Abstract
Clonal complex 59 (CC59) Staphylococcus aureus in Taiwan includes both methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA). As the most prominent community-associated MRSA (CA-MRSA) in Taiwan, CC59 has two major clones characterized as PVL-negative SCCmec IV (carrying the staphylococcal cassette chromosome mec IV but Panton-Valentine leukocidin-negative) and PVL-positive SCCmec V (5C2&5). We investigated the drug resistance, phylogeny and the distribution and sequence variation of SCCmec, staphylococcal bacteriophage φSA3, genomic island νSaβ and MES (an enterococcal mobile genetic element conferring multidrug resistance) in 195 CC59 S. aureus. Sequencing and PCR mapping revealed that all of the CC59/SCCmec V (5C2&5) MRSA strains had acquired MESPM1 or its segregants, and obtained a φSA3-related fragment in νSaβ. In contrast, MES6272-2 and MES4578, which showed gentamicin resistance that was not encoded by MESPM1, were dominant in SCCmec IVg MRSA. Translocation of a whole φSA3 into νSaβ instead of only a φSA3-related fragment was common in SCCmec IVg MRSA. However, the non-subtype-g SCCmec IV MRSA (SCCmec IVa is the major) still carried MES and νSaβ structures similar to those in SCCmec V (5C2&5) MRSA. A minimum spanning tree constructed by multiple-locus variable-number tandem repeat analysis revealed that SCCmec IVg MRSA and SCCmec V (5C2&5) MRSA grouped respectively in two major clades. The CC59 MSSA was equally distributed among the two clades, while the non-subtype-g SCCmec IV MRSA mostly clustered with SCCmec V (5C2&5) MRSA. Our findings strongly suggest that CC59 MSSA acquired divergent mobile genetic elements and evolved to SCCmec IVg MRSA and SCCmec V (5C2&5) MRSA/non-subtype-g SCCmec IV MRSA independently. The evolutionary history of CC59 S. aureus explains how mobile genetic elements increase the antimicrobial resistance and virulence and contribute to the success of CA-MRSA in Taiwan.
Introduction
Staphylococcus aureus poses significant public health challenges worldwide. It is well known that early acquisition of the staphylococcal chromosome cassette (SCC) mec in 1961 led to methicillin-resistant S. aureus (MRSA) [1, 2]. Since then, MRSA has imposed a heavy burden in healthcare environments, where it is also known as healthcare-associated MRSA (HA-MRSA) [1, 2]. In the late 1990s, another genetically distinct MRSA, designated community-associated MRSA (CA-MRSA), emerged in the community, causing skin and soft tissue infections (SSTIs) and severe clinical disease, such as necrotizing pneumonia, in children and young adults without antecedent healthcare exposure [2–5]. CA-MRSA is usually susceptible to non-β-lactam antibiotics, in contrast to HA-MRSA [2, 4, 6, 7]. However, accumulation of increased drug resistance and increasing incidence in healthcare facilities has been noted in some CA-MRSA strains, such as the USA300 clone, which emerged in the United States [8].
Currently, five major CA-MRSA clones with sequence type (ST) 1, ST8, ST30, ST59 and ST80 are distributed in specific geographic areas [9, 10]. In Taiwan, the dominant CA-MRSA clone is clonal complex (CC) 59 MRSA, which is responsible for 56% of pediatric CA-MRSA infections [11]. CC59 MRSA is composed of two genotypes: (i) the Asian-Pacific clone, characterized as Panton-Valentine leukocidin (PVL)-negative ST59/SCCmec IV MRSA, which is prevalent among colonizing isolates and in hospitals; and (ii) the Taiwan clone, characterized as PVL-positive ST59/SCCmec V (5C2&5) MRSA, which is dominant in the clinical isolates [10, 12]. The Taiwan clone possesses a novel SCCmec with two distinct ccrC genes (ccrC1 allele 2 and ccrC1 allele 8) [13]. The novel SCCmec was firstly named SCCmec VT [14] and later tentatively designated as SCCmec VII [15]. Finally, it was reclassified as SCCmecV (5C2&5) by the International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements [13].The CC59 MRSA strain has also been reported in Singapore [16], Hong Kong [17], Japan [18, 19], western Australia [20] and Europe [21].
The Taiwan clone possesses several features that are rarely found in other CA-MRSA: (i) resistance to at least four classes of non-β-lactam antimicrobials since it was first isolated in 1997 [14, 22]; (ii) acquisition of MESPM1, which originated in enterococci, encoding resistance to erythromycin, clindamycin, kanamycin, streptomycin and chloramphenicol [23]; (iii) truncation of type I restriction-modification (R-M) system genes (hsdS and hsdM) in genomic island νSaβ [23]; and (iv) retention of the immune evasion cluster (IEC) type C genes chp (encoding CHIPS, chemotaxis inhibitory protein) and scn (encoding SCIN, staphylococcal complement inhibitor) and loss of the Hlb-converting prophage φSA3 [23, 24]. S. aureus strains carry the IEC genes chp, sak (encoding SAK, staphylokinase), scn, sea (encoding SEA, staphylococcal enterotoxin A) and sep (encoding SEP, staphylococcal enterotoxin P) with different combinations [25]. The IEC genes usually cluster on the 3ˈ-end of φSA3 and integrate into hlb [25]. However, the Taiwan clone harbors IEC type C in the νSaβ rather than in φSa3. The acquisition disrupts hsdS and hsdM, which leads to defects in the type I R-M system that is known to block horizontal gene transfer [26] and thus may partly contribute to the introduction of foreign DNA (e.g., MESPM1) in the Taiwan clone [23].
In contrast, the Asian-Pacific clone carries an intact φSA3 with IEC type G, which carries sak (encoding SAK, staphylokinase), sep (encoding SEP, staphylococcal enterotoxin P) and scn based on microarray analysis [24]. The Asian-Pacific clone is also resistant to various non-β-lactam antibiotics, such as erythromycin, clindamycin, kanamycin, streptomycin and gentamicin, although more isolates with resistance to gentamicin and fewer isolates with resistance to streptomycin were noted [27, 28]. However, the genetic characteristics of IEC and multidrug resistance genes remain unknown.
In Taiwan, CC59 S. aureus also comprised 8.8% and 8% of the methicillin-susceptible S. aureus (MSSA) infection and nasal carriage isolates, respectively [29]. An enterococcal IS1216V-mediated multidrug resistance structure nearly identical to MESPM1 has been reported in the PVL-positive ST59 MSSA strain KS1 [19]. Moreover, PVL-positive ST59 MSSA displayed high similarity in PFGE pattern (>80%) and had an identical genetic profile (spa-CC c2:441/437 and agr group I) to PVL-positive ST59/SCCmec V (5C2&5) MRSA [30].
CC59 S. aureus is the only genotype in Taiwan that may be either MRSA or MSSA [30, 31]. The co-existence of CC59 MSSA, SCCmec IV MRSA and SCCmec V (5C2&5) MRSA with unique features in Taiwan may represent a novel evolutionary history. Therefore, we collected 195 CC59 S. aureus strains isolated from northern Taiwan in 2000, 2005, 2010 and 2011 and investigated the distribution of mobile genetic elements, including SCCmec, νSaβ, φSA3 and MES-related elements, within these isolates. The phylogenetic relationships of the CC59 S. aureus strains were determined by multiple-locus variable-number tandem repeat analysis (MLVA) [32].
Materials and Methods
Bacterial isolates
A total of 195 CC59 S. aureus isolates obtained from the National Taiwan University Hospital in 2000, 2005, 2010 and 2011 were investigated in this study (S1 Table); 176 strains were collected from blood culture, and 19 strains were isolated from various specimens (skin and soft tissue, sputum, abscess, etc.) that were characterized as CA-MRSA in our previous study [33]. All isolates were identified as CC59 S. aureus through spa type identification using the RIDOM spa server database [34]. The sequence types of 25 isolates were further confirmed by multilocus sequence typing (MLST) [35]. Three ST59 CA-MRSA strains were used as reference strains: strain PM1 (SCCmec V (5C2&5)) was characterized in our previous study [23], and TSGH17 (SCCmec V (5C2&5)) from Taiwan [14] and USA1000 (SCCmec IVa) from the United States [36] were kindly provided by C. C. Wang and by L. K. McDougal and L. C. McDonald, respectively.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed by the agar dilution method according to the CLSI 2015 guidelines [37] for erythromycin, kanamycin, streptomycin, gentamicin and chloramphenicol. S. aureus ATCC 29213 was used as the reference strain.
SCCmec typing
Characterization of SCCmec and subtyping of SCCmec IV elements were performed by PCR as previously described [33, 38, 39].
Whole genome sequencing
The genome sequence of the multidrug-resistant ST59/SCCmec IVg MRSA strain 4578 was analyzed with an Illumina Genome Analyzer (Illumina, San Diego, CA). Sequencing yielded 1,158,721,894 bp of raw sequence in 11,472,494 reads, which was approximately 413 times the size of the genome. The 913,070 reads were mapped to the genome of ST59/SCCmec V (5C2&5) MRSA strain M013 (accession number CP003166). Contigs were obtained using de novo assembly with an algorithm of Velvet [40]. The gaps between contigs related to mobile genetic elements were filled by PCR and sequencing. Open reading frames were analyzed using the DNAman software package (Version 6) (Lynnon Biosoft, Quebec, Canada).
Detection of resistance determinants and virulence genes
The presence of resistance determinants (ermB, aph(3')-IIIa, aadE, aacA-aphD and cat) and virulence genes (lukPVSF, hlb, chp, sak, scn, sea and sep) was determined by PCR as previously described [25, 27].
Detection of MES, νSaβ and φSA3 structures
The MES structure was mapped by PCR using primer sets a to d. The νSaβ structure was mapped using primer sets e to f. φSA3 integrated within hlb was shown using primer set i and j. All of the primer sets are listed in S2 Table, and the positions of the primers are indicated in S1 Fig. Combing the PCR mapping results and the profiles of resistance determinants or virulence genes, the MES, νSaβ and φSA3 structures were determined.
Multiple-locus variable-number tandem repeat analysis (MLVA)
The MLVA-16orsay PCR with 16 loci was performed as previously described by Sobral et al. [32]. The PCR products were resolved by gel electrophoresis in 1.2 or 3% agarose-0.5× Tris-borate-EDTA. PCR products showing different sizes for each loci were chosen for sequencing to determine the exact numbers of tandem repeats. The MLVA codes were provided in the order corresponding to the genome position in reference strain Mu50 (accession number NC_002758): Sa0122, Sa0266, Sa0311, Sa0704, Sa1132, Sa1194, Sa1291, Sa1729, Sa1866, Sa2039, Sa0387, Sa0550, Sa0684, Sa0964, Sa1097 and Sa2511. The MLVA profile data were imported into BioNumerics (Applied-Maths, Sint-Martens-Latem, Belgium) to construct the minimum spanning tree.
Selection for loss of antibiotic resistance in strain PM1
Loss of antibiotic resistance to erythromycin, kanamycin, streptomycin and chloramphenicol in the ST59/SCCmecV MRSA strain PM1 was tested. After growing overnight on Mueller Hinton II agar (Difco) supplemented with 32 ug/ml erythromycin and 32 ug/ml chloramphenicol, the PM1 was suspended into fresh Mueller Hinton broth (Difco) without antibiotics and inoculated in 37°C for 24 h. The growing cultures were then diluted and spread on Mueller Hinton II agar plates. The resulting colonies were picked and subcultured onto plates containing erythromycin (32 ug/ml) or chloramphenicol (32 ug/ml). Of the 1920 colonies tested, five (PM1-1 to PM1-5) failed to appear on the appropriate antibiotic plate.
Pulsed-field gel electrophoresis (PFGE)
Pulsed-field gel electrophoresis (PFGE) was performed as previously described [41]. In brief, The DNAs of PM1 and PM1-1 to PM1-5 were digested with SmaI (New England BioLabs, Ipswich, MA) and then were separated using a CHEF-DRIII apparatus (Bio-Rad Laboratories). PFGE was carried out at 200 V and 12°C for 20 h with the pulse times ranging from 5 to 60 s.
Southern blot
The DNAs digested with SmaI and separated by the PFGE were transferred to nylon membranes (Amersham Hybond™-N; GE Healthcare) by vacuum blotting. The blotted membranes were prehybridized overnight in DIG Easy Hyb (Roche) at 42°C. The digoxigenin (DIG)-labeled probes specific to IS1216V, ermB and cat were prepared using DIG PCR Probe Synthesis Kit (Roche). The membrane was hybridized with the specific probe overnight in DIG Easy Hyb (Roche) at 42°C. The detection of hybridization was performed with an anti-DIG antibody conjugated to alkaline phosphatase, and CSPD (Roche) was used as a substrate according to the manufacturer’s instructions.
Nucleotide sequences
The nucleotide sequences of SCCmec IVg, νSaβ (including φSA34578) and MES4578 in strain 4578, MES6272-2 in strain 6272–2, MES2250 in strain 2250 and νSaβ in strain 187–4 have been deposited in the GenBank database under accession numbers LC125348 to LC125353.
Statistics
The incidences of drug resistance, virulence genes and IEC types among SCCmec IV MRSA, SCCmec V (5C2&5) MRSA and MSSA were compared using Fisher's exact test. Statistical significance was defined as a P value of <0.05.
Results
Antimicrobial susceptibility and virulence gene distribution among the CC59 S. aureus strains
A total of 195 CC59 S. aureus strains were included in this study (S1 Table). The non-β-lactam antibiograms and distribution of virulence genes lukPVSF, hlb and IEC of the CC59 S. aureus strains are summarized in Table 1. Resistance to erythromycin, kanamycin and chloramphenicol was evenly distributed among the CC59 S. aureus strains. However, gentamicin resistance was predominantly found in SCCmec IV MRSA, while streptomycin resistance was mainly present in SCCmec V (5C2&5) MRSA and MSSA (Fisher’s exact test, P value < 0.05). Resistance to both gentamicin and streptomycin was found in only one SCCmec IV MRSA strain, two SCCmec V (5C2&5) MRSA strains and one MSSA strain (S3 Table). Moreover, strains resistant to gentamicin or streptomycin were always simultaneously resistant to both erythromycin and kanamycin (S3 Table).
Table 1. Drug resistance to non-β-lactams and presence of virulence genes in CC59 S. aureus strains.
| No. (%) of strains | |||
|---|---|---|---|
| SCCmec IV (n = 91) | SCCmec V (5C2&5) (n = 74) | MSSA (n = 30) | |
| Drug resistance rate (%) | |||
| Erythromycin | 85 (93.4) | 66 (89.2) | 27 (90.0) |
| Kanamycin | 80 (87.9) | 66 (89.2) | 27 (90.0) |
| Streptomycin a | 20 (22.0) | 66 (89.2) | 25 (83.3) |
| Gentamicin a | 58 (63.7) | 2 (2.7) | 3 (10.0) |
| Chloramphenicol | 60 (65.9) | 46 (62.2) | 16 (53.3) |
| Susceptible to above antibiotics | 3 (3.3) | 6 (8.0) | 3 (10.0) |
| Presence of virulence genes (%) | |||
| lukPVSF b | 12 (13.2) | 71 (95.9) | 15 (50.0) |
| hlb c | 80 (87.9) | 74 (100) | 30 (100) |
| Immune evasion cluster (IEC) | |||
| Type B: sak, chp, scn c | 13 (14.3) | 0 (0) | 1 (3.3) |
| Type C: chp, scn b | 8 (8.8) | 74 (100) | 25 (83.3) |
| Type D: sea, sak, scn | 3 (3.3) | 0 (0) | 0 (0) |
| Type G: sep, sak, scn b | 63 (69.2) | 0 (0) | 4 (13.3) |
| No IEC | 4 (4.4) | 0 (0) | 0 (0) |
a Statistically significant difference between SCCmec IV MRSA/SCCmec V (5C2&5) MRSA and between SCCmec IV MRSA/MSSA.
b Statistically significant difference among SCCmec IV MRSA, SCCmec V (5C2&5) MRSA and MSSA.
c Statistically significant difference between SCCmec IV MRSA/SCCmec V (5C2&5) MRSA.
Of the virulence genes, the lukPVSF gene (encoding PVL) was more prevalent in SCCmec V (5C2&5) MRSA compared with SCCmec IV MRSA or MSSA. The hlb gene, which is usually truncated due to φSA3 integration [23], was intact in all CC59 S. aureus isolates except 11 SCCmec IV MRSA strains. However, the IEC, which is usually carried by φSA3 [25], was still present in all CC59 S. aureus strains except 4 SCCmec IV MRSA strains. Moreover, the distribution of major IEC types was different (type G in SCCmec IV MRSA vs. type C in SCCmec V (5C2&5) MRSA and MSSA).
Comparative genomics of the Asian-Pacific clone and the Taiwan clone
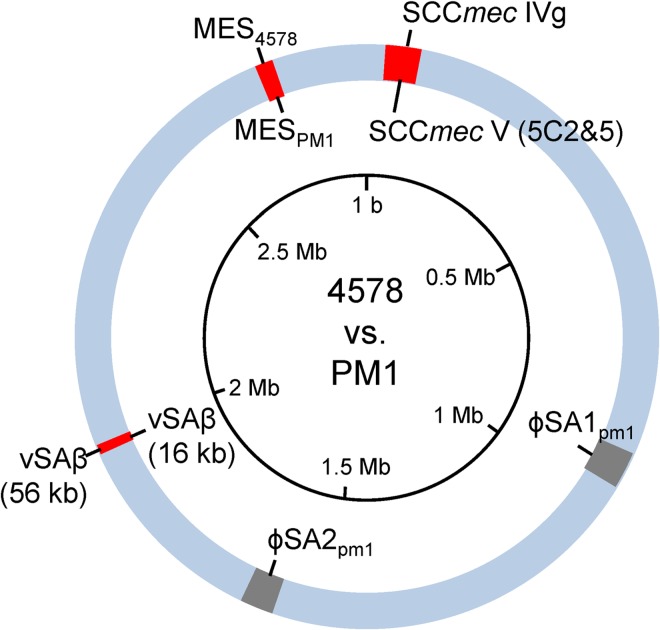
Because the Asian-Pacific clone showed distinct differences in gentamicin/streptomycin resistance and IEC types (Table 1), we were curious if any novel genetic organization was present, similar to MESPM1 and νSAβ in the Taiwan clone [23]. Therefore, the multidrug-resistant SCCmec IV MRSA strain 4578 was chosen for whole genome sequencing, and the results were compared with those from the SCCmec V (5C2&5) MRSA strain PM1 that we previously analyzed [23]. As summarized in Fig 1, two large deletions and three mobile genetic elements with marked divergence were identified: (i) the bacteriophages φSA1pm1 and φSA2pm1 (carrying the lukPVSF gene) were absent in SCCmec IV MRSA strain 4578; and (ii) three mobile genetic elements, SCCmec IVg, MES4578 and a 56-kb νSAβ, were unique in SCCmec IV MRSA strain 4578. The structures of MES4578 and νSAβ are discussed below.
Fig 1. Comparative genomics of 4578 (ST59/SCCmec IVg MRSA) and PM1 (ST59/SCCmec V (5C2&5) MRSA).
Information on the 4578 and PM1 genomes is presented outside and inside the genome circle, respectively. Colored regions: blue, conserved region; gray, deletion in strain 4578; red, distinct mobile genetic elements.
Structures of multidrug resistance elements
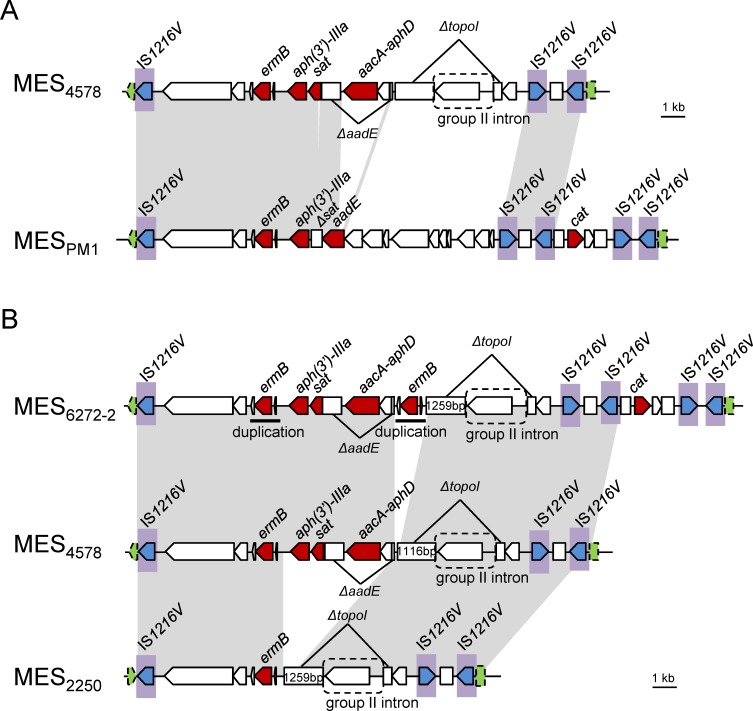
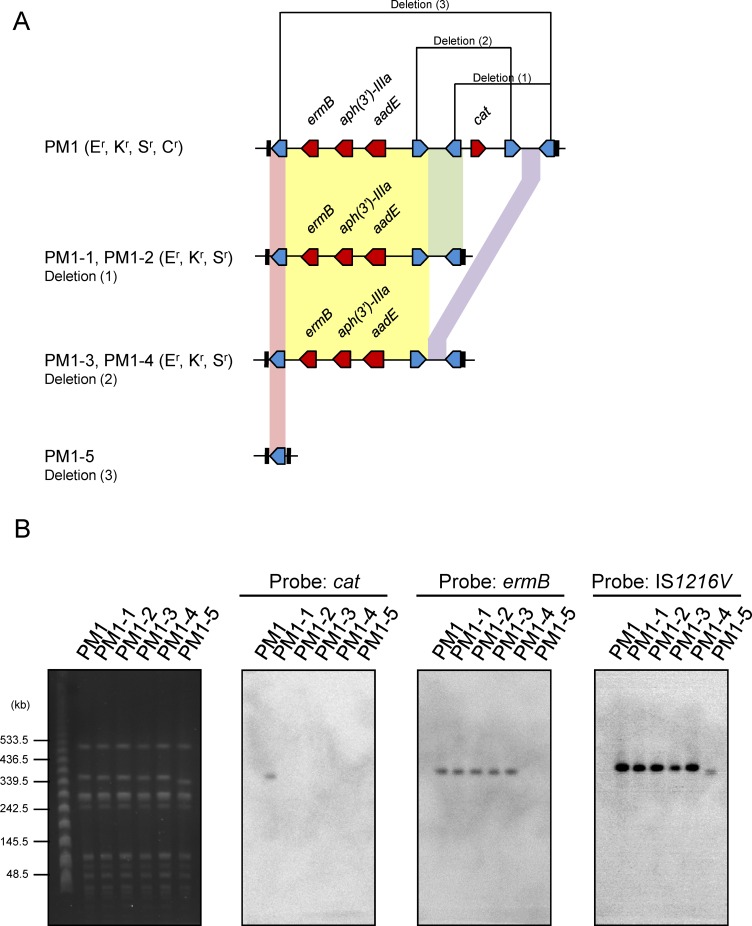
Sequence analysis revealed that MES4578 was flanked with enterococcal IS1216V and inserted into the 8-bp att duplication site within the sasK gene, similar to MESPM1 (Fig 2A). The left side of MES4578 contained an antibiotic resistance gene cluster ermB-(aph(3')-IIIa)-sat-aadE-(aacA-aphD), which is responsible for erythromycin/clindamycin, kanamycin, streptothricin, streptomycin and gentamicin resistance. The antibiotic resistance gene cluster was present in both MES4578 and MESPM1, albeit with some deletions or insertions (Fig 2A and S4 Table). The sat gene remained intact in MES4578, although it was truncated in MESPM1 due to a 62-bp deletion. In contrast, the aadE gene was intact in MESPM1 but was disrupted by the insertion of aacA-aphD and an ORF in MES4578, which explained the marked difference in the streptomycin and gentamicin resistance pattern in SCCmec IV MRSA and SCCmec V (5C2&5) MRSA. The region downstream of the antibiotic resistance gene cluster to the second IS1216V was quite different in MES4578 and MESPM1. The 3′ region of MES4578, between two IS1216V elements, showed 99.9% DNA sequence identity to that in MESPM1. However, the cat gene (responsible for chloramphenicol resistance) and the additional two IS1216V elements were lacking in MES4578. Our previous studies on clinical isolates revealed that excision/insertion event may take place between two direct repeats of IS1216V, leading to the distinct resistance patterns among the ST59/SCCmec V (5C2&5) clinical isolates [23]. In the present study, the excision/insertion event was further shown by selection for loss of antibiotic resistance in PM1, generating five strains with varied drug resistance patterns and MES structures (Fig 3A). PFGE separation of SmaI-digested genomic DNAs followed by Southern blot hybridization with the DIG-labeled cat-, ermB- and IS1216V-specific probes further confirmed the excision/insertion event in strain PM1 (Fig 3B).
Fig 2. The comparison of MES structures integrated into the sasK gene.
(A) MES4578 in ST59/SCCmec IVg MRSA strain 4578 is compared to MESPM1 in ST59/SCCmec V (5C2&5) MRSA strain PM1 (accession number AB699882) [23]. (B) MES6272-2, MES4578 and MES2250 in three ST59/SCCmec IVg MRSA strains 6272–2, 4578 and 2250, respectively, are compared. Homologous regions are shaded in gray. A Greek delta symbol indicates a truncated coding sequence. IS1216V with a transposase gene (tnp) is shaded in blue. The sasK gene is indicated in green arrow and box with dashed line.
Fig 3. Selection for loss of antibiotic resistance in ST59/SCCmec V (5C2&5) MRSA strain PM1.
(A) Cartoon representation of MES structures in PM1 and the generated colonies PM1-1 to PM1-5. (B) SmaI-digested PFGE of PM1 and PM1-1 to PM1-5 and the Southern blot. The SmaI-digested DNA separated by PFGE is shown in the left. The DNA was transferred to a nylon membrane and detected by Southern blot hybridization with DIG-labeled cat-, ermB- and IS1216V-specific probes, which are shown on the right. Abbreviations: Er, erythromycin resistant; Kr, kanamycin resistant; Sr, streptomycin resistant; Cr, chloramphenicol resistant.
The SCCmec IV MRSA showed more complex antimicrobial resistance patterns (S3 Table). Therefore, two SCCmec IV MRSA strains, 6272–2 (resistant to erythromycin, kanamycin, gentamicin and chloramphenicol) and 2250 (resistant to erythromycin only), displaying different antimicrobial resistance patterns were included to determine the full sequences of the antibiotic resistance elements. Sequence analysis revealed that the two elements, MES6272-2 and MES2250, were also flanked with enterococcal IS1216V elements and inserted into the sasK gene (Fig 2B). MES6272-2 had an additional ermB downstream of the aacA-aphD compared to MES4578. MES2250 lacked the antibiotic resistance gene cluster from aph(3')-IIIa to aacA-aphD and was therefore resistant to erythromycin only. Different lengths of the 5'-end-truncated topoisomerase I gene in MES4578 (1661 bp) and in MES6272-2 and MES2250 (1799 bp and 1800 bp) indicated that MES2250 and MES4578 may be generated from MES6272-2 independently. Moreover, MES6272-2 harbored the cat gene (chloramphenicol resistance), which was surrounded by four copies of IS1216V, displaying 99.2% DNA sequence similarity to that in MESPM1. The rightmost sides of the MES4578 and MES2250 sequences both contained two IS1216V elements and lacked the cat gene, suggesting that excision/insertion event also took place in SCCmec IV MRSA between two direct repeats of IS1216V, as we previously described in SCCmec V (5C2&5) MRSA. Detailed comparisons of ORFs in each MES are shown in S4 Table.
The structure of νSAβ
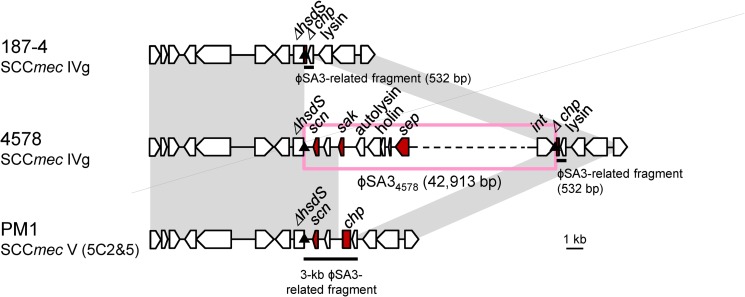
A comparison of the νSaβ structures in 4578 (SCCmec IV MRSA) and PM1 (SCCmec V (5C2&5) MRSA) is shown in Fig 4 and S5 Table. The two strains both carried truncated hsdS genes but harbored different IEC types. PM1 carried IEC type C (chp and scn), while 4578 carried IEC type G (sak, scn and sep). Remarkably, IEC type G in 4578 was carried by a 42.9-kb bacteriophage (φSA34578). The φSA34578, which belonged to the Siphoviridae family, was assigned to the φSA3 group based on the integrase gene sequence. As previously described, the φSA3 group is usually specifically integrated within the hlb gene and produces a 13-bp att sequence duplication at both ends [25]. However, φSA34578 was translocated into νSaβ and was still demarcated by the same att sequences. Interestingly, the νSaβ in 4578 also harbored a 141-bp chp remnant and the lysin gene (φSA3-related fragment) just downstream of φSA34578. This region (532 bp) showed 99.9% DNA sequence similarity to the corresponding region in PM1.
Fig 4. The νSaβ structure of strains 4578, PM1 and 187–4.
The strains 4578 and 187–4 are ST59/SCCmec IVg MRSA carrying IEC type G or no IEC, respectively. Strain PM1 is ST59/SCCmec V (5C2&5) MRSA with IEC type C. Homologous regions are shaded. A Greek delta symbol indicates a truncated coding sequence. The att sequence is indicated by a triangle. Arrows with dashed lines indicate nearly complete coding sequence.
The IEC-lacking SCCmec IV MRSA strain 187–4 was subsequently used to analyze the genetic organization of νSaβ. As shown in Fig 4, the sequence of νSaβ in strain 187–4 was almost identical to the corresponding region in strain 4578. However, the φSA3 sequence was absent in strain 187–4, in accordance to its IEC-lacking genotype.
Comparisons of mobile genetic elements among CC59 S. aureus strains
Based on the resolved sequences of the MES-related structures φSA3 and νSaβ, the 195 CC59 S. aureus strains were analyzed to determine the distribution of the mobile genetic elements. As shown in Table 2 and S3 Table, the multidrug-resistant structure MES6272-2 (gentamicin-resistant but streptomycin-susceptible) and the related structures MES4578 and MES2250 were found exclusively in SCCmec IV MRSA except for two MSSA strains. MESPM1 (streptomycin-resistant but gentamicin-susceptible) was mainly distributed in SCCmec V (5C2&5) MRSA and MSSA, although 22% of SCCmec IV MRSA strains carried MESPM1. The IS1216V/cat-related segregants, which could result from IS1216V-mediated excision/insertion in any MES and cause the loss of erythromycin, kanamycin, streptomycin and gentamicin resistance, were found to be evenly distributed in SCCmec IV MRSA, SCCmec V (5C2&5) MRSA or MSSA.
Table 2. Distribution of mobile genetic elements related to multidrug resistance or IEC.
| No. (%) of strains | |||
|---|---|---|---|
| SCCmec IV (n = 91) | SCCmec V (5C2&5) (n = 74) | MSSA (n = 30) | |
| Antibiotic resistance elements or related structures inserted into sasK a | |||
| MES6272-2 (Er, Kr, Gr) b | 51 (56.7) | 0 (0) | 1 (3.3) |
| MES4578 (Er, Kr, Gr) b | 8 (8.8) | 0 (0) | 1 (3.3) |
| MES2250 (Er) b | 6 (6.6) | 0 (0) | 0 (0) |
| MESPM1 (Er, Kr, Sr) b | 20 (22.0) | 66 (89.2) | 25 (83.3) |
| IS1216V or cat-related segregants | 5 (5.5) | 8 (10.8) | 3 (10) |
| Untypable | 1 (1.1) | 0 (0) | 0 (0) |
| Elements harboring IEC | |||
| φSA3 translocated into νSaβ: | |||
| IEC type B | 1 (1.1) | 0 (0) | 0 (0) |
| IEC type D | 3 (3.3) | 0 (0) | 0 (0) |
| IEC type G | 61 (67) | 0 (0) | 4 (13.3) |
| φSA3 integrated into hlb: IEC type B c | 11 (12.1) | 0 (0) | 0 (0) |
| φSA3-related fragment in νSaβ: IEC type C | 8 (8.8) | 74 (100) | 25 (83.3) |
| Loss of IEC | 4 (4.4) | 0 (0) | 0 (0) |
| Untypable | 3 (3.3) | 0 (0) | 1 (3.3) |
a Er: erythromycin resistant; Kr: kanamycin resistant; Gr: gentamicin resistant; Sr: streptomycin resistant.
b Classification of MES types is based on sequences of the antibiotic resistance gene cluster; the cat gene and its surrounding regions are not included because they are universal among different MES types.
c All the strains showed a "loss of IEC" pattern in νSaβ except strain 7576, which was untypable.
The νSaβ region with φSA3 translocation or with φSA3-related fragment was found in all of the CC59 S. aureus strains. For the distribution, SCCmec V (5C2&5) MRSA harbored φSA3-related fragment with IEC type C with no exception. In MSSA, the φSA3-related fragment with IEC type C was dominant (n = 25), followed by φSA3 translocation in νSaβ with IEC type G (n = 4). For SCCmec IV MRSA, although φSA3 translocation in νSaβ with IEC type G was frequently found in SCCmec IVg MRSA, the elements harboring IEC were quite different in the remaining SCCmec IV MRSA strains (see below). Eleven strains harbored φSA3 with IEC type B integrated into its regular site hlb. Within the 11 strains, 10 strains lacked any IEC in the νSaβ, similar to strain 187–4 (Fig 4), while one strain remained untypable. Eight strains acquired IEC type C via a φSA3-related fragment in νSaβ, similar to that in SCCmec V (5C2&5) MRSA. Four strains lacked any IEC in the entire genome. Three strains harbored IEC in the νSaβ with untypable structure and were without φSA3 integrated into hlb.
Diversity of the SCCmec IV MRSA
To further understand the genetic relatedness of the 91 SCCmec IV MRSA strains, SCCmec IV subtypes were determined. As shown in Table 3, a total of 81 strains (89%) carried SCCmec IVg, in agreement with the genomic analysis of ST59/SCCmec IV MRSA type strain 4578, which showed 98.8% nucleotide sequence similarity to the SCCmec IVg sequence (accession number DQ106887). For the 10 non-subtype-g SCCmec IV strains, SCCmec IVa was the dominant subtype (n = 7), followed by SCCmec IV nontypable (n = 2) and SCCmec IVc (n = 1). The distributions of the virulence factor gene lukPVSF, the elements harboring IEC (φSA3-related fragment in νSaβ or φSA3 translocated into νSaβ) and the MES structures (MESPM1 or MES6272-2 and related elements) were quite different in non-subtype-g SCCmec IV MRSA and SCCmec IVg MRSA (Table 3). For the 10 strains with non-subtype-g SCCmec IV, the characteristics (presence of lukPVSF gene, φSA3-related fragment in νSaβ and the MESPM1 structures) were much more similar to SCCmec V (5C2&5) MRSA than to SCCmec IVg MRSA.
Table 3. Distribution of virulence genes or mobile genetic elements within SCCmec IV MRSA.
| No. (%) of strains | ||
|---|---|---|
| Non-subtype-g SCCmec IV a (n = 10) | SCCmec IVg (n = 81) | |
| lukPVSF b | 8 (80) | 4 (49.4) |
| Elements harboring IEC | ||
| φSA3-related fragment in νSaβ b | 8 (80) | 0 (0) |
| φSA3 integrated into hlb | 1 (10) | 10 (12.3) |
| φSA3 translocated into νSaβ b | 0 (0) | 69 (85.2) |
| Untypable | 1 (10) | 2 (2.5) |
| Antibiotic resistance elements or related structures inserted into sasK | ||
| MESPM1 b | 9 (90) | 11 (13.6) |
| MES6272-2 and related elements b | 0 (0) | 65 (80.2) |
| IS1216V or cat-related segregants | 1 (10) | 4 (4.9) |
a Subtypes of non-subtype-g SCCmec IV MRSA: SCCmec IVa (n = 7), SCCmec IVc (n = 1) and SCCmec IV nontypable (n = 2).
b Statistically significant difference between non-subtype-g SCCmec IV and SCCmec IVg MRSA strains (P value <0.05 by Fisher’s exact test).
Considering the unique patterns of non-subtype-g SCCmec IV MRSA within the ST59/SCCmec IV MRSA, the PVL-positive ST59/SCCmec IVa MRSA strain USA1000 isolated from the United States was included for investigation. The 3-kb φSA3-related fragment was present in νSaβ of USA1000, similar to the structure in SCCmec V (5C2&5) MRSA. However, no IS1216V or MES-related structure was inserted into the sasK gene, which was quite different from the case for CC59 S. aureus strains isolated from Taiwan.
Cluster analysis of CC59 S. aureus strains
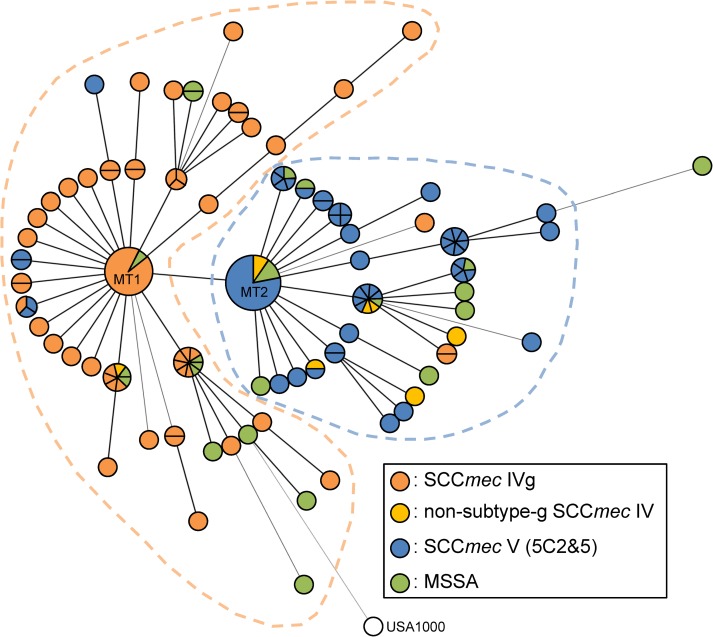
A minimum spanning tree based on the MLVA-16Orsay (with 16 loci) is shown in Fig 5. There were two dominant MLVA types, MT1 (MLVA profile: 7-7-2-2-2-2-2-4-2-3-1.5-0-3-10-16-3) and MT2 (MLVA profile: 7-7-2-2-2-2-2-4-2-3-1.5-0-3-7-16-3). The major difference between MT1 and MT2 was the repeat number in locus sa0964 (10 in MT1 and 7 in MT2); the repeat numbers in the other 15 loci were identical. MT1 and its adjacent neighbors formed a clade that was predominantly SCCmec IVg MRSA (orange dashed-line circle, Fig 5); MT2 and its relatives formed the other clade that mostly consisted of SCCmec V (5C2&5) MRSA and non-subtype-g SCCmec IV MRSA (blue dashed-line circle, Fig 5); MSSA was distributed in the both clades. In addition, the USA1000 was grouped with SCCmec IVg MRSA clade with a longer branch.
Fig 5. Minimum spanning tree of CC59 S. aureus strains constructed by MLVA.
Each circle represents an MLVA type. Circles or sectors of circles with orange, yellow, blue or green colors denote SCCmec IVg MRSA, non-subtype-g SCCmec IV MRSA, SCCmec V (5C2&5) MRSA or MSSA, respectively. The USA1000, which is ST59 MRSA in the United States, is shown in white. The size of each circle is proportional to the number of strains.
Discussion
The CC59 S. aureus in Taiwan has been reported to have unique multidrug resistance mechanisms and νSaβ structures, which are distinct from those of other lineages of S. aureus [19, 23]. The present study, combining molecular characterization of the mobile genetic elements and phylogenetic analysis based on MLVA, further elucidated these differences, as follows: (i) genetic relatedness within the CC59/SCCmec IV MRSA strains was diverse, with the non-subtype-g SCCmec IV MRSA phylogenetically closer to CC59/SCCmec V (5C2&5) MRSA than to CC59/SCCmec IVg MRSA; and (ii) CC59 MSSA strains were phylogenetically clustered within the SCCmec IVg MRSA or SCCmec V (5C2&5) MRSA, sharing both of their features.
Among the mobile genetic elements that we analyzed, νSaβ containing a φSA3-related fragment or translocated φSA3 were found in all CC59 S. aureus, including the USA1000 strain isolated from the United States, which suggested that the common progenitor of CC59 S. aureus may have already undergone chromosomal rearrangements to obtain IEC genes in the νSaβ. Acquisition of νSaβ containing a φSA3-related fragment leading to disruption of type I restriction-modification system was found exclusively in CC59 S. aureus, especially dominant in SCCmec V (5C2&5) MRSA as we previously reported [23]. In the current study, another unique νSaβ structure harboring translocated φSA3 in CC59 S. aureus, especially in SCCmec IVg MRSA was found, which indicated that the above chromosomal rearrangement events in νSaβ are the key features to CC59 S. aureus.
As for the enterococcal IS1216V-mediated MES structures, MESPM1 and MES6272-2 and related elements were found in all CC59 S. aureus strains, but the MES type distribution was quite different among SCCmec IVg, non-subtype-g SCCmec IV and SCCmec V (5C2&5) MRSA (Tables 2 and 3). The 5′ region of MESPM1 was identical to the corresponding region of a plasmid (pLG2) of Enterococcus faecalis [23, 42], while the left side of MES6272-2 was highly similar to the IS1216V-ermB-(aph(3')-IIIa)-sat-ΔaadE-(aacA-aphD)-ΔaadE structure of Enterococcus faecium isolated in Taiwan (our unpublished results). These results suggest that two independent events of acquisition from enterococci led to the varied MES distributions.
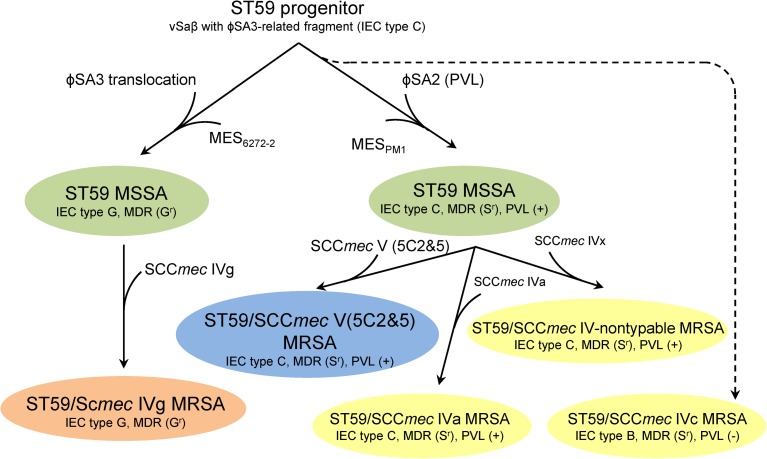
Based on the above findings, we propose a hypothetical ST59 MRSA evolutionary history in Taiwan (Fig 6). For the two major ST59 MRSA clones (SCCmec IVg and SCCmec V (5C2&5)), a common CC59 MSSA progenitor appeared first, and its νSaβ harbored a φSA3-related fragment with IEC type C. Next, two separate evolutionary routes were followed. In one route, strains acquired MESPM1 and the PVL-positive φSA2, and finally, CC59 MSSA (IEC type C, MESPM1 and PVL-positive) acquired SCCmec V (5C2&5) to become MRSA; in the other route, strains underwent φSA3 translocation and acquired MES6272-2, and finally, the MSSA strains (IEC type G, MES6272-2 and PVL-negative) acquired SCCmec IVg to become one of the dominant clones in CC59 MRSA. For the three minor ST59 MRSA clones characterized as SCCmec IVa, SCCmec IVc and SCCmec IV nontypable, they were phylogenetically related to SCCmec V (5C2&5) MRSA based on the minimum spanning tree constructed by MLVA (Fig 5). The acquisition events in SCCmec IVa and SCCmec IV nontypable MRSA were similar to that in SCCmec V (5C2&5) MRSA except for the last step to acquire SCCmec elements. In contrast, the SCCmec IVc MRSA strain was PVL-negative and was characterized as untypable νSaβ with IEC type B, indicating this strain underwent different chromosomal rearrangement events.
Fig 6. Proposed evolutionary history of CC59 S. aureus in Taiwan.
SCCmec IVg MRSA, non-subtype-g SCCmec IV MRSA, SCCmec V (5C2&5) MRSA or MSSA with the characteristics found in this study are shaded in orange, yellow, blue or green, respectively. Dash line indicates an uncertain evolutionary route. MDR: multidrug resistance; Sr: streptomycin resistance; Gr: gentamicin resistance.
CC59 S. aureus is the predominant CA-MRSA clone in Asia, especially in Taiwan and China [10, 43]. Previous studies have indicated that the SCCmec V (5C2&5) sequences of CC59 MRSA in the two areas are divergent. In strains from Taiwan, SCCmec V (5C2&5) has two distinct ccrC genes (ccrC1 allele 2 and allele 8), while strains isolated from China carry only one ccrC gene (SCCmec V (5C2)) [44, 45]. Our results further indicated that the genetic backgrounds of the major SCCmec IV MRSA strains circulating in Taiwan and China are also different. ST59/SCCmec IVa MRSA carrying the PVL gene at a high rate was found to be the dominant clone among the pediatric population in China [45, 46]. However, the major clone of the 91 SCCmec IV MRSA strains that we analyzed is PVL-negative SCCmec IVg MRSA; the isolation rate of PVL-positive SCCmec IVa MRSA was low (7.7%, 7/91) (Table 3).
MLVA is a permissive molecular typing tool with stronger phylogenetic value than MLST or spa typing [47]. However, only one locus can effectively discriminate ST59/SCCmec IVg MRSA and ST59/SCCmec V (5C2&5) MRSA within the 16 loci used in the present study. These results implied that the branching of ST59/SCCmec IVg MRSA and ST59/SCCmec V (5C2&5) MRSA may have happened recently. Further studies using whole genome sequencing combined with molecular clock analysis are needed to precisely elucidate the evolutionary history of CC59 S. aureus.
The IEC, which can counteract antibacterial activity of the human innate immune system [48, 49], is widely distributed among S. aureus strains of human origin [25, 50]. CC59 S. aureus has acquired IEC in the νSaβ without inactivation of hlb, ensuring beta-hemolysin production. Beta-hemolysin contributes to the first stages of nasal colonization, which was shown by comparing S. aureus NCTC 8325–4 strains with an intact hlb or a hlb disrupted by φSA3 [51, 52]. The spontaneous and precise excision of φSA3 in the CA-MRSA clone MW2 was shown to significantly increase its skin colonization ability [53]. Indeed, previous molecular epidemiology studies in Taiwan indicated ST59 clones accounted for >77% of MRSA isolates carried by healthy children and adolescents [54–56]. In addition, the φSA3 dynamics during infections that lead to complete restoration of beta-hemolysin production and promote host adaption and increase virulence have been reported [52, 53, 57]. Hence, spontaneously harboring IEC and intact hlb may increase fitness for colonization and infection in the majority (180/195) of CC59 S. aureus.
In conclusion, our analyses support a proposed depiction of the localized evolution of CC59 S. aureus. The acquisition of several mobile genetic elements increased antimicrobial resistance and adaptation, which has led CC59 S. aureus to become the most successful CA-MRSA clone in Taiwan.
Supporting Information
Schematic maps of (A) MES, (B) νSaβ and (C) φSA3 integrated within hlb are shown. The arrows below the structures indicate PCR primers, which are listed in S2 Table.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
The nucleotide sequences have been deposited in the DDBJ database under accession numbers LC125348 to LC125353. The information has been released.
Funding Statement
This work was supported by grants from the Ministry of Science and Technology of Taiwan (MOST 103-2320-B-002-056-MY3) and from the Kaohsiung Medical University Research Foundation (KMU-Q104012 and KMU-Q105006).
References
- 1.Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006; 368: 874–885. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto T, Hung WC, Takano T, Nishiyama A. Genetic nature and virulence of community-associated methicillin-resistant Staphylococcus aureus. BioMed. 2013; 3: 2–18. [Google Scholar]
- 3.Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008; 16: 361–369. 10.1016/j.tim.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010; 23: 616–687. 10.1128/CMR.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto T, Nishiyama A, Takano T, Yabe S, Higuchi W, Razvina O, et al. Community-acquired methicillin-resistant Staphylococcus aureus: community transmission, pathogenesis, and drug resistance. J Infect Chemother. 2010; 16: 225–254. 10.1007/s10156-010-0045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhlemann AC, Otto M, Lowy FD, DeLeo FR. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus. Infect Genet Evol. 2014; 21: 563–574. 10.1016/j.meegid.2013.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. Antimicrobial resistance: Not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician's guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis. 2011; 52: 99–114. 10.1093/cid/ciq067 [DOI] [PubMed] [Google Scholar]
- 8.Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller LG, Han LL, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008; 148: 249–257. [DOI] [PubMed] [Google Scholar]
- 9.Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr Opin Microbiol. 2012; 15: 588–595. 10.1016/j.mib.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 10.Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect. 2014; 20: 605–623. 10.1111/1469-0691.12705 [DOI] [PubMed] [Google Scholar]
- 11.Huang YC, Ho CF, Chen CJ, Su LH, Lin TY. Comparative molecular analysis of community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus isolates from children in northern Taiwan. Clin Microbiol Infect. 2008; 14: 1167–1172. 10.1111/j.1469-0691.2008.02115.x [DOI] [PubMed] [Google Scholar]
- 12.Huang YC, Chen CJ. Community-associated meticillin-resistant Staphylococcus aureus in children in Taiwan, 2000s. Int J Antimicrob Agents. 2011; 38: 2–8. 10.1016/j.ijantimicag.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 13.International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC). Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009; 53: 4961–4967. 10.1128/AAC.00579-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle-Vavra S, Ereshefsky B, Wang CC, Daum RS. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel Staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J Clin Microbiol. 2005; 43: 4719–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higuchi W, Takano T, Teng LJ, Yamamoto T. Structure and specific detection of staphylococcal cassette chromosome mec type VII. Biochem Biophys Res Commun. 2008; 377:752–756. 10.1016/j.bbrc.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 16.Hsu LY, Tristan A, Koh TH, Bes M, Etienne J, Kurup A, et al. Community associated methicillin-resistant Staphylococcus aureus, Singapore. Emerg Infect Dis. 2005; 11: 341–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho PL, Cheung C, Mak GC, Tse CW, Ng TK, Cheung CH, et al. Molecular epidemiology and household transmission of community-associated methicillin-resistant Staphylococcus aureus in Hong Kong. Diagn Microbiol Infect Dis. 2007; 57: 145–151. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi W, Hung WC, Takano T, Iwao Y, Ozaki K, Isobe H, et al. Molecular characteristics of the Taiwanese multiple drug-resistant ST59 clone of Panton-Valentine leucocidin-positive community-acquired methicillin-resistant Staphylococcus aureus from pediatric cellulitis. J Infect Chemother. 2010; 16: 144–149. 10.1007/s10156-010-0029-9 [DOI] [PubMed] [Google Scholar]
- 19.Sawanobori E, Hung WC, Takano T, Hachuda K, Horiuchi T, Higuchi W, et al. Emergence of Panton-Valentine leukocidin-positive ST59 methicillin-susceptible Staphylococcus aureus with high cytolytic peptide expression in association with community-acquired pediatric osteomyelitis complicated by pulmonary embolism. J Microbiol Immunol Infect. 2015; 48: 565–573. 10.1016/j.jmii.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 20.Coombs GW, Monecke S, Ehricht R, Slickers P, Pearson JC, Tan HL, et al. Differentiation of clonal complex 59 community-associated methicillin-resistant Staphylococcus aureus in Western Australia. Antimicrob Agents Chemother. 2010; 54: 1914–1921. 10.1128/AAC.01287-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasner C, Pluister G, Westh H, Arends JP, Empel J, Giles E, et al. Staphylococcus aureus spa type t437: identification of the most dominant community-associated clone from Asia across Europe. Clin Microbiol Infect. 2015; 21: 163 e161-168. 10.1016/j.cmi.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 22.Wang CC, Lo WT, Chu ML, Siu LK. Epidemiological typing of community-acquired methicillin-resistant Staphylococcus aureus isolates from children in Taiwan. Clin Infect Dis. 2004; 39: 481–487. [DOI] [PubMed] [Google Scholar]
- 23.Hung WC, Takano T, Higuchi W, Iwao Y, Khokhlova O, Teng LJ, et al. Comparative genomics of community-acquired ST59 methicillin-resistant Staphylococcus aureus in Taiwan: novel mobile resistance structures with IS1216V. PLoS One. 2012; 7: e46987 10.1371/journal.pone.0046987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CJ, Unger C, Hoffmann W, Lindsay JA, Huang YC, Gotz F. Characterization and comparison of 2 distinct epidemic community-associated methicillin-resistant Staphylococcus aureus clones of ST59 lineage. PLoS One. 2013; 8: e63210 10.1371/journal.pone.0063210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J Bacteriol. 2006; 188: 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldron DE, Lindsay JA. Sau1: a novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineages. J Bacteriol. 2006; 188: 5578–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takano T, Higuchi W, Otsuka T, Baranovich T, Enany S, Saito K, et al. Novel characteristics of community-acquired methicillin-resistant Staphylococcus aureus strains belonging to multilocus sequence type 59 in Taiwan. Antimicrob Agents Chemother. 2008; 52: 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JL, Wang JT, Chen SY, Chen YC, Chang SC. Distribution of Staphylococcal cassette chromosome mec Types and correlation with comorbidity and infection type in patients with MRSA bacteremia. PLoS One. 2010; 5: e9489 10.1371/journal.pone.0009489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen FJ, Siu LK, Lin JC, Wang CH, Lu PL. Molecular typing and characterization of nasal carriage and community-onset infection methicillin-susceptible Staphylococcus aureus isolates in two Taiwan medical centers. BMC Infect Dis. 2012; 12: 343 10.1186/1471-2334-12-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen FJ, Hiramatsu K, Huang IW, Wang CH, Lauderdale TL. Panton-Valentine leukocidin (PVL)-positive methicillin-susceptible and resistant Staphylococcus aureus in Taiwan: identification of oxacillin-susceptible mecA-positive methicillin-resistant S. aureus. Diagn Microbiol Infect Dis. 2009; 65: 351–357. 10.1016/j.diagmicrobio.2009.07.024 [DOI] [PubMed] [Google Scholar]
- 31.Ho CM, Lin CY, Ho MW, Lin HC, Peng CT, Lu JJ. Concomitant genotyping revealed diverse spreading between methicillin-resistant Staphylococcus aureus and methicillin-susceptible Staphylococcus aureus in central Taiwan. J Microbiol Immunol Infect. [DOI] [PubMed] [Google Scholar]
- 32.Sobral D, Schwarz S, Bergonier D, Brisabois A, Fessler AT, Gilbert FB, et al. High throughput multiple locus variable number of tandem repeat analysis (MLVA) of Staphylococcus aureus from human, animal and food sources. PLoS One. 2013; 7: e33967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YH, Tseng SP, Hu JM, Tsai JC, Hsueh PR, Teng LJ. Clonal spread of SCCmec type IV methicillin-resistant Staphylococcus aureus between community and hospital. Clin Microbiol Infect. 2007; 13: 717–724. [DOI] [PubMed] [Google Scholar]
- 34.Koreen L, Ramaswamy SV, Graviss EA, Naidich S, Musser JM, Kreiswirth BN. spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J Clin Microbiol. 2004; 42: 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000; 38: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003; 41: 5113–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Twenty-fifth informational supplement M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA, USA. 2015.
- 38.Milheirico C, Oliveira DC, de Lencastre H. Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphylococcus aureus: 'SCCmec IV multiplex'. J Antimicrob Chemother. 2007; 60: 42–48. [DOI] [PubMed] [Google Scholar]
- 39.Higuchi W, Takano T, Teng LJ, Yamamoto T. Structure and specific detection of staphylococcal cassette chromosome mec type VII. Biochem Biophys Res Commun. 2008; 377: 752–756. 10.1016/j.bbrc.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 40.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008; 18: 821–829. 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung WC, Chen HJ, Lin YT, Tsai JC, Chen CW, Lu HH, et al. Skin commensal staphylococci may act as reservoir for fusidic acid resistance genes. PLoS One. 2015; 10: e0143106 10.1371/journal.pone.0143106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laverde Gomez JA, Hendrickx AP, Willems RJ, Top J, Sava I, et al. Intra- and interspecies genomic transfer of the Enterococcus faecalis pathogenicity island. PLoS One. 2011; 6: e16720 10.1371/journal.pone.0016720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chuang YY, Huang YC. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Asia. Lancet Infect Dis. 2013; 13: 698–708. 10.1016/S1473-3099(13)70136-1 [DOI] [PubMed] [Google Scholar]
- 44.Qu T, Feng Y, Jiang Y, Zhu P, Wei Z, Chen Y, et al. Whole genome analysis of a community-associated methicillin-resistant Staphylococcus aureus ST59 isolate from a case of human sepsis and severe pneumonia in China. PLoS One. 2014; 9: e89235 10.1371/journal.pone.0089235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geng W, Yang Y, Wu D, Huang G, Wang C, Deng L, et al. Molecular characteristics of community-acquired, methicillin-resistant Staphylococcus aureus isolated from Chinese children. FEMS Immunol Med Microbiol. 2010; 58: 356–362. 10.1111/j.1574-695X.2010.00648.x [DOI] [PubMed] [Google Scholar]
- 46.Li J, Wang L, Ip M, Sun M, Sun J, Huang G, et al. Molecular and clinical characteristics of clonal complex 59 methicillin-resistant Staphylococcus aureus infections in Mainland China. PLoS One. 2013; 8: e70602 10.1371/journal.pone.0070602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pourcel C, Hormigos K, Onteniente L, Sakwinska O, Deurenberg RH, Vergnaud G. Improved multiple-locus variable-number tandem-repeat assay for Staphylococcus aureus genotyping, providing a highly informative technique together with strong phylogenetic value. J Clin Microbiol. 2009; 47: 3121–3128. 10.1128/JCM.00267-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rooijakkers SH, van Kessel KP, van Strijp JA. Staphylococcal innate immune evasion. Trends Microbiol. 2005; 13: 596–601. [DOI] [PubMed] [Google Scholar]
- 49.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol. 2015; 13: 529–543. 10.1038/nrmicro3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung JM, Lloyd DH, Lindsay JA. Staphylococcus aureus host specificity: comparative genomics of human versus animal isolates by multi-strain microarray. Microbiology. 2008; 154: 1949–1959. 10.1099/mic.0.2007/015289-0 [DOI] [PubMed] [Google Scholar]
- 51.Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS One. 2011; 6: e17936 10.1371/journal.pone.0017936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salgado-Pabon W, Herrera A, Vu BG, Stach CS, Merriman JA, Spaulding AR, et al. Staphylococcus aureus beta-toxin production is common in strains with the beta-toxin gene inactivated by bacteriophage. J Infect Dis. 2014; 210: 784–792. 10.1093/infdis/jiu146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katayama Y, Baba T, Sekine M, Fukuda M, Hiramatsu K. Beta-hemolysin promotes skin colonization by Staphylococcus aureus. J Bacteriol. 2013; 195: 1194–1203. 10.1128/JB.01786-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen CJ, Wang SC, Chang HY, Huang YC. Longitudinal analysis of methicillin-resistant and methicillin-susceptible Staphylococcus aureus carriage in healthy adolescents. J Clin Microbiol. 2014; 51: 2508–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen CJ, Hsu KH, Lin TY, Hwang KP, Chen PY, Huang YC. Factors associated with nasal colonization of methicillin-resistant Staphylococcus aureus among healthy children in Taiwan. J Clin Microbiol. 2011; 49: 131–137. 10.1128/JCM.01774-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang JT, Liao CH, Fang CT, Chie WC, Lai MS, Lauderdale TL, et al. Prevalence of and risk factors for colonization by methicillin-resistant Staphylococcus aureus among adults in community settings in Taiwan. J Clin Microbiol. 2009; 47: 2957–2963. 10.1128/JCM.00853-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goerke C, Wirtz C, Fluckiger U, Wolz C. Extensive phage dynamics in Staphylococcus aureus contributes to adaptation to the human host during infection. Mol Microbiol. 2006; 61: 1673–1685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic maps of (A) MES, (B) νSaβ and (C) φSA3 integrated within hlb are shown. The arrows below the structures indicate PCR primers, which are listed in S2 Table.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
The nucleotide sequences have been deposited in the DDBJ database under accession numbers LC125348 to LC125353. The information has been released.