Abstract
Purpose
To determine the relationship between carotid intima–media thickness (IMT), coronary artery calcification (CAC), and myocardial blood flow (MBF) at rest and during vasomotor stress in type 2 diabetes mellitus (DM).
Methods
In 68 individuals, carotid IMT was measured using high-resolution vascular ultrasound, while the presence of CAC was determined with electron beam tomography (EBT). Global and regional MBF was determined in milliliters per gram per minute with 13N-ammonia and positron emission tomography (PET) at rest, during cold pressor testing (CPT), and during adenosine (ADO) stimulation.
Results
There was neither a relationship between carotid IMT and CAC (r=0.10, p=0.32) nor between carotid IMT and coronary circulatory function in response to CPT and during ADO (r=−0.18, p=0.25 and r=0.10, p=0.54, respectively). In 33 individuals, EBT detected CAC with a mean Agatston-derived calcium score of 44±18. There was a significant difference in regional MBFs between territories with and without CAC at rest and during ADO-stimulated hyperemia (0.69±0.24 vs. 0.74±0.23 and 1.82±0.50 vs. 1.95±0.51 ml/g/min; p≤0.05, respectively) and also during CPT in DM but less pronounced (0.81±0.24 vs. 0.83±0.23 ml/g/min; p=ns). The increase in CAC was paralleled with a progressive regional decrease in resting as well as in CPT- and ADO-related MBFs (r=−0.36, p≤0.014; r=−0.46, p≤0.007; and r=−0.33, p≤0.041, respectively).
Conclusions
The absence of any correlation between carotid IMT and coronary circulatory function in type 2 DM suggests different features and stages of early atherosclerosis in the peripheral and coronary circulation. PET-measured MBF heterogeneity at rest and during vasomotor stress may reflect downstream fluid dynamic effects of coronary artery disease (CAD)-related early structural alterations of the arterial wall.
Keywords: Blood flow, Carotid IMT, Circulation, Cold pressor test, Coronary artery calcification, Diabetes mellitus, Flow heterogeneity, Tomography, Vasomotor function
Introduction
Positron emission tomography (PET) affords the noninvasive identification and characterization of coronary vascular dysfunction that may precede or accompany coronary artery disease (CAD)-related structural alterations of the arterial wall [1–6]. On the other hand, early structural alterations of the atherosclerotic process, for example, increases in carotid intima–media thickness (IMT) by vascular ultrasound, can also be identified. Increases in carotid IMT have been suggested to serve as surrogate marker for subintimal thickening of the coronary arteries [7, 8]. Furthermore, coronary artery calcification (CAC), thought of as an indicator of the atherosclerotic burden of the arterial wall, can be routinely identified by electron beam tomography (EBT) or, more recently, by multislice detector computed tomography (MDCT) [9, 10]. The interrelation of the structural and functional determinants of subclinical CAD, however, still remains to be elucidated. To current knowledge, focal coronary artery lesions between 60% and 85% diameter stenosis commonly do not significantly impair resting myocardial blood flow (MBF) owing to a compensatory vasodilation of the downstream arteriolar resistance vessels [11]. This adaptive vasodilation under resting conditions is insufficient to compensate for greater increases in epicardial resistance, i.e., for more severe coronary stenoses (greater than 85%) [11–14]. Pharmacologically stimulated hyperemic MBFs, however, begin to decline in the presence of a coronary lesion of about 50%. Recent investigations [2, 15–17] suggest that hyperemic MBFs may also be reduced in the presence of diffuse CAD with coronary lesions less than 50% and/or coronary circulatory dysfunction. In view of these findings, a new concept has evolved that even subclinical CAD-related structural alterations of the arterial wall may exert downstream fluid dynamic effects that may manifest as mild myocardial perfusion heterogeneity at rest [18] or as longitudinal, base-to-apex perfusion gradient during hemodynamic stress [15, 19–21].
With this in mind, we intended to evaluate whether abnormal increases in carotid IMT or CAC, as surrogate marker for coronary arterial vessel stiffness, affect regional MBFs and thereby lead to MBF heterogeneity at rest, during sympathetic stimulation with cold pressor testing (CPT), or during pharmacologic vasodilation in type 2 diabetes mellitus (DM) patients.
Materials and methods
Study population and design
The study population comprised 32 asymptomatic individuals without traditional coronary risk factors such as arterial hypertension, smoking, and hypercholesterolemia as healthy controls (CON) and 36 patients with type 2 DM either normotensive (NDM) or hypertensive (HDM) (Table 1). Diabetic patients were not treated with hypoglycemic medications at the time of the study. They were classified as type 2 DM based on standard criteria including fasting plasma glucose levels determined on at least two occasions of >126 mg/dL and high glycosylated hemoglobin (HbA1c) >5.9%. Each study participant was screened by complete history, physical examination, resting electrocardiogram (ECG), and routine blood chemistry. Excluded were patients with vascular complications of diabetes, a history of CAD, cardiac disease, and any other disease. In addition, no study participant was on vasoactive medication such as angiotensin-converting enzyme inhibitors, calcium channel blockers, or statins at the time of study inclusion.
Table 1.
Characteristics of study population (n=68)
| CON (n=32) | DM (n=36) | NDM (n=23) | HDM (n=13) | |
|---|---|---|---|---|
| Age (years) | 41 ± 12 | 51 ± 10* | 48±9* | 57±9* |
| Sex (F/M) | 19/13 | 18/18 | 10/13 | 8/5 |
| BMI (kg/m ) | 27±3 | 31±5* | 31±6* | 30±4* |
| Lipid status | ||||
| Total cholesterol (mg/dL) | 173 ±32 | 202±48* | 198±44* | 210±54* |
| LDL cholesterol (mg/dL) | 106±28 | 120±33 | 117±27 | 126±42 |
| HDL cholesterol (mg/dL) | 44±11 | 42±11 | 40±12* | 46±9 |
| Triglyceride level (mg/dL) | 109±85 | 220±171 | 226±198* | 208±14* |
| Glucose level (mg/dL) | 85±11 | 188±77* | 195±81* | 177±71* |
| Insulin (mcU/mL) | 8±4 | 15±15* | 14±15* | 15±17 |
| HOMA | 1.7±0.96 | 6.4±7.0* | 6.2±7.9* | 5.5±5.6* |
| HbA1c (%) | 5.5±0.25 | 9.4±2.8* | 9.5±2.6* | 9.4±3.3* |
Values are presented as the mean±SD
CON controls, DM diabetes mellitus, NDM normotensive diabetes mellitus, HDM hypertensive diabetes mellitus, BMI body mass index, HOMA homeostasis model assessment, HbA1c hemoglobin A1c
p<0.05 vs. CON
At the time of the PET study to assess coronary circulatory function, blood samples were obtained by venipuncture, and blood chemistry included total cholesterol, HDL and LDL cholesterol, triglycerides, glucose, insulin, HbA1c, and high-sensitive C-reactive protein (hs-CRP) (Table 1). Within 20 days of the PET study, high-resolution vascular ultrasound and EBT were performed to assess carotid IMT and CAC, respectively [22, 23]. The study was approved by the University of California at Los Angeles Human Subject Protection Committee and each participant signed an informed consent form.
Noninvasive assessment of MBF by PET
MBF in milliliters per gram per minute was measured noninvasively with intravenous 13N-ammonia, serial image acquisition by PET (ECAT EXACT HR+, Siemens/CTI model 931/08-12, Siemens AG, Munich, Germany), and a two-compartment tracer kinetic model [3, 20]. MBF measurements were performed first at baseline and then during CPT. For the CPT, study participants immersed their left hand into ice water for 60 s, and 13N-ammonia was injected again while CPT was maintained for another 60 s to allow the trapping of 13N-ammonia in the myocardium [3]. Hyperemic MBF was induced with intravenous standard dose adenosine (140 μg/kg/min). The relative distribution of MBF was assessed visually on reorientated static 13N-ammonia images. Using the two-compartment tracer kinetic model [3, 20], regional MBFs of the LAD, LCx, and RCA territory were averaged on a polar map and the resulting mean MBF of the LV was defined as global MBF. As the global MBF was averaged over the LV, it may have resulted in relatively lower but more consistent hyperemic MBF values. Heart rate (HR), blood pressure (BP), and a 12-lead ECG were recorded continuously. From the average of HR and BP during the first 2 min of each image acquisition, the rate–pressure product (RPP) was derived as an index of cardiac work. To account for interindividual variations in coronary driving pressure, an index of coronary vascular resistance (CVR) was determined as the ratio of mean arterial blood pressure (in millimeters of mercury) to MBF (in milliliters per gram per minute). Regional MBFs are denoted for myocardial territories with or without CAC.
Assessment of carotid IMT
Measurements were obtained by an experienced vascular sonographer who was blinded to the clinical or laboratory profile of the study participants. High-resolution B-mode ultrasound images of the common carotid artery were obtained with a 10-MHz linear array transducer attached to an ATL Ultramark IV (Bothell, WA, USA) ultrasound machine. The right and left common carotid arteries were imaged according to a predetermined, standardized scanning protocol [22]. A longitudinal section of the common carotid artery 1 cm proximal to the carotid bulb was scanned. The reported IMT for each study participant resulted from the average of ten measurements (five measurements from the right and five from the left common carotid artery).
Calcium scanning
CAC was measured using EBT (GE Imatron, Milwaukee, WI, USA). Two prospective cardiac gated (at 60% R–R interval) scans were acquired using 3 mm collimation CVS mode. Utilizing the Accu-Image workstation areas of CAC considered present if three or more contiguous pixels with a signal intensity of >130 HU were identified [24]. The size of the lesion was automatically calculated, and the CAC was scored using the Agatston algorithm [23]. The coronary artery calcium score (CCS) was assessed across all lesions identified within the left main, LAD, LCx, and RCA, and the sum of all lesion scores yielded the total CCS. The Agatston score [23] defined normal and pathological range based on the total CCS related to the age and gender of the individual. An abnormal CAC was defined as an increase in CCS >75th percentile of age-related CAC. Furthermore, in a more simplified scoring system [25] based on the total CCS, study participants were assigned into groups of minor, moderate or severe CAC with CCS of ≤100, >100, and >400 U, respectively.
Statistical analysis
Data are presented as the mean±SD for quantitative and absolute frequencies for qualitative variables. For comparison of differences, appropriate t tests for independent or paired samples were used. Pearson’s correlation coefficient (r), assuming a linear regression, was calculated to investigate the associations between resting MBF and its changes during vasomotor stress, carotid IMT, and CAC. According to previous investigations [26], the study population was grouped according to normal and increased carotid IMT levels (<0.8 and ≥0.8 mm). Furthermore, study participants were also grouped accordingly to tertiles of the carotid IMT findings with <0.6933 mm (lowest tertile), between 0.6933 to 0.82 mm (medium tertile), and >0.82 mm (highest tertile). Since coronary calcium scores (CCS) followed a skewed distribution, the CCS were logarithmically transformed (log-CCS) and related to the MBF data. To current knowledge, there are no generally accepted cut-off values that would denote clinical significance of calcium scores in study participants predominantly with relative CCS <100. Consequently, study participants were grouped by tertiles: log-CCS <−0.0292 (lowest tertile), log-CCS −0.0292 to 0.7628 (medium tertile), and log-CCS >0.7628 (highest tertile). Multivariate analysis was performed with the logistic regression model. Statistical significance was assumed if a null hypothesis could be rejected at p=0.05. All statistical analysis was performed with SPSS for Windows 12.0 (SPSS).
Results
Group characteristics
Table 1 summarizes the clinical characteristics of the CON, DM, NDM, and HDM. No study participant presented regional perfusion defects on the adenosine-induced hyperemic PET perfusion images, arguing against the presence of hemodynamically significant obstructive CAD.
Carotid IMT and CAC
As denoted in Table 2, the mean carotid IMT was significantly higher in DM than in CON. When DM was subgrouped in NDM and HDM, carotid IMT was similar between CON and NDM, while it was significantly higher in HDM. As regards the CAC, as reflected by the CCS (Table 2), it was nonsignificantly higher in DM than in CON, while similar between NDM and HDM. For the entire study population and for DM, the CCS did not correlate with carotid IMT (r=0.10, p=0.32 and r=0.25, p=0.35, respectively). Also, when the log-CCS was compared to the carotid IMT, no association was observed for both groups (r=0.31, p=0.26 and r=0.21, p=0.56, respectively). For the entire study population, CACs were found in 49% (33/68). In the CON group, the prevalence of CAC was 28% (9/32), which was significantly lower to that of 66% (24/36) of CAC in type 2 DM. Regarding the CAC distribution, the highest prevalence of CAC was in the LAD, followed by the LCx and RCA (Table 3). As can be appreciated, the average CCS of 44±18 was relatively low. CAC, as reflected by the log-CCS, did correlate with the age of the study participants (r= 0.35, p<0.015). Only five in DM and one in CON had abnormally increased CCS, when it was related to the age of the individual study participant (>75th percentile).
Table 2.
IMT, CAC, and PET study
| CON | DM | NDM | HDM | |
|---|---|---|---|---|
| IMT and CAC | ||||
| Carotid IMT (mm) | 0.70±11 | 0.83±0.19* | 0.76±0.17 | 0.95±0.17* |
| CCS | 5.87±28 | 35.84±82 | 34.21±67 | 38.73±105 |
| Hemodynamics at rest | ||||
| Heart rate (bpm) | 67±7 | 71 ± 13 | 71±9 | 72±19 |
| SBP (mmHg) | 117±16 | 138±26* | 122±12 | 165±20* |
| DBP (mmHg) | 71 ± 10 | 76±9* | 74±7 | 81±11* |
| RPP | 7,866±1,387 | 9,887±3,142* | 8,696± 1,660 | 11,096±4,027* |
| Cold pressor testing | ||||
| Heart rate (bpm) | 74±10 | 77±14 | 76±12 | 78±17 |
| SBP (mmHg) | 140±20 | 161 ±30* | 144±22 | 190±17* |
| DBP (mmHg) | 84±11 | 90±13* | 88±14 | 93±12* |
| RPP | 10,374±2,107 | 12,433±3,341* | 11,186±2,885 | 14,640±3,007* |
| ΔRPP | 2,509±1,606 | 2,546±1,786 | 2,440± 1,211 | 2,644±1,953 |
| Pharmacologic vasodilation | ||||
| Heart rate (bpm) | 94±16 | 96±17 | 95±13 | 99±22 |
| SBP (mmHg) | 117±14 | 131 ±22* | 121±18 | 148±18* |
| DBP (mmHg) | 73±12 | 73±10 | 72±9 | 73±11 |
| ΔRPP | 11,020±2,124 | 12,746±3,705* | 11,593±2,655 | 14,785±4,479* |
| ΔRPP | 3,155±1,779 | 2,858±1,857 | 2,496± 1,793 | 2,790±2,137 |
| Global MBF (ml/min/g myocardium) | ||||
| At rest | 0.67±0.15 | 0.77±0.26* | 0.65±0.15 | 0.99±0.28* |
| During CPT | 0.90±0.20 | 0.83±0.27 | 0.73±0.20* | 1.01 ±0.28 |
| Change to CPT | 0.23±0.09 | 0.06±0.10* | 0.08±0.10* | 0.03±0.07* |
| Pharmacologic vasodilation | 2.02±0.48 | 1.78±0.36* | 1.74±0.39* | 1.83±0.29 |
| MFR | 3.16±0.99 | 2.45±0.61* | 2.74±0.49* | 1.95±0.46* |
| Global CVR (mmHg/ml/min/g) | ||||
| At rest | 135±32 | 135±31 | 145±30 | 120±25* |
| During CPT | 119±27 | 146±37* | 154±37* | 131 ±33 |
| Change to CPT | -16±19 | 12±23* | 9±27* | 16±14* |
| CVR to ADO | 46±14 | 54±11* | 53±11* | 55± 11* |
Values are presented as the mean±SD
CON controls, DM diabetes mellitus, NDM normotensive diabetes mellitus, HDM hypertensive diabetes mellitus, CCS coronary artery calcium score, SBP systolic blood pressure, DBP diastolic blood pressure, RPP rate-pressure product, MBF myocardial blood flow, MFR myocardial flow reserve, CVR coronary vascular resistance
p<0.05 vs. CON
Table 3.
Coronary artery calcification
| CAC distribution | |||||
|---|---|---|---|---|---|
|
|
|||||
| LMS | LAD | LCx | RCA | Total CCS | |
| CON | 1 | 3 | 4 | 2 | 6±28 |
| NDM | 0 | 13 | 8 | 9 | 34±67 |
| HDM | 0 | 7 | 7 | 3 | 39±105 |
| Total | 1 | 23 | 19 | 14 | 44±18 |
CCS coronary artery calcium score
p=NS for CCS between groups by ANOVA
Hemodynamics during PET study
Hemodynamic parameters during MBF measurements at baseline and during CPT are listed in Table 2. In all groups, CPT induced a significant increase in heart rate, systolic blood pressure, and diastolic blood pressure, so that the RPP was significantly higher during CPT than at baseline. The increase in the RPP (ΔRPP) as a result of the CPT-induced sympathetic stimulation was not significantly different between the CON, DM, NDM, and HDM, indicating comparable increases in myocardial workload for all study groups. As regards the pharmacologic vasodilation with adenosine, there was a significant increase in heart rate and RPP in all groups. The adenosine-induced change in RPP (ΔRPP) remained similar among these groups.
Global MBF responses to CPT and during adenosine
Left ventricular global MBF and global CVR at rest, during CPT, and pharmacologic vasodilation with adenosine are indicated in Table 2. At baseline, global MBF was significantly higher in the entire study group of DM and HDM compared with CON and NDM. The change in global MBF from rest to CPT (global ΔMBF) was significantly less in DM as well as in the subgroups of NDM and HDM than in CON (p<0.0001, respectively). The group comparison between the CPT-induced global ΔMBF in CON was significant compared to DM, NDM, and HDM (p<0.0001 by ANOVA). Global MBFs during adenosine-induced pharmacologic vasodilation and, thus, the total vasodilator capacity were statistically significantly lower in DM and NDM than in CON, while it tended to be lower in HDM. The group comparison of adenosine-stimulated MBFs in CON was significant compared to DM, NDM, and HDM (p≤0.05 by ANOVA).
Regional MBFs subtended and not subtended to CAC
In study participants with CAC, the intrapatient comparison of resting MBF between calcified and noncalcified territories demonstrated significantly lower resting MBF in territories with CAC than in those without CAC (Table 4). Regional MBFs during CPT did not differ significantly between territories with and without CAC, while adenosine-stimulated MBFs in regions subtended by CAC vessels were significantly lower than those in regions subtended by non-CAC vessels. When the study population was subgrouped in CON, DM, and further into NDM and HDM, resting regional MBF was also significantly lower in the calcified than in the noncalcified coronary territory, apart from the HDM group (Table 4). Furthermore, CPT-related regional MBFs tended to be lower in CAC territories than in those without CAC in DM and NDM, but not in CON and HDM. Finally, regional hyperemic MBFs during adenosine stimulation were lower in the CAC than in the non-CAC territories in CON, DM, and NDM. In DM and NDM, this difference in regional MBF during adenosine stimulation reached borderline significance (p=0.06 and p=0.07, respectively), while no difference was observed for the HDM group.
Table 4.
MBF in CAC and non-CAC myocardial territory
| MBF at rest |
MBF during CPT |
MBF during adenosine |
|
|---|---|---|---|
| Whole study group | |||
| CAC territory | 0.69±0.24 | 0.81±0.23 | 1.82±0.50 |
| Non-CAC territory | 0.74±0.23* | 0.81±0.22 | 1.95±0.51* |
| CON | |||
| CAC territory | 0.61 ±0.15 | 0.81±0.22 | 1.96±0.64 |
| Non-CAC territory | 0.65±0.19* | 0.77±0.31 | 2.10±0.58 |
| DM | |||
| CAC territory | 0.75±0.27 | 0.81±0.24 | 1.71 ±0.33 |
| Non-CAC territory | 0.80±0.24* | 0.83±0.23 | 1.83±0.44 |
| NDM | |||
| CAC territory | 0.61 ±0.13 | 0.73±0.19 | 1.76±0.34 |
| Non-CAC territory | 0.68±0.13* | 0.77±0.13 | 1.98±0.48 |
| HDM | |||
| CAC territory | 0.97±0.29 | 0.91±0.25 | 1.64±0.32 |
| Non-CAC territory | 0.99±0.23 | 0.91±0.30 | 1.61 ±0.25 |
Paired analysis of the vascular territory of the LAD, LCx and RCA CAC coronary artery calcification, CON controls, NDM normotensive diabetes mellitus, HDM hypertensive diabetes mellitus
p<0.02 vs. CAC territory
Carotid IMT, CAC, and global MBFs
To evaluate whether increases in carotid IMT were associated with alterations in global MBFs, the study population was divided according to normal and increased carotid IMT levels (<0.8 and ≥0.8 mm). As can be appreciated in Table 5, no differences in global MBFs at rest and during vasomotor stress between the two groups of normal and increased carotid IMT levels were found. Furthermore, we divided study participants with CAC by tertiles of carotid IMT and related these to global MBFs (Table 6). And also here, no significant differences in global MBFs were observed. The regression analysis between the extent of carotid IMT and global MBFs at rest and during CPT demonstrated a positive correlation (r= 0.48, p≤0.001 and r=0.38, p≤0.013, respectively), while it was not observed for adenosine-stimulated global MBFs (r= −0.08, p=0.63). When we accounted for interindividual differences in hemodynamics by calculating the CVR, however, there was no significant association between carotid IMT and global CVR at rest (r=−0.24, p=0.14) and during CPT (r=−0.l8, p=0.25) or adenosine stimulation (r=0.10, p= 0.54). As regards the relationship between global CAC, global log-CCS did not correlate significantly with global MBFs at rest, during CPT, or adenosine stimulation (r=−0.21, p=0.25; r=−0.23, p=0.21; and r=−0.36, p=0.06, respectively). Also, when global log-CCS was related to global CVR at rest or during vasomotor stress with CPT or adenosine, no significant association was observed (r=0.19, p=0.30; r=0.17, p=0.35; and r=0.14, p=0.44, respectively).
Table 5.
Carotid IMT
| <0.8 mm | ≥0.8 mm | p value | |
|---|---|---|---|
| Global MBF at rest | 0.69±0.15 | 0.77±0.21 | 0.15 |
| Global CVR at rest | 136±29 | 137±33 | 0.92 |
| Global MBF during CPT | 0.83±0.19 | 0.89±0.23 | 0.31 |
| Global CVR during CPT | 135±35 | 137±34 | 0.83 |
| Global MBF during ADO | 1.91±0.42 | 1.84±0.36 | 0.59 |
| Global CVR during ADO | 50±12 | 52±11 | 0.56 |
| Global MFR | 2.86±0.77 | 2.50±0.54 | 0.10 |
ADO adenosine, CPT cold pressor test, MBF myocardial blood flow, CVR coronary vascular resistance, MFR myocardial flow reserve p=NS by t test
Table 6.
Global MBF in study participants with CAC grouped according to tertiles of carotid IMT
| Lowest | Medium | Highest | p value | |
|---|---|---|---|---|
| Global MBF at rest | 0.63±0.10 | 0.74±0.17 | 0.79±0.24 | 0.09 |
| Global CVR at rest | 142±29 | 130±30 | 140±33 | 0.47 |
| Global MBF during | 0.80±0.18 | 0.87±0.20 | 0.79±0.24 | 0.46 |
| CPT | ||||
| Global CVR during | 134±35 | 134±35 | 135±32 | 0.99 |
| CPT | ||||
| Global MBF during | 1.89±0.54 | 1.96±0.37 | 1.75±0.22 | 0.40 |
| ADO | ||||
| Global CVR during | 52±14 | 48±11 | 56±07 | 0.17 |
| ADO | ||||
| Global MFR | 3.02±0.76 | 2.75±0.75 | 2.35±0.46 | 0.08 |
Group comparison by ANOVA
ADO adenosine, CPT cold pressor test, MBF myocardial blood flow, CVR coronary vascular resistance, MFR myocardial flow reserve
Regional MBF subtended to CAC
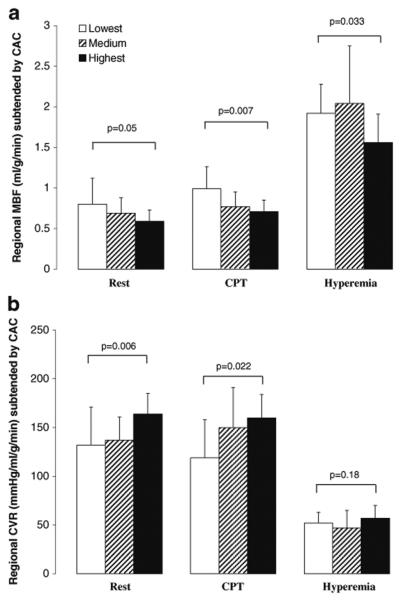
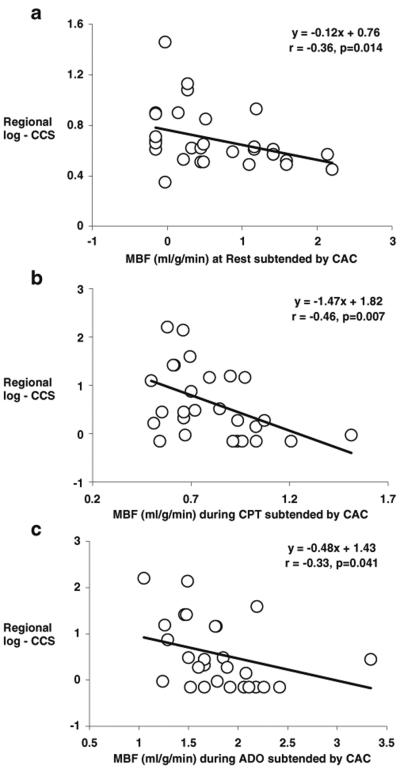
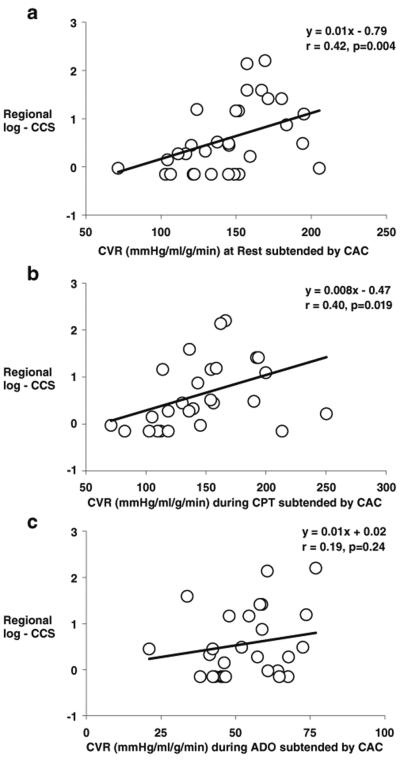
As regards the effects of regional CAC on corresponding MBF, it was evaluated by tertile analysis (Table 7). Increases in regional log-CCS were paralleled by a significant and progressive decrease in corresponding regional MBF at rest and during CPT (Fig. 1a), while it was not observed in the noncalcified coronary territory (Table 7). To account for possible interindividual differences in intracoronary driving pressure, the corresponding regional CVR was also evaluated and related to the corresponding regional log-CCS. And indeed, there was a significant progressive increase in regional CVR at rest and during CPT with increases in the corresponding regional log-CCS (Fig. 1b), substantiating the association between structural alterations of the arterial wall and lower regional MBFs. As regards the adenosine-stimulated MBFs, regional hyperemic MBF was lowest in the highest tertile of regional log-CCS (Fig. 1a). When we compensated for interindividual differences in intracoronary driving pressure with the regional CVR, the group comparison by ANOVA was not significant any more (Fig. 1b). In patients with CAC, there was an inverse correlation between regional log-CCS and corresponding regional MBF at rest and during CPT (r=−0.36, p<0.014 and r=−0.46, p<0.007, respectively) (Fig. 2a, b). This correlation was also maintained when corresponding regional CVR values were evaluated (r=0.42, p<0.004 and r=0.40, p<0.019, respectively) (Fig. 3a, b), indicating that CAD-related vessel stiffness may exert downstream effects on regional MBFs. The extent of regional log-CCS also inversely correlated with the regional hyperemic MBF (r=−0.33, p<0.041) (Fig. 2c). When we evaluated the relation between regional log-CCS and corresponding CVR during adenosine stimulation, the correlation did not reach statistical significance (r= 0.19, p=0.24) (Fig. 3c).
Table 7.
Analysis of regional MBF according to tertile of regional log-CCS
| Tertile | Lowest | Medium | Highest | p value |
|---|---|---|---|---|
| Rest | ||||
| MBF in CAC | 0.80±0.32 | 0.69±0.19 | 0.59±0.14 | 0.052 |
| MBF in NCAC | 0.82±0.27 | 0.73±0.21 | 0.67±0.18 | 0.18 |
| CVR in CAC | 132±39 | 137±24 | 164±21 | 0.006 |
| CVR in NCAC | 125±34 | 132±28 | 149±30 | 0.10 |
| CPT | ||||
| MBF in CAC | 0.99±0.27 | 0.77±0.18 | 0.71 ±0.14 | 0.007 |
| MBF in NCAC | 0.91±0.27 | 0.83±0.19 | 0.72±0.19 | 0.10 |
| CVR in CAC | 119±39 | 150±41 | 160±24 | 0.022 |
| CVR in NCAC | 128±38 | 138±33 | 168±51 | 0.08 |
| Adenosine | ||||
| MBF in CAC | 1.92±0.36 | 2.04±0.71 | 1.56±0.35 | 0.033 |
| MBF in NCAC | 2.07±0.50 | 2.16±0.54 | 1.67±0.40 | 0.031 |
| CVR in CAC | 52±11 | 47±18 | 57±13 | 0.18 |
| CVR in NCAC | 50±13 | 42±15 | 54±14 | 0.16 |
| MFR in CAC | 2.72±0.91 | 3.24±1.80 | 2.65±0.89 | 0.44 |
| MFR in NCAC | 2.79±0.95 | 3.38±1.83 | 2.54±0.68 | 0.22 |
Group comparison by ANOVA
MBF myocardial blood flow, CVR coronary vascular resistance, CCS coronary calcium score, CAC coronary artery calcification, NCAC noncoronary artery calcification
Fig. 1.
Comparison of regional MBF (a) and regional CVR (b) subtended to CAC among individuals with the lowest, medium, and highest tertile of log-CCS. Group comparison by ANOVA
Fig. 2.
Correlation between log-CCS and regional MBF subtended by CAC at rest (a), during CPT (b), and during adenosine stimulation (c)
Fig. 3.
Correlation between log-CCS and regional CVR subtended by CAC at rest (a), during CPT (b), and during adenosine stimulation (c)
Determinants of regional MBF subtended to CAC
In patients with CAC, on univariate analysis, gender, systolic blood pressure, HDL cholesterol, free fatty acids, HbA1c, and regional log-CCS were significantly associated with regional resting MBF and corresponding CVR in myocardial subtended by CAC vessels (Table 8). Multivariate analysis to assess the predictive value of regional log-CCS of the corresponding regional MBF at rest did not demonstrate log-CCS as a statistically significant independent predictor of the corresponding regional resting MBF. Independent predictors of the regional resting MBFs and its CVR in calcified territory were gender, BMI, LDL cholesterol, insulin, and glucose plasma levels. Similarly, CPT-related regional MBFs and corresponding CVR were correlated with HDL cholesterol, insulin, and also regional log-CCS, while no independent predictor was realized in the multivariate analysis (Table 8). The adenosine-stimulated regional MBFs and its CVR in the calcified coronary territory revealed a significant association only with age and plasma glucose levels.
Table 8.
Univariate and multivariate analysis in patients with CAC
| At rest | During CPT | During ADO | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Univariate p | Multivariate SC | p value | Univariate p | Multivariate SC | p value | Univariate p | Multivariate SC | p value | |
| MBF | |||||||||
| IMT | 0.022a | / | / | 0.48 | / | / | 0.15 | / | / |
| CAC | 0.014a | / | / | 0.007a | / | / | 0.041a | / | / |
| Age | 0.043a | / | / | 0.59 | / | / | 0.016a | / | / |
| Sex | ≤0.0001a | 0.47 | 0.046a | 0.019a | / | / | 0.85 | / | / |
| BMI | 0.92 | / | / | 0.46 | / | / | 0.44 | / | / |
| SBP | ≤0.0001a | / | / | 0.026a | / | / | 0.63 | / | / |
| Total cholesterol | 0.17 | / | / | 0.85 | / | / | 0.93 | / | / |
| LDL cholesterol | 0.91 | / | / | 0.38 | / | / | 0.72 | / | / |
| HDL cholesterol | 0.011a | / | / | 0.06 | / | / | 0.26 | / | / |
| Triglycerides | 0.85 | / | / | 0.56 | / | / | 0.09 | / | / |
| Glucose | 0.005a | 0.60 | 0.020a | 0.76 | / | / | 0.13 | / | / |
| Insulin | 0.07 | / | / | 0.035a | / | / | 0.053a | / | / |
| HOMA | 0.40 | / | / | 0.12 | / | / | 0.09 | −0.72 | 0.046a |
| Lactate | 0.38 | / | / | 0.66 | / | / | 0.75 | / | / |
| FFA | 0.002a | / | / | 0.038a | / | / | 0.98 | / | / |
| HbA1c | 0.001a | / | / | 0.24 | / | / | 0.72 | / | / |
| CVR | |||||||||
| IMT | 0.70 | / | / | 0.86 | / | / | 0.27 | / | / |
| CAC | 0.004a | / | / | 0.019a | / | / | 0.24 | / | / |
| Age | 0.35 | / | / | 0.75 | / | / | 0.053a | / | / |
| Sex | ≤0.0001a | −0.82 | ≤0.0001a | 0.043a | / | / | 0.54 | / | / |
| BMI | 0.23 | 0.08 | 0.007a | 0.20 | / | / | 0.55 | / | / |
| SBP | ≤0.037a | / | / | 0.40 | / | / | 0.17 | / | / |
| Total cholesterol | 0.63 | / | / | 0.68 | / | / | 0.78 | / | / |
| LDL cholesterol | 0.91 | −0.24 | ≤0.0001a | 0.63 | / | / | 0.18 | / | / |
| HDL cholesterol | 0.007a | / | / | 0.01a | / | / | 0.28 | / | / |
| Triglycerides | 0.44 | / | / | 0.23 | / | / | 0.020a | / | / |
| Glucose | 0.16 | / | / | 0.053a | / | / | 0.006a | / | / |
| Insulin | 0.003a | 0.50 | ≤0.0001a | 0.052a | / | / | 0.14 | / | / |
| HOMA | 0.10 | / | / | 0.06 | / | / | 0.10 | / | / |
| Lactate | 0.46 | / | / | 0.72 | / | / | 0.75 | / | / |
| FFA | 0.001a | / | / | 0.14 | / | / | 0.43 | / | / |
| HbA1c | 0.028a | / | / | 0.68 | / | / | 0.10 | / | / |
MBF myocardial blood flow, CVR coronary vascular resistance, IMT intima–media thickness, CAC coronary artery calcium score, BMI body mass index, SBP systolic blood pressure, LDL cholesterol low-density lipoprotein cholesterol, HDL cholesterol high-density lipoprotein cholesterol, HOMA homeostatic model assessment, HbA1c hemoglobin A1c, SC standardized coefficient
Significant difference by ANOVA
Discussion
The current study provides several novel findings. In study participants with evidence for CAC but without hemodynamically significant epicardial artery lesions, there was a significant difference in regional resting and hyperemic MBF between territories with and without CAC, which resulted in heterogeneity in MBF of the left ventricle. As it was observed, increases in CAC were significantly associated with relatively lower regional resting and hyperemic MBFs. The increases in regional CAC scores were inversely correlated with lower regional MBFs in the corresponding myocardial territories at rest and during vasomotor stress stimulation, suggesting, at least in part, direct downstream effects of CAC on coronary flows. The current and new observation may agree with a new concept, as first raised by Gould et al. [15], that a heterogeneity in myocardial perfusion may be related to direct downstream consequences of CAD-related vessels stiffness. According to this concept, an increase in CAD-related vessel stiffness is likely to have raised resistance to coronary flow [27, 28], which remained preserved and resulted in a proximal-to-distal decline in intracoronary perfusion pressure [28], associated with a relative decrease in regional MBF. Thus, as demonstrated in the current study for MBF at rest and during vasomotor stress, a vessel stiffness-related decline in intracoronary perfusion may indeed account for the relative decrease in regional MBF, resulting in heterogeneity in left ventricular MBF. While increases in CAC were accompanied by continuous decrease in resting and CPT-related regional MBF, no such continuous decline of regional MBF subtended by CAC was observed during pharmacologically stimulated hyperemic flow increases. The absence of a continuous decline in regional hyperemic MBFs with increases in the extent of CAC may accord with previous investigations in patients with hemodynamically obstructive CAD that described compensatory alterations of the coronary arteriolar vessels to occur in response to progressive CAC [11, 12]. Conceptually, it may be possible that an adaptive vasodilation of the coronary arteriolar resistance vessels may also manifest in patients with hemodynamically nonobstructive or diffuse CAD, aiming to balance the velocity-induced increases in epicardial coronary vascular resistance due to a vessel stiffness-related impairment of flow-mediated vasodilatory function, so that the overall coronary resistance is virtually kept low. Although the latter contention may be intuitively correct, currently, it remains speculative and further comparative evaluations between PET flow studies and invasive angiographic assessment of coronary morphology and function are needed.
Arterial structural alterations and coronary circulatory function
Previous investigations in 414 older adults with subclinical CAD described a weak though statistically significant association between CAC and carotid IMT (r=0.30) [29]. In the current study population with type 2 DM, however, we did not find such an association. Thus, it appears that carotid IMT and CAC identify different aspects and stages of developing atherosclerotic disease in type 2 DM. Other clinical studies have also investigated the relationship between structural and functional alterations of the peripheral arterial wall in early stages of developing atherosclerotic disease [30–32]. In these investigations in individuals with a high coronary risk profile, increases in carotid IMT demonstrated some inverse correlation with flow-mediated vasomotor function of the brachial artery. In contrast, in a cohort of middle-aged men without cardiovascular disease and with relatively few coronary risk factors, no such association was observed [33]. The latter findings [30–32], however, may emphasize that a systemic flow-mediated and, thus, endothelium-dependent vasomotor abnormality may affect, at least in part, the relation between risk factors and early structural alterations of the arterial wall. In the current study in type 2 DM, we did not find an association between carotid IMT and coronary circulatory function in response to vasomotor stress. The reasons for the discordant observation between the current and previous investigations [30–32] remain unclear but are likely to be related to differences in patients characteristics, differences in sample size studied, and/or different factors accounting for vasomotor (dys)function in the brachial artery and coronary circulation. It is also possible that the current study population was not large enough or, conversely, that the range of IMTs was not wide enough to bring out a statistically significant association. Moreover, as shown in the current study and by others [2, 34, 35], functional abnormalities of the coronary artery may precede or accompany CAD-related structural alterations. Thus, because coronary vasomotor function may deteriorate before the occurrence of any structural alteration, it may also have added to the dissociation between function and structure of the arterial wall.
The results of the current investigation, however, suggest that heterogeneity in left ventricular MBF at rest and during vasomotor stress may result, at least in part, from fluid dynamic effects of CAD-related structural alterations of the arterial wall. As EBT-determined CAC [9, 36] can be seen as a noninvasive surrogate of CAD-related vessel stiffness, which is commonly associated with an impairment of flow-mediated vasodilation [27, 37], it is intriguing to hypothesize that coronary vessel stiffness contributed to the extent of coronary vascular dysfunction in DM [15]. As seen on univariate analysis, CAC, apart from effects of diabetes-related coronary risk factors on coronary vasomotor function, was indeed significantly associated with relatively lower regional MBFs at rest as well as during vasomotor stress. The additionally performed multivariate analysis, however, did not reveal CAC as an independent predictor of relative reductions in corresponding regional MBFs. Thus, it appears that CAD-related coronary vessel stiffness, as denoted by the presence of CAC, adds to the relative reductions in regional MBF in concert with diabetes-related coronary vasomotor dysfunction. Noteworthy, according to the Hagen–Poiseuille law, the intracoronary resistance relates not only to the length of the coronary artery and inversely to the vessel diameter, but also to the velocity of the blood flow [11]. According to the latter, higher increases in global hyperemic MBFs should also lead to relatively higher increases in intracoronary resistance in the calcified and stiff artery than in the noncalcified coronary artery where a flow-mediated vasodilation may still exist and counterbalances an increase coronary vascular resistance during flow increases. If this holds true, then differences in regional hyperemic MBF between calcified and noncalcified coronary vessels should be more distinct with higher increases in hyperemic MBFs. And indeed, as observed in the current study, the MBF heterogeneity was evident in those individuals with CAC in controls and NDM during relatively high global hyperemic MBF increases, while the MBF heterogeneity was not observed in HDM with markedly reduced global hyperemic flows reflecting a severe degree of diabetes-related vasomotor dysfunction.
Recent investigations [18], also reported a heterogeneity in resting myocardial perfusion of the left ventricle as a reflection of a heterogeneous pattern of impaired flow-mediated vasodilation, while this was not appreciated during hyperemic flow increases during pharmacologic vasodilation. The latter finding may contrast our observation where MBF heterogeneity was not only observed at resting condition but also during higher coronary flows owing to adenosine stimulation. The reason for these discordant findings remains to be investigated and may be related to differences in patients’ characteristics and/or to differences in the extent of CAD-related structural alterations of the arterial wall.
Conclusions
The absence of any correlation between carotid IMT and coronary circulatory function in type 2 DM suggests different features and stages of early atherosclerosis in the peripheral and coronary circulation. PET-measured MBF heterogeneity at rest and during vasomotor stress may reflect downstream fluid dynamic effects of CAD-related early structural alterations of the arterial wall. The assessment of heterogeneity in MBF of the left ventricle could be a promising noninvasive index to identify early structural stages of the CAD process.
Acknowledgments
This work was supported by Research Grant HL 33177, National Heart, Lung and Blood Institute, Bethesda, MD, USA.
Abbreviations
- ADO
adenosine
- IMT
intima–media thickness
- CAC
coronary artery calcification
- CCS
coronary artery calcium score
- CPT
cold pressor test
- CVR
coronary vascular resistance
- EBT
electron beam tomography
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- MBF
myocardial blood flow
- RPP
rate–pressure product
- PET
positron emission tomography
Footnotes
Conflict of interest None.
Contributor Information
Thomas H. Schindler, Department of Molecular and Medical Pharmacology, Radiological Science, David Geffen School of Medicine, University of California at Los Angeles, 10833 Le Conte Avenue, 23-120 CHS, P.O. Box 173517, Los Angeles, CA 90095-1735, USA Department of Internal Medicine, Cardiovascular Center, Nuclear Cardiology, University Hospital of Geneva, Geneva, Switzerland.
Alvaro D. Facta, Department of Molecular and Medical Pharmacology, Radiological Science, David Geffen School of Medicine, University of California at Los Angeles, 10833 Le Conte Avenue, 23-120 CHS, P.O. Box 173517, Los Angeles, CA 90095-1735, USA
John O. Prior, Department of Molecular and Medical Pharmacology, Radiological Science, David Geffen School of Medicine, University of California at Los Angeles, 10833 Le Conte Avenue, 23-120 CHS, P.O. Box 173517, Los Angeles, CA 90095-1735, USA
Jerson Cadenas, Department of Molecular and Medical Pharmacology, Radiological Science, David Geffen School of Medicine, University of California at Los Angeles, 10833 Le Conte Avenue, 23-120 CHS, P.O. Box 173517, Los Angeles, CA 90095-1735, USA.
Xiao-Li Zhang, Department of Molecular and Medical Pharmacology, Radiological Science, David Geffen School of Medicine, University of California at Los Angeles, 10833 Le Conte Avenue, 23-120 CHS, P.O. Box 173517, Los Angeles, CA 90095-1735, USA.
Yanjie Li, Atherosclerosis Research Unit, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
James Sayre, Department of Molecular and Medical Pharmacology, Radiological Science, David Geffen School of Medicine, University of California at Los Angeles, 10833 Le Conte Avenue, 23-120 CHS, P.O. Box 173517, Los Angeles, CA 90095-1735, USA.
Jonathan Goldin, Department of Molecular and Medical Pharmacology, Radiological Science, David Geffen School of Medicine, University of California at Los Angeles, 10833 Le Conte Avenue, 23-120 CHS, P.O. Box 173517, Los Angeles, CA 90095-1735, USA.
Heinrich R. Schelbert, Email: hschelbert@mednet.ucla.edu, Department of Molecular and Medical Pharmacology, Radiological Science, David Geffen School of Medicine, University of California at Los Angeles, 10833 Le Conte Avenue, 23-120 CHS, P.O. Box 173517, Los Angeles, CA 90095-1735, USA
References
- 1.Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115:1464–80. doi: 10.1161/CIRCULATIONAHA.106.629808. [DOI] [PubMed] [Google Scholar]
- 2.Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833–43. doi: 10.1016/0735-1097(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 3.Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188–95. doi: 10.1016/j.jacc.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 4.Di Carli MF, Dorbala S, Hachamovitch R. Integrated cardiac PET-CT for the diagnosis and management of CAD. J Nucl Cardiol. 2006;13:139–44. doi: 10.1007/BF02971234. [DOI] [PubMed] [Google Scholar]
- 5.Schindler TH, Zhang XL, Vincenti G, Mhiri L, Lerch R, Schelbert HR. Role of PET in the evaluation and understanding of coronary physiology. J Nucl Cardiol. 2007;14:589–603. doi: 10.1016/j.nuclcard.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prior JO, Schindler TH, Facta AD, Hernandez-Pampaloni M, Campisi R, Dahlbom M, et al. Determinants of myocardial blood flow response to cold pressor testing and pharmacologic vasodilation in healthy humans. Eur J Nucl Med Mol Imaging. 2007;34:20–7. doi: 10.1007/s00259-006-0193-4. [DOI] [PubMed] [Google Scholar]
- 7.Graner M, Varpula M, Kahri J, Salonen RM, Nyyssönen K, Nieminen MS, et al. Association of carotid intima–media thickness with angiographic severity and extent of coronary artery disease. Am J Cardiol. 2006;97:624–9. doi: 10.1016/j.amjcard.2005.09.098. [DOI] [PubMed] [Google Scholar]
- 8.Ogata T, Yasaka M, Yamagishi M, Seguchi O, Nagatsuka K, Minematsu K. Atherosclerosis found on carotid ultrasonography is associated with atherosclerosis on coronary intravascular ultrasonography. J Ultrasound Med. 2005;24:469–74. doi: 10.7863/jum.2005.24.4.469. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann U, Ferencik M, Cury RC, Pena AJ. Coronary CT angiography. J Nucl Med. 2006;47:797–806. [PubMed] [Google Scholar]
- 10.Hoffmann U, Nagurney JT, Moselewski F, Pena A, Ferencik M, Chae CU, et al. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation. 2006;114:2251–60. doi: 10.1161/CIRCULATIONAHA.106.634808. [DOI] [PubMed] [Google Scholar]
- 11.Gould KL, Lipscomb K, Calvert C. Compensatory changes of the distal coronary vascular bed during progressive coronary constriction. Circulation. 1975;51:1085–94. doi: 10.1161/01.cir.51.6.1085. [DOI] [PubMed] [Google Scholar]
- 12.Di Carli M, Czernin J, Hoh CK, Gerbaudo VH, Brunken RC, Huang SC, et al. Relation among stenosis severity, myocardial blood flow, and flow reserve in patients with coronary artery disease. Circulation. 1995;91:1944–51. doi: 10.1161/01.cir.91.7.1944. [DOI] [PubMed] [Google Scholar]
- 13.Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330:1782–8. doi: 10.1056/NEJM199406233302503. [DOI] [PubMed] [Google Scholar]
- 14.Beanlands RS, Muzik O, Melon P, Sutor R, Sawada S, Muller D, et al. Noninvasive quantification of regional myocardial flow reserve in patients with coronary atherosclerosis using nitrogen-13 ammonia positron emission tomography. Determination of extent of altered vascular reactivity. J Am Coll Cardiol. 1995;26:1465–75. doi: 10.1016/0735-1097(95)00359-2. [DOI] [PubMed] [Google Scholar]
- 15.Gould KL, Nakagawa Y, Nakagawa K, Sdringola S, Hess MJ, Haynie M, et al. Frequency and clinical implications of fluid dynamically significant diffuse coronary artery disease manifest as graded, longitudinal, base-to-apex myocardial perfusion abnormalities by noninvasive positron emission tomography. Circulation. 2000;101:1931–9. doi: 10.1161/01.cir.101.16.1931. [DOI] [PubMed] [Google Scholar]
- 16.Gould KL. Assessing progression or regression of CAD: the role of perfusion imaging. J Nucl Cardiol. 2005;12:625–38. doi: 10.1016/j.nuclcard.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Graf S, Khorsand A, Gwechenberger M, Novotny C, Kletter K, Sochor H, et al. Typical chest pain and normal coronary angiogram: cardiac risk factor analysis versus PET for detection of microvascular disease. J Nucl Med. 2007;48:175–81. [PubMed] [Google Scholar]
- 18.Johnson NP, Gould KL. Clinical evaluation of a new concept: resting myocardial perfusion heterogeneity quantified by markovian analysis of PET identifies coronary microvascular dysfunction and early atherosclerosis in 1,034 subjects. J Nucl Med. 2005;46:1427–37. [PubMed] [Google Scholar]
- 19.Hernandez-Pampaloni M, Keng FY, Kudo T, Sayre JS, Schelbert HR. Abnormal longitudinal, base-to-apex myocardial perfusion gradient by quantitative blood flow measurements in patients with coronary risk factors. Circulation. 2001;104:527–32. doi: 10.1161/hc3001.093503. [DOI] [PubMed] [Google Scholar]
- 20.Schindler TH, Facta AD, Prior JO, Campisi R, Inubushi M, Kreissl MC, et al. PET-measured heterogeneity in longitudinal myocardial blood flow in response to sympathetic and pharmacologic stress as a non-invasive probe of epicardial vasomotor dysfunction. Eur J Nucl Med Mol Imaging. 2006;33:1140–9. doi: 10.1007/s00259-006-0069-7. [DOI] [PubMed] [Google Scholar]
- 21.Schindler TH, Zhang XL, Vincenti G, Mhiri L, Nkoulou R, Just H, et al. Diagnostic value of PET-measured heterogeneity in myocardial blood flows during cold pressor testing for the identification of coronary vasomotor dysfunction. J Nucl Cardiol. 2007;14:688–97. doi: 10.1016/j.nuclcard.2007.06.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvisalo MJ, Jartti L, Nanto-Salonen K, Irjala K, Roennemaa T, Hartiala JJ, et al. Increased aortic intima–media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104:2943–7. doi: 10.1161/hc4901.100522. [DOI] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 24.Goldin JG, Yoon HC, Greaser LE, 3rd, Heinze SB, McNitt-Gray MM, Brown MS, et al. Spiral versus electron-beam CT for coronary artery calcium scoring. Radiology. 2001;221:213–21. doi: 10.1148/radiol.2211001038. [DOI] [PubMed] [Google Scholar]
- 25.Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243–52. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 26.Hodis HN, Mack WJ, Zheng L, Li Y, Torres M, Sevilla D, et al. Effect of peroxisome proliferator-activated receptor gamma agonist treatment on subclinical atherosclerosis in patients with insulin-requiring type 2 diabetes. Diabetes Care. 2006;29:1545–53. doi: 10.2337/dc05-2462. [DOI] [PubMed] [Google Scholar]
- 27.Zeiher AM, Drexler H. Coronary hemodynamic determinants of epicardial artery vasomotor responses during sympathetic stimulation in humans. Basic Res Cardiol. 1991;86(Suppl 2):203–13. doi: 10.1007/978-3-642-72461-9_20. [DOI] [PubMed] [Google Scholar]
- 28.De Bruyne B, Hersbach F, Pijls NH, Bartunek J, Bech JW, Heyndrickx GR, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “normal” coronary angiography. Circulation. 2001;104:2401–6. doi: 10.1161/hc4501.099316. [DOI] [PubMed] [Google Scholar]
- 29.Newman AB, Naydeck BL, Sutton-Tyrrell K, Edmundowicz D, O’Leary D, Kronmal R, et al. Relationship between coronary artery calcification and other measures of subclinical cardiovascular disease in older adults. Arterioscler Thromb Vasc Biol. 2002;22:1674–9. doi: 10.1161/01.atv.0000033540.89672.24. [DOI] [PubMed] [Google Scholar]
- 30.Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Roennemaa T, et al. Interrelations between brachial endothelial function and carotid intima–media thickness in young adults: the cardiovascular risk in young Finns study. Circulation. 2004;110:2918–23. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 31.Jarvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, et al. Endothelial dysfunction and increased arterial intima–media thickness in children with type 1 diabetes. Circulation. 2004;109:1750–5. doi: 10.1161/01.CIR.0000124725.46165.2C. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto M, Eto M, Akishita M, Kozaki K, Ako J, Iijima K, et al. Correlation between flow-mediated vasodilatation of the brachial artery and intima–media thickness in the carotid artery in men. Arterioscler Thromb Vasc Biol. 1999;19:2795–800. doi: 10.1161/01.atv.19.11.2795. [DOI] [PubMed] [Google Scholar]
- 33.Yan RT, Anderson TJ, Charbonneau F, Title L, Verma S, Lonn E. Relationship between carotid artery intima–media thickness and brachial artery flow-mediated dilation in middle-aged healthy men. J Am Coll Cardiol. 2005;45:1980–6. doi: 10.1016/j.jacc.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 34.Schachinger V, Zeiher AM. Quantitative assessment of coronary vasoreactivity in humans in vivo. Importance of baseline vasomotor tone in atherosclerosis. Circulation. 1995;92:2087–94. doi: 10.1161/01.cir.92.8.2087. [DOI] [PubMed] [Google Scholar]
- 35.Zeiher AM, Drexler H, Wollschlaeger H, Saurbier B, Just H. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol. 1989;14:1181–90. doi: 10.1016/0735-1097(89)90414-2. [DOI] [PubMed] [Google Scholar]
- 36.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 37.Zeiher AM, Drexler H, Wollschlager H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]