minority who may be undertreated is at the heart of the controversy surrounding the use of breast MR imaging. Patient decisions for diagnosis and treatment may depend on the relative weight placed on either of these options.
Advances in Knowledge
■ Multicentric cancers that were detected only at MR imaging and not at mammography were most frequently invasive carcinomas smaller than 1 cm (median size, 0.6 cm; range, 0.1–6.3 cm), although in 25% (18 of 73; 95% confidence interval [CI]: 15, 36) of patients they were larger than 1 cm.
■ Multicentric cancers that were detected only at MR imaging were larger (1.3 cm; range, 0.2–5.0 cm) than the known index cancer in 17 of 73 patients (23%; 95% CI: 14, 35).
■ Two of 73 patients (3%; 95% CI: 0.3, 10) had unsuspected multicentric invasive cancer in the setting of a known ductal carcinoma in situ, altering the disease stage, and two of 73 patients (3%; 95% CI: 0.3, 10) had unsuspected multicentric invasive cancer of a higher histologic grade in the setting of known invasive index cancer.
Implication for Patient Care
■ Multicentric cancer detected at breast MR imaging that is occult at mammography appears to represent a larger tumor burden in approximately one-quarter of patients and can upstage the diagnosis, resulting in potential changes in treatment.
Acknowledgments
Acknowledgment
The authors thank Charles Fry for his helpful support with data retrieval.
Received April 15, 2015; revision requested June 1; revision received July 7; accepted July 31; final version accepted August 27.
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Disclosures of Conflicts of Interest: C.I. disclosed no relevant disclosed no relevant relationships. L.G. disclosed no relevant disclosed no relevant relationships. J.Z. disclosed no relevant disclosed no relevant relationships. V.S. disclosed no relevant disclosed no relevant relationships. E.J.S. disclosed no relevant disclosed no relevant relationships. D.D. disclosed no relevant disclosed no relevant relationships. E.A.M. disclosed no relevant disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- DCIS
- ductal carcinoma in situ
- ER
- estrogen receptor
- HER2
- human epidermal growth factor receptor type 2
- IDC
- invasive ductal cancer
References
- 1.Sung JS, Malak SF, Bajaj P, Alis R, Dershaw DD, Morris EA. Screening breast MR imaging in women with a history of lobular carcinoma in situ. Radiology 2011;261(2):414–420. [DOI] [PubMed] [Google Scholar]
- 2.Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology 2007;244(2):381–388. [DOI] [PubMed] [Google Scholar]
- 3.Pediconi F, Catalano C, Roselli A, et al. Contrast-enhanced MR mammography for evaluation of the contralateral breast in patients with diagnosed unilateral breast cancer or high-risk lesions. Radiology 2007;243(3):670–680. [DOI] [PubMed] [Google Scholar]
- 4.Cilotti A, Iacconi C, Marini C, et al. Contrast-enhanced MR imaging in patients with BI-RADS 3-5 microcalcifications. Radiol Med (Torino) 2007;112(2):272–286. [DOI] [PubMed] [Google Scholar]
- 5.Liberman L. Breast MR imaging in assessing extent of disease. Magn Reson Imaging Clin N Am 2006;14(3):339–349, vi. [DOI] [PubMed] [Google Scholar]
- 6.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007;356(13):1295–1303. [DOI] [PubMed] [Google Scholar]
- 7.Liberman L, Morris EA, Kim CM, et al. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR Am J Roentgenol 2003;180(2):333–341. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010;375(9714):563–571. [DOI] [PubMed] [Google Scholar]
- 9.Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg 2013;257(2):249–255. [DOI] [PubMed] [Google Scholar]
- 10.Bernardi D, Ciatto S, Pellegrini M, Valentini M, Houssami N. EUSOMA criteria for performing pre-operative MRI staging in candidates for breast conserving surgery: hype or helpful? Breast 2012;21(3):406–408. [DOI] [PubMed] [Google Scholar]
- 11.American College of Radiology BI-RADS Committee . Breast imaging reporting and data system. 5th ed . Reston, Va: American College of Radiology, 2013. [Google Scholar]
- 12.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011;260(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer: I—the value of histological grade in breast cancer—experience from a large study with long-term follow-up. Histopathology 1991;19(5):403–410. [DOI] [PubMed] [Google Scholar]
- 14.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28(16):2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25(1):118–145. [DOI] [PubMed] [Google Scholar]
- 16.Rao JN, Scott AJ. On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat 1984;12(1):46–60. [Google Scholar]
- 17.Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology 2001;220(1):13–30. [DOI] [PubMed] [Google Scholar]
- 18.Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1-2 breast carcinomas: implications for clinical trials of breast-conserving surgery. Cancer 1985;56(5):979–990. [DOI] [PubMed] [Google Scholar]
- 19.Santiago RJ, Fox K, Schultz D, Glick J, Solin LJ. Fifteen-year results of breast-conserving surgery and definitive irradiation for stage I and II breast carcinoma: the University of Pennsylvania experience. 2004 1;58(1):233–240. [DOI] [PubMed] [Google Scholar]
- 20.Sung JS, Li J, Da Costa G, et al. Preoperative breast MRI for early-stage breast cancer: effect on surgical and long-term outcomes. AJR Am J Roentgenol 2014;202(6):1376–1382. [DOI] [PubMed] [Google Scholar]