Abstract
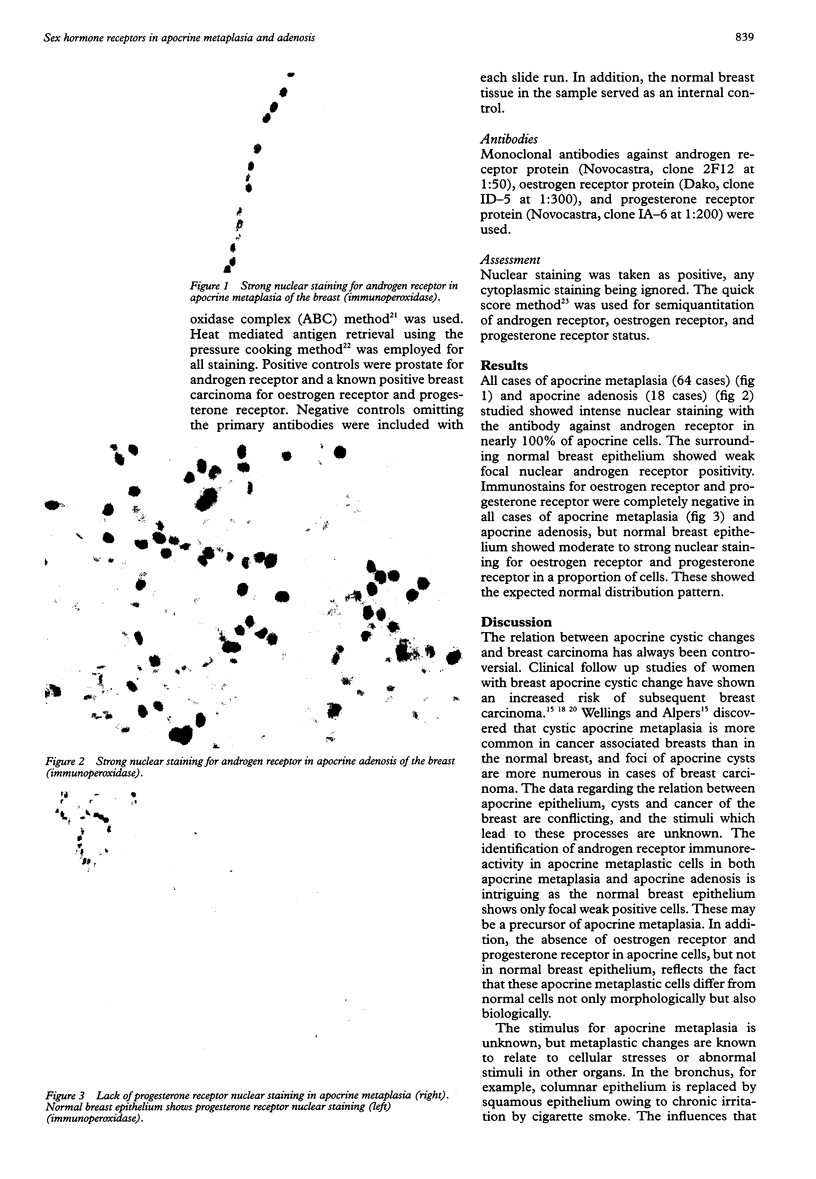
AIM: To investigate the receptor status of the sex steroid hormones in apocrine metaplasia of the breast. METHODS: 82 cases of apocrine metaplasia, including 18 of the rare lesion apocrine adenosis, were studied immunohistochemically for the expression of androgen receptor, oestrogen receptor, and progesterone receptor proteins on formalin fixed, paraffin embedded tissue sections. The standard avidin biotin complex (ABC) technique was followed and appropriate positive and negative controls were used. RESULTS: All the studied cases (82/82) were positive for androgen receptor, but were negative for oestrogen receptor and progesterone receptor. CONCLUSIONS: Apocrine metaplastic epithelium, unlike the normal breast epithelium, is responsive to androgens, through androgen receptors, rather than to the female sex hormones. This may have clinical implications.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeli A., Bradlow H. L., Bodian C. A., Chasalow F. I., Dogliotti L., Haagensen D. E., Jr Criteria for classifying breast cyst fluids. Ann N Y Acad Sci. 1990;586:49–52. doi: 10.1111/j.1749-6632.1990.tb17788.x. [DOI] [PubMed] [Google Scholar]
- Bruzzi P., Dogliotti L., Naldoni C., Bucchi L., Costantini M., Cicognani A., Torta M., Buzzi G. F., Angeli A. Cohort study of association of risk of breast cancer with cyst type in women with gross cystic disease of the breast. BMJ. 1997 Mar 29;314(7085):925–928. doi: 10.1136/bmj.314.7085.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger A., Caron S., Labrie F., Naldoni C., Dogliotti L., Angeli A. Levels of eighteen non-conjugated and conjugated steroids in human breast cyst fluid: relationships with cyst type. Eur J Cancer. 1990 Mar;26(3):277–281. doi: 10.1016/0277-5379(90)90222-f. [DOI] [PubMed] [Google Scholar]
- Dixon J. M., Lumsden A. B., Miller W. R. The relationship of cyst type to risk factors for breast cancer and the subsequent development of breast cancer in patients with breast cystic disease. Eur J Cancer Clin Oncol. 1985 Sep;21(9):1047–1050. doi: 10.1016/0277-5379(85)90289-5. [DOI] [PubMed] [Google Scholar]
- Dixon J. M., McDonald C., Elton R. A., Miller W. R. Risk of breast cancer in women with palpable breast cysts: a prospective study. Edinburgh Breast Group. Lancet. 1999 May 22;353(9166):1742–1745. doi: 10.1016/s0140-6736(98)06408-3. [DOI] [PubMed] [Google Scholar]
- Dupont W. D., Page D. L. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985 Jan 17;312(3):146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- Ellis L. M., Wittliff J. L., Bryant M. S., Hogancamp W. E., Sitren H. S., Bland K. I. Correlation of estrogen, progesterone, and androgen receptors in breast cancer. Am J Surg. 1989 Jun;157(6):577–581. doi: 10.1016/0002-9610(89)90704-6. [DOI] [PubMed] [Google Scholar]
- Gatalica Z. Immunohistochemical analysis of apocrine breast lesions. Consistent over-expression of androgen receptor accompanied by the loss of estrogen and progesterone receptors in apocrine metaplasia and apocrine carcinoma in situ. Pathol Res Pract. 1997;193(11-12):753–758. doi: 10.1016/S0344-0338(97)80053-2. [DOI] [PubMed] [Google Scholar]
- Haagensen D. E., Jr, Dilley W. G., Mazoujian G., Wells S. A., Jr Review of GCDFP-15. An apocrine marker protein. Ann N Y Acad Sci. 1990;586:161–173. doi: 10.1111/j.1749-6632.1990.tb17804.x. [DOI] [PubMed] [Google Scholar]
- Hackenberg R., Hawighorst T., Filmer A., Nia A. H., Schulz K. D. Medroxyprogesterone acetate inhibits the proliferation of estrogen- and progesterone-receptor negative MFM-223 human mammary cancer cells via the androgen receptor. Breast Cancer Res Treat. 1993;25(3):217–224. doi: 10.1007/BF00689836. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kuenen-Boumeester V., Van der Kwast T. H., van Putten W. L., Claassen C., van Ooijen B., Henzen-Logmans S. C. Immunohistochemical determination of androgen receptors in relation to oestrogen and progesterone receptors in female breast cancer. Int J Cancer. 1992 Oct 21;52(4):581–584. doi: 10.1002/ijc.2910520415. [DOI] [PubMed] [Google Scholar]
- Langer M., Kubista E., Schemper M., Spona J. Androgen receptors, serum androgen levels and survival of breast cancer patients. Arch Gynecol Obstet. 1990;247(4):203–209. doi: 10.1007/BF02389545. [DOI] [PubMed] [Google Scholar]
- Liberato M. H., Sonohara S., Brentani M. M. Effects of androgens on proliferation and progesterone receptor levels in T47D human breast cancer cells. Tumour Biol. 1993;14(1):38–45. doi: 10.1159/000217823. [DOI] [PubMed] [Google Scholar]
- Ness J. C., Sedghinasab M., Moe R. E., Tapper D. Identification of multiple proliferative growth factors in breast cyst fluid. Am J Surg. 1993 Sep;166(3):237–243. doi: 10.1016/s0002-9610(05)80965-1. [DOI] [PubMed] [Google Scholar]
- Norton A. J., Jordan S., Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994 Aug;173(4):371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- Page D. L., Dupont W. D., Jensen R. A. Papillary apocrine change of the breast: associations with atypical hyperplasia and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1996 Jan;5(1):29–32. [PubMed] [Google Scholar]
- Page D. L., Vander Zwaag R., Rogers L. W., Williams L. T., Walker W. E., Hartmann W. H. Relation between component parts of fibrocystic disease complex and breast cancer. J Natl Cancer Inst. 1978 Oct;61(4):1055–1063. [PubMed] [Google Scholar]
- Roberts M. M., Jones V., Elton R. A., Fortt R. W., Williams S., Gravelle I. H. Risk of breast cancer in women with history of benign disease of the breast. Br Med J (Clin Res Ed) 1984 Jan 28;288(6413):275–278. doi: 10.1136/bmj.288.6413.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. D., Ashton M., Lefkowitz M. Atypical apocrine adenosis of the breast: a clinicopathologic study of 37 patients with 8.7-year follow-up. Cancer. 1996 Jun 15;77(12):2529–2537. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2529::AID-CNCR16>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Tavassoli F. A., Norris H. J. Intraductal apocrine carcinoma: a clinicopathologic study of 37 cases. Mod Pathol. 1994 Oct;7(8):813–818. [PubMed] [Google Scholar]
- Wales N. A., Ebling F. J. The control of the apocrine glands of the rabbit by steroid hormones. J Endocrinol. 1971 Dec;51(4):763–770. doi: 10.1677/joe.0.0510763. [DOI] [PubMed] [Google Scholar]
- Wellings S. R., Alpers C. E. Apocrine cystic metaplasia: subgross pathology and prevalence in cancer-associated versus random autopsy breasts. Hum Pathol. 1987 Apr;18(4):381–386. doi: 10.1016/s0046-8177(87)80169-7. [DOI] [PubMed] [Google Scholar]
- Wells C. A., McGregor I. L., Makunura C. N., Yeomans P., Davies J. D. Apocrine adenosis: a precursor of aggressive breast cancer? J Clin Pathol. 1995 Aug;48(8):737–742. doi: 10.1136/jcp.48.8.737. [DOI] [PMC free article] [PubMed] [Google Scholar]





