Abstract
Background
SDF1 and its cognate receptors CXCR4 and CXCR7 are involved in myocardial repair and are associated with outcome in cardiovascular patients. Hence, we aimed to investigate clinically significant SDF1 SNPs for their prognostic impact in patients with cardiovascular disease.
Methods and Results
Genotyping for selected SDF1 variants (rs1065297, rs2839693, rs1801157, rs266087, rs266085 and rs266089 was performed in patients (n = 872) who underwent percutaneous coronary intervention. Carriers of variant rs2839693 and rs266089 showed significantly higher cumulative event-free survival compared with non-carriers. All other polymorphisms had no relevant influence on outcome. Multivariate Cox regression analysis showed a significant correlation of these SNPs with cardiovascular outcome after inclusion of clinical and prognostic relevant variables (hazard ratio (HR) 0.51 (95% CI 0.30–0.88), p = 0.015 and [HR 0.51 (95% CI 0.30–0.88), p = 0.016, respectively). In addition, multivariate Cox regression with SDF1 haplotypes revealed a significantly reduced risk for the haplotype carrying the minor alleles of rs2839693 and rs266089 (HR 0.47 (95% CI 0.27–0.84), p = 0.011).
Conclusion
Distinct SDF1 polymorphisms are associated with improved cardiovascular prognosis in CAD patients. Further studies are warranted to validate these results and to better describe the endogenous regeneration potential in carriers of these SNPs. Targeted, genotype guided therapeutic approaches to foster myocardial regeneration and thus cardiovascular prognosis should be evaluated in future.
Introduction
SDF1 (CXCL-12) is a CXC chemokine and is expressed in a variety of tissues where it acts as a potent chemoattractant for hematopoetic cells.[1,2,3] SDF1 is involved in homing of hematopoietic stem cells to the bone marrow and controlling human- and murine progenitor cell proliferation- and survival.[4,5,6] SDF1 creates a stem cell-attracting environment which possibly results in organ- and tissue repair.[7] Several experimental studies have shown, that high SDF1 levels in ischemic myocardium lead to myocardial protection and improved function after myocardial infarction in vivo.[8,9]
Platelets are a relevant source for SDF1. Platelet surface expression of SDF1 is higher in patients with acute coronary syndrome (ACS) compared with those suffering from stable coronary artery disease (CAD).[10,11] Recently, we could demonstrate an influence of SDF-1 platelet surface expression on endothelial progenitor cell recruitment after myocardial ischemia. Furthermore, our group demonstrated an enhanced LVEF% recovery in patients with high platelet SDF-1 expression levels following ACS.[12] SDF1 effects are mediated by its two receptors CXCR4 and CXCR7. Several studies suggest that the SDF1/CXCR4/CXCR7 axis is crucially involved in endothelial progenitor cell recruitment and functional recovery after myocardial ischaemia.[13,14,15] Human platelets constitutively express both CXCR4 and CXCR7. Platelet expressions levels of those two receptors correlate with platelet expression of SDF1. Previously, we showed that platelet surface expression of CXCR7 is elevated in ACS patients when compared to patients with stable CAD. In the same study, high platelet CXCR7 surface expression was associated with improvement of LVEF% after ACS.[15] In a patient collective with symptomatic CAD we found baseline platelet CXCR4 levels to be significantly lower in CAD patients suffering subsequent death or myocardial infarction. Both baseline values of CXCR4 and CXCR7 were significantly associated with all-cause mortality in patients with symptomatic CAD.[16] Several single nucleotide polymorphisms (SNPs) of the SDF1 gene might alter prognosis in cardiovascular patients. There are however little data for SDF1 SNPs in patients with cardiovascular disease. Single nucleotide polymorphisms (SNPs) of the SDF1 gene, including rs1801157, rs2839693, rs1065297, and rs266085 might be associated with the occurrence and prognosis of childhood immune thrombocytopenia (ITP). [17] The SDF1 SNP rs1801157 is associated with premature CAD in Chinese patients.[18] Since SDF1 and its cognate receptors CXCR4 and CXCR7 are involved in myocardial repair and are associated with outcome in cardiovascular patients we aimed to investigate clinically significant SDF1 SNPs for their prognostic impact in patients with cardiovascular disease.
Methods
Subjects
SDF1 single-nucleotide polymorphism analysis was performed in 943 consecutive patients with coronary artery disease. All subjects gave written informed consent. Patients were admitted to the department of cardiology of the University of Tuebingen, Germany. The study was approved by the institutional ethics committee (Ethik-Kommission an der Medizinischen Fakultät der Eberhard-Karls-Universität und am Universitätsklinikum Tübingen) (270/2011BO1) and complies with the declaration of Helsinki and the good clinical practice guidelines.[19,20,21]
Genotyping of SDF1 variants
Genomic DNA was isolated from ethylenediaminetetraacetic acid (EDTA) blood samples using the QIAmp® DNA Blood Mini Kit System (Qiagen, Hilden, Germany). Candidate genetic variants of SDF1 were selected on the basis of previous literature reporting on either clinical importance or functional importance on protein expression profile. The criteria for selection of variants in these genes were a representative allele frequency in Caucasians and clear evidence for functional consequences based on in vitro/in vivo data.[22,23,24,25,26] Thus, the following polymorphisms of SDF1 were analysed: rs1065297, rs2839693, rs1801157, rs266087, rs266085 and rs266089. Genotyping for SDF1 variants was performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using the MassARRAY® Compact system (Sequenom, CA, USA) as previously described.[27] Details of primers and assays are available upon request. Approximately 10% of samples within each assay were retyped as a quality control. Study personal assessing outcome was blinded to the case status of the study participants during the entire genotyping process. Minor allele frequencies of SDF1 variants in the study cohort are provided in S1 Table. Linkage disequilibrium (LD) map is shown in S1 Fig.
Follow-Up
All patients were tracked after initial PCI for clinical events including all cause death, myocardial infarction and ischemic stroke for 360 days after study inclusion. The combined primary endpoint consisted of either time to death, MI or ischemic stroke. Secondary endpoints included the single events of all-cause death, MI and ischemic stroke. 71 patients were lost to follow up (7.5%). The patients lost to follow up did not significantly differ in their baseline characteristics as compared to the group remaining in the study. Follow-up for the primary combined endpoint was performed until first occurrence of one of the pre-defined endpoints. Follow-up was performed by telephone interview and/or review of patients´ charts on readmission by investigators blinded to the results of laboratory testing.
Statistical analysis
Most statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Crosstabulations with Chi-square analysis were performed to analyse baseline characteristics and outcome differences between homozygote carriers of major allele and carriers of minor allele. A two-tailed alpha level <0.05 was considered statistically significant. Unless otherwise stated, p-values were not corrected for multiple testing. Cox regression analysis was applied to compare the association of SDF1 SNPs with the combined endpoint and after adjustment for epidemiological factors influencing cardiovascular outcome. The time-dependent covariate method was used to check the proportional hazard assumption of the model. Survival functions for overall survival and time to death were estimated by Kaplan-Meier curves. The log-rank test was applied to compare survival functions between homozygote carriers of major allele and carriers of minor allele. Package qvalue_2.2.2 of statistical software R- 3.2.3 was used to estimate corresponding q-values, defined as the minimal positive false discovery rate at which the considered log-rank test is called significant. Observed and expected allele and genotype frequencies within populations were compared by means of Hardy—Weinberg equilibrium calculations.[28] Linkage disequilibrium map was created using Haploview (Barrett et al. Bioinformatics 2005). Haplotype analyses were performed with packages haplo.stats_1.7.7 and survival_2.38–3. To be more precise, haplotypes of 6 SDF1-polymorphisms were estimated with function haplo.em. Associations between haplotypes and the combined endpoint were then investigated by weighted uni- and multivariate Cox models, with weights given by the posterior probabilities of haplotype pairs for each patient. Here, rare haplotypes (i.e., with haplotype probability <5%) were combined prior to Cox analysis and haplotype effects were investigated in the dominant model (i.e. combining heterozygote and homozygote carriers of a particular haplotype).
Patients’ characteristics (age, gender, cardiovascular risk factors, co-medication) of the prospective cohort (n = 872) stratified according to SDF1 SNPs are provided in Tables 1 and 2. SDF-1 rs2839693 and rs266089 as well as rs266087 and rs266085 are highly correlated with each other. Thus, we omitted rs266089 and rs266085 from the detailed analysis. Covariates such as cardiovascular risk factors and medication on admission were collected based on patient history and diagnosis during hospital stay. We decided to include common risk factors and medication in a cardiovascular patient collective in our baseline characteristics section. Furthermore, we included important cardiovascular risk factors, clinical factors and medication, that are well known to influence prognosis in our multivariate analysis.
Table 1. Baseline characteristics of the complete cohort (n = 872) (hc = homozygote carriers).
| Characteristics | All (n = 872) | rs1065297 (n = 870) | p | rs2839693 (n = 870) | p | ||
|---|---|---|---|---|---|---|---|
| (n = 799) hc of major allele | (n = 71) minor allele carriers | (n = 633) hc of major allele | (n = 237) minor allele carriers | ||||
| No. of males | 662 (70%) | 610 (71%) | 52 (68%) | 0.701 | 486 (71%) | 176 (68%) | 0.442 |
| Age (years ± SD) | 68 (±12) | 68 (±12) | 66 (±14) | 0.251 | 68 (±13) | 68 (±12) | 0.346 |
| CVRF | |||||||
| Arterial hypertension | 760 (81%) | 702 (81%) | 58 (76%) | 0.405 | 551 (81%) | 209 (81%) | 0.756 |
| Hyperlipidemia | 522 (55%) | 478 (55%) | 43 (57%) | 0.635 | 395 (58%) | 126 (49%) | 0.016 |
| Diabetes mellitus type II | 298 (32%) | 276 (32%) | 22 (29%) | 0.837 | 211 (31%) | 87 (34%) | 0.275 |
| Smoking | 375 (40%) | 345 (40%) | 29 (38%) | 0.835 | 276 (40%) | 98 (38%) | 0.568 |
| Clinical factors | |||||||
| Gensini Score | 39 (±42) | 39 (±43) | 36 (±32) | 0.520 | 40 (±43) | 36 (±38) | 0.209 |
| Atrial fibrillation | 195 (21%) | 180 (21%) | 14 (18%) | 0.782 | 137 (20%) | 57 (22%) | 0.364 |
| LVEF% normal | 447 (47%) | 413 (48%) | 33 (43%) | 0.457 | 330 (48%) | 116 (45%) | 0.525 |
| LVEF% mild impairment | 241 (26%) | 224 (26%) | 17 (22%) | 166 (24%) | 75 (29%) | ||
| LVEF% moderate impairment | 155 (17%) | 143 (17%) | 11 (14%) | 113 (17%) | 41 (16%) | ||
| LVEF% severe impairment | 92 (10%) | 81 (9%) | 11 (14%) | 68 (10%) | 24 (9%) | ||
| LVEF% unknown | 8 (1%) | 4 (0%) | 4 (5%) | 7 (1%) | 1 (0%) | ||
| Renal function (creatinine ±SD) | 1.1 (±0.7) | 1.0 (±0.6) | 1.1 (±1.3) | 0.542 | 1.1 (±0.7) | 1.0 (±0.6) | 0.902 |
| Medication on admission | |||||||
| Acetyl salicylic acid | 495 (52%) | 451 (52%) | 43 (57%) | 0.433 | 359 (52%) | 135 (53%) | 0.919 |
| Clopidogrel | 108 (11%) | 93 (11%) | 15 (20%) | 0.018 | 80 (12%) | 28 (11%) | 0.717 |
| Prasugrel | 17 (2%) | 15 (2%) | 2 (3%) | 0.571 | 11 (2%) | 6 (2%) | 0.461 |
| Ticagrelor | 38 (4%) | 34 (4%) | 4 (5%) | 0.568 | 28 (4%) | 10 (4%) | 0.880 |
| Oral anticoagulants | 80 (8%) | 73 (8%) | 6 (8%) | 0.950 | 54 (8%) | 25 (10%) | 0.208 |
| ACE inhibitors | 393 (42%) | 365 (42%) | 28 (37%) | 0.386 | 286 (42%) | 107 (42%) | 0.915 |
| AT1-receptor antagonists | 173 (18%) | 158 (18%) | 15 (20%) | 0.731 | 117 (17%) | 56 (22%) | 0.105 |
| Ca-channel inhibitors | 180 (19%) | 172 (20%) | 8 (11%) | 0.049 | 131 (19%) | 49 (19%) | 0.950 |
| Beta blockers | 528 (56%) | 485 (56%) | 41 (54%) | 0.764 | 377 (55%) | 149 (58%) | 0.475 |
| Statins | 425 (45%) | 386 (45%) | 38 (50%) | 0.339 | 314 (46%) | 110 (43%) | 0.360 |
| Reason of admission | |||||||
| ACS | 350 (40%) | 326 (41%) | 24 (34%) | 0.063 | 258 (41%) | 92 (39%) | 0.879 |
| Stable CAD | 355 (41%) | 314 (39%) | 39 (55%) | 255 (40%) | 98 (41%) | ||
| Other | 167 (19%) | 159 (20%) | 8 (11%) | 120 (19%) | 47 (20%) | ||
| Missing values for SDF1 polymorphisms | 2 (0.2%) | 2 (0.2%) | |||||
Table 2. Baseline characteristics of the complete cohort (n = 872).
| Characteristics | All (n = 872) | rs266087 (n = 872) | p | rs1801157 (n = 872) | p | ||
|---|---|---|---|---|---|---|---|
| (n = 385) hc of major allele | (n = 487) minor allele carriers | (n = 572) hc of major allele | (n = 300) minor allele carriers | ||||
| No. of males | 662 (70%) | 283 (69%) | 379 (72%) | 0.320 | 425 (69%) | 237 (72%) | 0.426 |
| Age (years ± SD) | 68 (±12) | 69 (±12) | 67 (±13) | 0.053 | 68 (±13) | 67 (±12) | 0.776 |
| CVRF | |||||||
| Arterial hypertension | 760 (81%) | 340 (82%) | 420 (79%) | 0.407 | 499 (81%) | 261 (79%) | 0.472 |
| Hyperlipidemia | 521 (55%) | 233 (56%) | 289 (55%) | 0.793 | 341 (56%) | 181 (55%) | 0.953 |
| Diabetes mellitus type II | 298 (32%) | 124 (30%) | 174 (33%) | 0.333 | 184 (30%) | 114 (35%) | 0.314 |
| Smoking | 374 (40%) | 163 (39%) | 212 (40%) | 0.723 | 237 (39%) | 138 (42%) | 0.261 |
| Clinical factors | |||||||
| Gensini Score | 39 (±42) | 40 (±39) | 43 (±43) | 0.793 | 37 (±38) | 43 (±48) | 0.061 |
| Atrial fibrillation | 194 (21%) | 87 (21%) | 108 (20%) | 0.450 | 121 (20%) | 74 (22%) | 0.561 |
| LVEF% normal | 447 (47%) | 190 (46%) | 257 (48%) | 0.840 | 287 (47%) | 160 (48%) | 0.929 |
| LVEF% mild impairment | 241 (26%) | 111 (27%) | 130 (25%) | 159 (26%) | 82 (25%) | ||
| LVEF% moderate impairment | 155 (17%) | 69 (17%) | 86 (16%) | 101 (16%) | 54 (16%) | ||
| LVEF% severe impairment | 92 (10%) | 40 (10%) | 52 (10%) | 62 (10%) | 30 (9%) | ||
| LVEF% unknown | 8 (1%) | 4 (1%) | 4 (1%) | 4 (1%) | 4(1%) | ||
| Renal function (creatinine ±SD) | 1.1 (±0.7) | 1.1 (±0.7) | 1.0 (±0.6) | 0.471 | 1.1 (±0.8) | 1.0 (±0.4) | 0.005 |
| Medication on admission | |||||||
| Acetyl salicylic acid | 494 (52%) | 206 (50%) | 289 (55%) | 0.103 | 318 (52%) | 177 (54%) | 0.561 |
| Clopidogrel | 108 (11%) | 47 (11%) | 61 (12%) | 0.887 | 67 (11%) | 41 (12%) | 0.484 |
| Prasugrel | 17 (2%) | 6 (1%) | 11 (2%) | 0.458 | 9 (1%) | 8 (2%) | 0.290 |
| Ticagrelor | 38 (4%) | 18 (4%) | 20 (4%) | 0.684 | 29 (5%) | 9 (3%) | 0.137 |
| Oral anticoagulants | 79 (8%) | 37 (9%) | 43 (8%) | 0.508 | 53 (9%) | 27 (8%) | 0.754 |
| ACE inhibitors | 393 (42%) | 173 (42%) | 222 (42%) | 0.908 | 254 (41%) | 141 (43%) | 0.677 |
| AT1-receptor antagonists | 173 (18%) | 64 (15%) | 109 (21%) | 0.039 | 108 (18%) | 65 (20%) | 0.420 |
| Ca-channel inhibitors | 180 (19%) | 78 (19%) | 102 (19%) | 0.837 | 120 (20%) | 60 (18%) | 0.616 |
| Beta blockers | 526 (56%) | 222 (54%) | 306 (58%) | 0.167 | 341 (56%) | 187 (57%) | 0.726 |
| Statins | 424 (45%) | 177 (43%) | 248 (47%) | 0.183 | 270 (44%) | 155 (47%) | 0.369 |
| Reason of admission | |||||||
| ACS | 350 (40%) | 149 (39%) | 201 (41%) | 0.217 | 227 (40%) | 123 (41%) | 0.821 |
| Stable CAD | 355 (41%) | 170 (44%) | 185 (38%) | 237 (41%) | 118 (39%) | ||
| Other | 167 (19%) | 66 (17%) | 101 (21%) | 108 (19%) | 59 (20%) | ||
| Missing values for SDF1 Polymorphisms | 0 (0%) | 0 (0%) | |||||
Results
Number- and categories of events are shown in Table 3.
Table 3. Events and incident rate (IR)/ 100 person years (PY) in the overall cohort (n = 872) (71 patients lost to follow-up).
| Variable | Number of events rs1065297 (hc of major allele / minor allele carriers) | IR/100 PY (hc major allele / minor allele carriers) | p |
| All cause mortality | N = 51 (48/3) | 6.0/4.2 | 0.540 |
| Myocardial infarction | N = 54 (52/2) | 6.5/2.8 | 0.217 |
| Ischemic stroke | N = 13 (12/1) | 1.5/1.4 | 0.965 |
| Combined endpoint | N = 98 (93/5) | 11.6/7.0 | 0.240 |
| Variable | Number of events rs2839693 (hc of major allele / minor allele carriers) | p | |
| All cause mortality | N = 51 (41/10) | 6.5/4.2 | 0.207 |
| Myocardial infarction | N = 54 (45/9) | 7.1/3.8 | 0.072 |
| Ischemic stroke | N = 13 (10/3) | 1.6/1.3 | 0.798 |
| Combined endpoint | N = 98 (80/18) | 12.6/7.6 | 0.036 |
| Variable | Number of events rs1801157 (hc of major allele / minor allele carriers) | p | |
| All cause mortality | N = 51 (30/21) | 5.2/7.0 | 0.294 |
| Myocardial infarction | N = 54 (35/19) | 6.1/6.3 | 0.901 |
| Ischemic stroke | N = 13 (5/8) | 0.9/2.7 | 0.118 |
| Combined endpoint | N = 98 (61/37) | 10.7/12.3 | 0.459 |
| Variable | Number of events rs266087 (hc of major allele / minor allele carriers) | p | |
| All cause mortality | N = 51 (23/28) | 6.0/5.7 | 0.888 |
| Myocardial infarction | N = 54 (19/35) | 4.9/7.2 | 0.171 |
| Ischemic stroke | N = 13 (2/11) | 0.5/2.3 | 0.127 |
| Combined endpoint | N = 98 (38/60) | 9.9/12.3 | 0.255 |
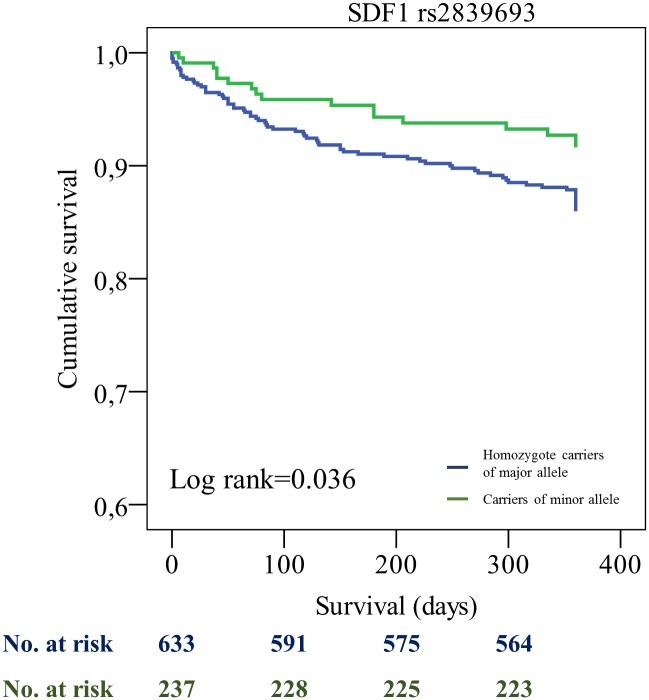
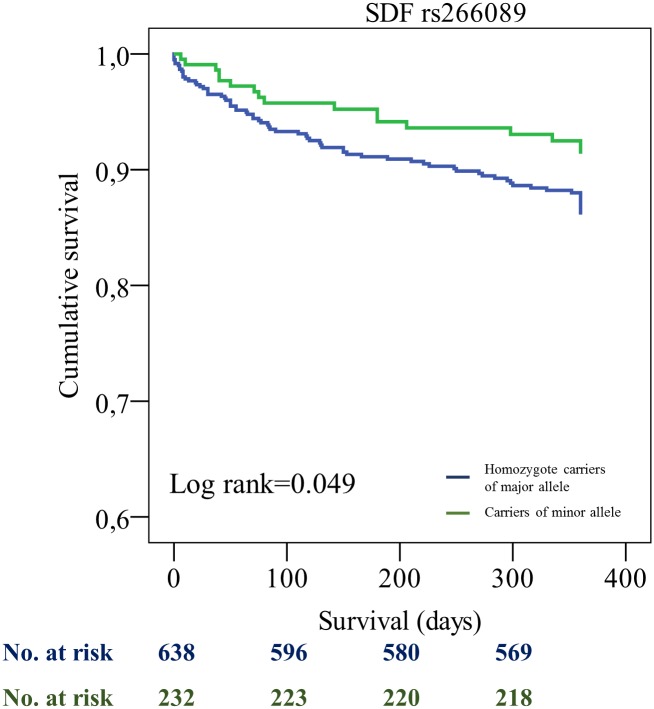
All patients were tracked after initial PCI. We found a significant difference for the combined endpoint for SDF1 rs2839693 [incidence rate (IR)/100PY 7.6 (minor allele) vs. 12.6 (major allele), p = 0.036] and SDF1 rs266089 [IR/100PY 7.8 (minor allele) vs. 12.5 (major allele), p = 0.049], respectively. Furthermore, we found differences for the secondary endpoint myocardial infarction for the SDF1 SNPs rs2839693 [IR/100PY 3.8 (minor allele) vs. 7.1 (major allele), p = 0.072] and rs266089 [IR/100PY 3.9 (minor allele) vs. 7.1 (major allele), p = 0.086]. These results however failed to be significant. We could not find any significant differences for the SDF1 SNPs rs1065297, rs1801157 and rs266087 (see Table 3). Multivariate Cox regression analysis showed that rs2839693 as well as rs266089 were associated with the primary combined endpoint after adjustment for epidemiological factors [hazard ratio (HR) 0.51 (95% CI 0.30–0.88), p = 0.015] and [HR 0.51 (95% CI 0.30–0.88), p = 0.016] (Table 4).
Table 4. Cox Regression analysis for the combined endpoint as dependent variable and clinical factors as covariates in the overall cohort of cardiovascular patients.
| Variable | HR rs2839693 (95% CI) ** | p | q-value |
|---|---|---|---|
| CVRF | |||
| Hypertension | 0.82 (0.45/1.50) | 0.527 | 0.184 |
| Hyperlipoproteinaemia | 0.78 (0.50/1.22) | 0.278 | 0.166 |
| Smoker | 0.77 (0.47/1.28) | 0.320 | 0.176 |
| Diabetes mellitus type II | 1.18 (0.76/1.84) | 0.459 | 0.184 |
| Medication | |||
| ASA | 2.08 (1.24/3.49) | 0.006 | 0.017 |
| Clopidogrel | 1.07 (0.59/1.96) | 0.820 | 0.212 |
| ACE inhibitors | 0.69 (0.44/1.10) | 0.119 | 0.1 |
| Beta blockers | 1.17 (0.70/1.93) | 0.552 | 0.184 |
| Statins | 0.85 (0.53/1.39) | 0.524 | 0.184 |
| Clinical factors | |||
| Age | 1.06 (1.03/1.08) | <0.001 | 0.017 |
| Gender | 0.98 (0.61/1.57) | 0.975 | 0.224 |
| LVEF% class* | 1.52 (1.24/1.87) | <0.001 | 0.017 |
| Groups | |||
| SDF1 SNP | 0.51 (0.30/0.88) | 0.015 | 0.021 |
*numerically coded as 0 (normal), 1 (mild), 2 (moderate), and 3 (severe impairment)
**the SNP rs2839693 is highly correlated with rs266089 (LD = 98, r2 = 94), as described in the Supporting Information.
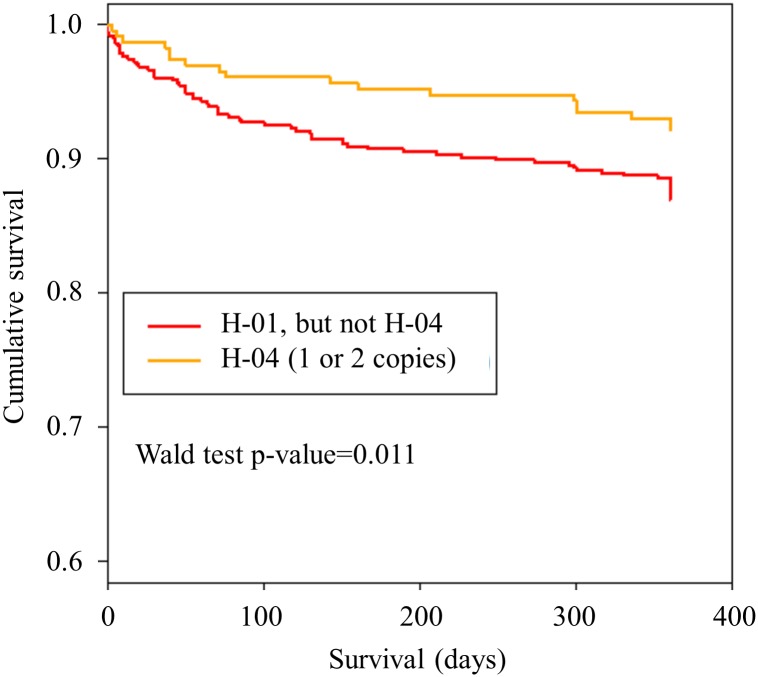
Patients who were SDF1 rs2839693 and rs266089 minor allele carriers showed a significantly better event-free survival probability compared to homozygote carriers of major allele (log rank 0.039 and log rank 0.049 for primary combined endpoint) (Figs 1 and 2). Moreover, for the haplotype carrying minor alleles of rs2839693 and rs266089, a borderline association in univariate and a significant effect on the combined endpoint in multivariate Cox regression was revealed (Table 5, Fig 3, and S2 Table).
Fig 1. Kaplan-Meier curves showing cumulative survival (combined endpoint all-cause death and/or MI and/or ischemic stroke) stratified according to SDF1 rs2839693 homozygote carriers of major allele and carriers of minor allele.
No. at risk: blue = homozygote carriers of major allele, green = carriers of minor allele.
Fig 2. Kaplan-Meier curves showing cumulative survival (combined endpoint all-cause death and/or MI and/or ischemic stroke) stratified according to SDF1 rs266089 homozygote carriers of major allele and carriers of minor allele.
No. at risk: blue = homozygote carriers of major allele, green = carriers of minor allele.
Table 5. SDF1 haplotypes and their effects on the combined endpoint in uni- and multivariate Cox regression.
| SDF1 variants* | Univariate analysis | Multivariate analysis† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Haplotype‡ | 1 | 2 | 3 | 4 | 5 | 6 | Frequency (%) | HR (95% CI) | p (Wald test) | HR (95% CI) | p (Wald test) |
| ReferenceH-01 | 47.3 | - | - | - | - | ||||||
| H-02 | 18.7 | 0.98 (0.62/1.53) | 0.922 | 0.76 (0.46/1.25) | 0.285 | ||||||
| H-03 | 15 | 1.09 (0.71/1.66) | 0.687 | 1.00 (0.63/1.59) | 0.996 | ||||||
| H-04 | 13.8 | 0.59 (0.35/1.00) | 0.052 | 0.47 (0.27/0.84) | 0.0107 | ||||||
1 rs1065297, 2 rs1801157, 3 rs266089, 4 rs266087, 5 rs266085, 6 rs2839693
* variants are ordered according to chromosomal position (GRCh38); light grey/dark grey = carriers of major/minor allele
† with covariates as listed in Table 4
‡ listed are only haplotypes with frequencies ≥ 5%
Fig 3. Kaplan-Meier curves showing cumulative survival (combined endpoint all-cause death and/or MI and/or ischemic stroke) stratified according to carriers of reference haplotype H-01 and haplotype H-04 (applying a dominant haplotype model).
Wald test p-value refers to our multivariate Cox regression for SDF1 haplotypes (see Table 5). Red = carriers of 1 or 2 copies of H-01, but not H-04, Orange = carriers of 1 or 2 copies of H-04.
Discussion
The major findings of our study are that the SDF1 SNPs rs2839693 and rs266089 are associated with a better prognosis in patients with cardiovascular disease.
SDF1 is involved in progenitor cell proliferation, traffic, adhesion and regulates cell survival.[2,3,4,5,6,7,29] We have previously shown that platelet SDF1- and CXCR7 expression levels have a significant impact on myocardial function recovery in ACS patients,[12,15] possibly due to a contribution of the SDF1/CXCR4/CXCR7 axis to progenitor cell survival after organ hypoxia.[30] As mentioned before, several experimental studies have suggested that high expression levels of SDF1 in ischemic myocardium result in cardioprotection and improved myocardial function after myocardial infarction in vivo.[8,31] Our group developed a bifunctional protein consisting of a SDF1- and a glycoprotein VI (GPVI) domain with high binding affinity to CXCR4, which supports this hypothesis. SDF1-GPVI attracts CXCR4-positive cells, preserves cell survival, enhances endothelial differentiation of bone marrow cells (BMCs) and reveals proangiogenic effects. In vivo, administration of the bifunctional protein leads to enhanced recruitment of BMCs, increased capillary density, reduced infarct size, and preserved cardiac function.[32,33] On the other hand, our group could recently show, that high platelet SDF-1 levels might alter outcomes in patients with cardiovascular disease in a negative way (unpublished). SDF1 possesses different functions in vivo and SNPs in the SDF1 gene play various roles in many pathophysiologic processes. The gene encoding SDF-1 is located on the human chromosome 10q11.1, which has been previously identified as a susceptibility locus for cardiovascular disease by genome-wide association studies (GWASs).[34,35] Several studies have shown, that certain SDF-1 SNPs might alter SDF-1 protein expression levels.[36,37] However, in our present collective we could neither find a significant alteration of SDF-1 plasma- nor platelet levels within the different SDF-1 haplotypes (data not shown). The lack of alteration in the SDF-1 levels might be explained by the moderate sample size or the inhomogeneity of our study collective. Furthermore, hyperlipidemia is more prevalent in homozygous carriers of major allele than in minor allele carriers, possibly explaining the observations in our current study. Hyperlipidemia is known to be a major cardiovascular risk factor. However, in our multivariate analysis, hyperlipidemia fails to be independently associated with outcome, probably to the moderate sample size and frequent co-medication such as statins. Finally, most traditional cardiovascular risk factors fail to show independent associations with the end point in our study. We believe, that our collective is very heterogeneous and treated in an aggressive way to lower e.g. hypertension, hyperlipidemia and serum glucose levels. In combination with the moderate sample size, this fact might diminish effects of those risk factors on prognosis in our collective. Thus, the mechanisms involved remain speculative and warrant further investigation. Since there are sparse reports dealing with SDF1 SNPs in cardiovascular disease we can hardly compare our results with previous reports. In a study by Feng et al., the SDF1 polymorphism rs1801157 was suggested to be an independent risk factor for development of coronary artery disease.[18] A meta analysis performed by Wu et al suggested that rs1801157 was significantly associated with a decreased risk of myocardial infarction.[38] Furthermore, SDF1 rs1801157 has been demonstrated to influence protein expression levels.[39] There exist controversial data on the influence of the afore mentioned polymorphism on cardiovascular disease, especially when Chinese and Caucasian population are compared.[38] As already mentioned we could not show any differences in plasma SDF-1- or platelet SDF-1 levels for the SNP rs1801157 and we did not investigate incidence of coronary artery disease. Interestingly, we can demonstrate, that minor allele carriers of SNP rs1801157 suffer markedly less from acute coronary syndromes as compared to major allele carriers of the same polymorphism. However, we could not find a significant influence of different SDF-1 polymorphisms on acuity and severity of coronary artery disease i.e. defined by the Gensini Score.[40]
The major clinical implication of the present study is that identification of SDF1 haplotypes (variant rs2839693 and rs266089) might help to characterise cardiovascular patients at high risk for future evens who might benefit from genotype guided targeted therapies. Previously, a number of studies have highlighted the benefits of genotype guided pharmacotherapies in cardiovascular cohorts.[41,42] Although we could not show effects of the investigated SNPs on protein expression and did not evaluate effects on protein function, it is tempting to speculate that therapies targeting SDF-1 mediated effects on cardiovascular regeneration are of potential benefit.
To conclude, this is to the best of our knowledge the first report of the prognostic impact of selected SDF1 SNPs in a large cohort of cardiovascular patients. Thus, we show that SDF1 SNPs rs2839693 and rs266089 are associated with the combined endpoint including death, stroke and myocardial infarction. In addition, multivariate haplotype analysis revealed that the haplotype carrying these two linked SDF1-polymorphisms is significantly associated with the combined endpoint.
There are other additional SDF1 SNPs that might be associated with cardiovascular prognosis. Thus, SDF1 SNPs with rare allele frequency need to be identified by novel sequencing techniques including deep sequencing and independent causal alleles should be evaluated by genome wide approaches.
The present results suggest, that identification of distinct SNPs for SDF1 might help to develop novel pharmacological strategies to foster regenerative processes and to improve cardiovascular outcome. We are aware that these associations warrant confirmation in genome wide association approaches and the effects of guided therapy need confirmation by interventional randomized pharmacogenetic trials.
Limitations
We are aware that our results are barely hypothesis generating. Our study has certain limitations mainly due to the observational character of the study and the moderate sample size. We did not account for other potential confounders including biomarkers that have previously been associated with outcome in cardiovascular cohorts. Since the study cohort consisted of mostly Caucasian individuals, it might be difficult to generalise the results for other ethnicities. A SNP study should definitely be validated in another cohort. However, a sample size calculation based on a log-rank test yielded that in order to validate the effect of rs266089 with 80% power and for a significance level of 5%, another cohort with at least n = 788 patients would be required. Unfortunately, an additional appropriate cohort of this size for validation was not available. We regard the present study as a first observational study which needs replication in further large scale studies.
Supporting Information
Linkage disequilibrium map was created using Haploview.
(DOCX)
(DOCX)
(PPTX)
(DOCX)
Acknowledgments
This work was supported in part by the German Ministry of Education and Research, the Deutsche Forschungsgemeinschaft (Grant Number: BO 3786/1-1), the Robert Bosch Stiftung Stuttgart, the Open Access Publishing Fund of the University of Tuebingen and the Klinische Forschergruppe KFO274 (Grant number 2133-0-0; SCHW858/1-1/2) ‘Platelets-Basic Mechanisms and Translational Implications’. We gratefully acknowledge Monika Elbl, Andrea Jarmuth, and Heidi Köhler for excellent technical assistance. Furthermore we would like to thank L. Laptev, E. Tavlaki, D. Lombardi, A. Hoffmann, A. Valera, D. Eppler, D. Tschernow, J. Metzger, J. P. Schwille, K. Hey, M. Schmid, M. Haas, S. Breuning, C. Eick, and Christina Flaum for the excellent support in data collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by the German Ministry of Education and Research, the Deutsche Forschungsgemeinschaft (Grant Number: BO 3786/1-1), the Robert Bosch Stiftung Stuttgart, the Open Access Publishing Fund of the University of Tuebingen and the Klinische Forschergruppe KFO274 (Grant number 2133-0-0; SCHW858/1-1/2) ‘Platelets-Basic Mechanisms and Translational Implications’. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.George JN, Pickett EB, Saucerman S, McEver RP, Kunicki TJ, Kieffer N, et al. Platelet surface glycoproteins. Studies on resting and activated platelets and platelet membrane microparticles in normal subjects, and observations in patients during adult respiratory distress syndrome and cardiac surgery. J Clin Invest. 1986;78:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Secchiero P, Celeghini C, Cutroneo G, Di Baldassarre A, Rana R, Zauli G. Differential effects of stromal derived factor-1 alpha (sdf-1 alpha) on early and late stages of human megakaryocytic development. Anat Rec. 2000;260:141–147. [DOI] [PubMed] [Google Scholar]
- 3.Wright LM, Maloney W, Yu X, Kindle L, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 binding to its chemokine receptor cxcr4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 2005;36:840–853. [DOI] [PubMed] [Google Scholar]
- 4.Gillette JM, Larochelle A, Dunbar CE, Lippincott-Schwartz J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat Cell Biol. 2009;11:303–311. 10.1038/ncb1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayakawa J, Migita M, Ueda T, Fukazawa R, Adachi K, Ooue Y, et al. Dextran sulfate and stromal cell derived factor-1 promote cxcr4 expression and improve bone marrow homing efficiency of infused hematopoietic stem cells. J Nippon Med Sch. 2009;76:198–208. [DOI] [PubMed] [Google Scholar]
- 6.Kyriakou C, Rabin N, Pizzey A, Nathwani A, Yong K. Factors that influence short-term homing of human bone marrow-derived mesenchymal stem cells in a xenogeneic animal model. Haematologica. 2008;93:1457–1465. 10.3324/haematol.12553 [DOI] [PubMed] [Google Scholar]
- 7.Janowski M. Functional diversity of sdf-1 splicing variants. Cell Adh Migr. 2009;3:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. 10.1016/j.stem.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 9.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. [DOI] [PubMed] [Google Scholar]
- 10.Stellos K, Bigalke B, Langer H, Geisler T, Schad A, Kögel A, et al. Expression of stromal-cell-derived factor-1 on circulating platelets is increased in patients with acute coronary syndrome and correlates with the number of CD34+ progenitor cells. Eur Heart J. 2009;30:584–593. 10.1093/eurheartj/ehn566 [DOI] [PubMed] [Google Scholar]
- 11.Stellos K, Ruf M, Sopova K, Kilias A, Rahmann A, Stamatelopoulos K, et al. Plasma levels of stromal cell-derived factor-1 in patients with coronary artery disease: Effect of clinical presentation and cardiovascular risk factors. Atherosclerosis. 2011;219:913–916. 10.1016/j.atherosclerosis.2011.09.022 [DOI] [PubMed] [Google Scholar]
- 12.Geisler T, Fekecs L, Wurster T, Chiribiri A, Schuster A, Nagel E, et al. Association of platelet-SDF-1 with hemodynamic function and infarct size using cardiac MR in patients with AMI. Eur J Radiol. 2012;81:486–490. [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, et al. Overexpression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281–292. 10.1016/j.yjmcc.2007.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rath D, Chatterjee M, Borst O, Müller K, Stellos K, Mack AF, et al. Expression of stromal cell-derived factor-1 receptors CXCR4 and CXCR7 on circulating platelets of patients with acute coronary syndrome and association with left ventricular functional recovery. Eur Heart J. 2014;35:386–394. 10.1093/eurheartj/eht448 [DOI] [PubMed] [Google Scholar]
- 16.Rath D, Chatterjee M, Borst O, Müller K, Langer H, Mack AF, et al. Platelet surface expression of stromal cell-derived factor-1 receptors CXCR4 and CXCR7 is associated with clinical outcomes in patients with coronary artery disease. J Thromb Haemost. 2015;13:719–728. 10.1111/jth.12870 [DOI] [PubMed] [Google Scholar]
- 17.Ku FC, Tsai CR, Der Wang J, Wang CH, Chang TK, et al. Stromal-derived factor-1 gene variations in pediatric patients with primary immune thrombocytopenia. Eur J Haematol. 2013;90:25–30. 10.1111/ejh.12025 [DOI] [PubMed] [Google Scholar]
- 18.Feng L, Nian SY, Hao YL, Xu WB, Ye D, Zhang XF, et al. A single nucleotide polymorphism in the stromal cell-derived factor 1 gene is associated with coronary heart disease in Chinese patients. Int J Mol Sci. 2014;15:11054–11063. 10.3390/ijms150611054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res. 1997;35:2–3. [PubMed] [Google Scholar]
- 20.ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice. J Postgrad Med. 2001;47:45–50: 121,–130: 199–203. [PubMed] [Google Scholar]
- 21.Directive 2001/20/EC of the European Parliament and of the Council of 4th April 2001 on the approximation of the laws, regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Med Etika Bioet. 2002;9:12–19. [PubMed] [Google Scholar]
- 22.Gerli G, Vanelli C, Turri O, Erario M, Gardellini A, Pugliano M, et al. SDF1-3'A gene polymorphism is associated with chronic myeloproliferative disease and thrombotic events. Clin Chem. 2005;51:2411–2414. [DOI] [PubMed] [Google Scholar]
- 23.Kimura R, Nishioka T, Soemantri A, Ishida T. Allele-specific transcript quantification detects haplotypic variation in the levels of the SDF-1 transcripts. Hum Mol Genet. 2005;14:1579–1585. [DOI] [PubMed] [Google Scholar]
- 24.Xiao Q, Ye S, Oberhollenzer F, Mayr A, Jahangiri M, Willeit J, et al. SDF1 gene variation is associated with circulating SDF1alpha level and endothelial progenitor cell number: the Bruneck Study. PLoS One. 2008;3:e4061 10.1371/journal.pone.0004061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu N, Zhang X, Jia P, Jia D. Lack of an Association between the SDF-1 rs1801157 Polymorphism and Coronary Heart Disease: A Meta-Analysis. Sci Rep. 2015;5:11803 10.1038/srep11803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu XL, Ma N, Xiang DC, Huang J, Dong ZH, Lei HY, et al. Polymorphism of stromal cell-derived factor-1 selectively upregulates gene expression and is associated with increased susceptibility to coronary artery disease. Biochem Biophys Res Commun. 2014;443:932–937. 10.1016/j.bbrc.2013.12.065 [DOI] [PubMed] [Google Scholar]
- 27.Schroth W, Antoniadou L, Fritz P, Schwab M, Muerdter T, Zanger UM, et al. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 2007;25:5187–5193. [DOI] [PubMed] [Google Scholar]
- 28.Hardy—Weinberg equilibrium. http://ihg.gsf.de/cgi-bin/hw/hwa1.pl.
- 29.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479–490. 10.1084/jem.20071903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012;7:e34608 10.1371/journal.pone.0034608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, et al. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206–215. [DOI] [PubMed] [Google Scholar]
- 32.Ziegler M, Elvers M, Baumer Y, Leder C, Ochmann C, Schönberger T, et al. The bispecific SDF1-GPVI fusion protein preserves myocardial function after transient ischemia in mice. Circulation. 2012;125:685–696. 10.1161/CIRCULATIONAHA.111.070508 [DOI] [PubMed] [Google Scholar]
- 33.Schesny MK, Monaghan M, Bindermann AH, Freund D, Seifert M, Eble JA, et al. Preserved bioactivity and tunable release of a SDF1-GPVI bi-specific protein using photo-crosslinked PEGda hydrogels. Biomaterials. 2014;35:7180–7187. 10.1016/j.biomaterials.2014.04.116 [DOI] [PubMed] [Google Scholar]
- 34.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myocardial Infarction Genetics Consortium, Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. 10.1038/ng.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soriano A, Martínez C, García F, Plana M, Palou E, Lejeune M, et al. Plasma stromal cell derived factor (SDF)-1 levels, SDF1-3’A genotype, and expression of CXCR4 on T lymphocytes: their impact on SDF-1 gene variations in childhood primary immune thrombocytopenia resistance to human immunodeficiency virus type 1 infection and its progression. J Infect Dis 2002;186:922–931 [DOI] [PubMed] [Google Scholar]
- 37.Kimura R, Nishioka T, Soemantri A, Ishida T. Allele-specific transcript quantification detects haplotypic variation in the levels of the SDF-1 transcripts. Hum Mol Genet;14:1579–1585. [DOI] [PubMed] [Google Scholar]
- 38.Wu N, Zhang X, Jia P, Jia D. Lack of an Association between the SDF-1 rs1801157 Polymorphism and Coronary Heart Disease: A Meta-Analysis. Sci Rep. 2015;5:11803 10.1038/srep11803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkler C, Modi W, Smith MW, Nelson GW, Wu X, Carrington M, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC). Science. 1998;279:389–393 [DOI] [PubMed] [Google Scholar]
- 40.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606 [DOI] [PubMed] [Google Scholar]
- 41.Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, et al. ; EU-PACT Group. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369:2294–2303 10.1056/NEJMoa1311386 [DOI] [PubMed] [Google Scholar]
- 42.Verschuren JJ, Trompet S, Wessels JA, Guchelaar HJ, de Maat MP, Simoons ML, et al. A systematic review on pharmacogenetics in cardiovascular disease: is it ready for clinical application? Eur Heart J. 2012;33:165–175. 10.1093/eurheartj/ehr239 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linkage disequilibrium map was created using Haploview.
(DOCX)
(DOCX)
(PPTX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.