Abstract
Over the past three decades, peptide nucleic acids have been employed in numerous chemical and biological applications. Peptide nucleic acids possess enormous potential because of their superior biophysical properties, compared to other oligonucleotide chemistries. However, for therapeutic applications, intracellular delivery of peptide nucleic acids remains a challenge. In this review, we summarize the progress that has been made in delivering peptide nucleic acids to intracellular targets. In addition, we emphasize recent nanoparticle-based strategies for efficient delivery of conventional and chemically-modified peptides nucleic acids. Keywords: nanoparticles; peptide nucleic acids; gene editing; antisense therapy
Keywords: nanoparticles, peptide nucleic acids, gene editing, antisense therapy
Graphical Abstract
This review highlight the novel nanoparticle based delivery strategies employed for delivery of regular as well as chemically modified PNAs both ex vivo as well as in vivo
1. Introduction
Nucleic acid structures play an important role in directing transcriptional as well as post-transcriptional events during protein synthesis and their subsequent effects on gene expression. Point mutations in DNA and RNA sequences lead to myriads of diseases, such as various forms of cancer and rare genetic disorders. Hence, small molecule based drugs targeting nucleic acids have attained significant attention as anticancer therapeutics [1–4]. Though promising results have been obtained, off target toxicities produced by small molecules often limit their clinical use. An alternate approach is to target diseases at the genetic level, using the specificity of complementary binding by nucleic acids to minimize off-target effects. Gene therapy approaches rely heavily on targeted reagents, which can recognize DNA and RNA sequences selectively.
Bio-organic reagents have been developed to mimic the chemical features of DNA and RNA but exhibit improved properties. Examples include phosphorothioates [5, 6], morpholinos [7, 8], and locked nucleic acids (LNA) [9, 10]. Rigorous chemical structure optimization has been done on these nucleic acids analogues to provide improved features such as reduced off-target binding, resistance to enzymatic degradation, and improved aqueous solubility. One promising class of reagents is peptide nucleic acids (PNAs) [11, 12]. PNAs, which were introduced by Nielsen and coworkers in 1991, are synthetic DNA analogues in which the phosphodiester backbone is replaced with unchanged 2-N-aminoethylglycine units (Fig 1). Importantly, PNAs are resistant to enzymatic degradation by nucleases and proteases [13]. This feature makes PNAs attractive candidates for numerous therapeutic and biomedical based applications [14]. Additionally, unlike other reagents, the neutral PNA backbone confers strong hybridization properties with its complementary DNA/RNA targets in a sequence specific manner [15, 16]. Over the last two decades, PNAs have been extensively studied as antisense (targeting mRNA or microRNAs) as well as antigene agents (targeting genomic DNA) to control the gene expression and regulation [17–19].
Fig. 1.
Chemical structures of DNA (RNA) and PNA
Intracellular delivery of PNAs is a challenge [20]. Therefore, a number of strategies have been investigated for enhancing PNA delivery into cells [21–23]. These strategies include electroporation, transfection-based methods, inclusion of cationic peptides, use of chemically modified PNAs, and entrapment of PNA in polymer nanoparticles [24, 25]. Though all of these strategies have yielded some interesting results, issues such as selectivity, endosomal entrapment, and unwieldy synthetic procedures limit their broader applications. Here, we review current PNA delivery strategies and their limitations, focusing on the use of nanoparticle-based delivery strategies. Table 1 summarizes recent reports on nanoparticle-based approaches for PNA delivery.
Table 1.
Nanoparticles for delivery of PNA
| Nanoparticles | Applications | Study design | Ref |
|---|---|---|---|
| PLGA | Gene editing | Cell culture (CD34+ and THP1) Preclinical (in mice) |
[26–28] |
| Antisense | Cell culture (THP1 and HeLa) | [29] | |
| Peptide coated PLGA | Antisense | Preclinical (in mice) | [30] |
| PBAE/PLGA | Gene editing | Preclinical (in mice) | [27] |
| MPG coated PBAE/PLGA | Gene editing | Preclinical (in mice) | [31] |
| Avidin-labeled nanoparticles | Delivery vehicle | Preliminary characterization | [32] |
| Zeolites-L-Nanocrystals | Delivery vehicle | Cell culture (HeLa) | [33] |
| Mesoporous silica nanoparticles |
Antisense | Cell culture (HeLa) | [34] |
| Cationic shell cross-linked knedel-like nanoparticles |
Antisense | Preclinical (in mice) | [35, 36] |
| Splice correction | Cell culture (HeLa) | [37] | |
| Imaging mRNAs | Cell culture (RAW 264.7) | [38, 39] | |
| Gold nanoparticles | RNA detection | Cell culture (Vero cells) | [40] |
| Cobalt ferrite core/metallic shell |
PNA/DNA based biosensors |
Solution based in vitro assay | [41] |
2. Peptide Nucleic Acids and their delivery methods
PNA-based agents have shown promise in vitro due to their favorable biophysical properties, but broader applications of PNAs have been more difficult to achieve due to their limited cellular uptake. To increase uptake, various methods have been tested for increasing PNA transport across the cell membrane (Fig. 2).
Fig. 2.
Different delivery methods of PNA
Hanvey et al. first demonstrated the antisense and antigene properties of PNAs by microinjecting the PNAs into fibroblast cells [19]. PNAs were delivered inside cells via electroporation for modulating alternative splicing [42] and for induction of human gamma globin gene [43]. Corey et al. found that co-transfection of PNA/DNA complexes can lead to significant uptake in cells [44–47]. Direct permeabilization of cells using streptolycin-O enhances permeability to PNAs, which leads to PNA based mutations in supFG1 reporter gene [48]. These direct delivery methods have been useful in showing the biological activity of PNAs when introduced into via mechanical or electrical transduction (microinjection, electroporation and transduction), but they appear to be restricted to small-scale experiments, and likely are not translatable into clinical applications.
Another promising strategy for PNA delivery involves conjugation to cell penetrating peptides (Table 2). However, PNAs delivered via conjugated peptides appear to be largely entrapped in endocytic vesicles, where they are degraded or recycled back to the cell surface: escape into the cytoplasm and transport to the nucleus appears limited [49]. On the other hand, one particular approach, using a peptide called pHLIP (pH low insertion peptide) [50, 51], appears to be particularly promising for selective delivery to cancer cells. pHLIP undergoes uptake only under acidic conditions through a pH-dependent cellular translocation mechanism. This technology is promising, but is limited to delivery to cells in acidic microenvironments [18].
Table 2.
Peptides used for delivery of PNA
| Name of the peptide | Sequence of the peptide | Ref |
|---|---|---|
| Penetratin | RQIKIWFQNRRMKWKK | [54, 55] |
| Transportan | GWTLNSAGYLLGKINLAALAKKIL | [56] |
| Retro-inverso penetratin | (D)-KKWKMRRNQFWIKIQR-(D) | [57] |
| Phenyl leucine | FLFLFL | [23] |
| Nuclear localization peptide | PKKKRKV | [57] |
| Cell wall/Membrane active Peptide |
KFFKFFKFFK | [60] |
| Tat peptide | GRKKRRQRRRPPQ | [58] |
| Polylysine | KKKK | [23] |
| pH low insertion peptide (pHLIP) |
AAEQNPIYWARYADWLFTTPLLLL DLALLVDADEGTCG |
[18] |
Further, PNAs have also been conjugated with lipophilic moieties such as adamantyl acetic acid to facilitate their cellular uptake [52]. In addition to adamantyl acetic acid, triphenyl phosphinum based cations have also been used for PNA delivery in the human fibroblast cells [53].
In a different strategy, chemical modifications are introduced in the PNA backbone to impart positive charge characteristics, which facilitates cellular uptake properties [59–61]. These positively-charged groups include guanidinium, which can be placed at the gamma or alpha position on the PNA backbone (Fig. 3).
Fig. 3.
Chemical structures of αGPNA and γGPNA
Studies have shown that PNA-peptide conjugates are more toxic then GPNAs. This is likely due to the amphipathic nature of the PNA peptide conjugates, which result in disruption or permeabilization of cell membranes. Guanidinium containing PNAs are less toxic because they are less amphipathic than PNA peptide conjugates [60].
These strategies have shown promise in intracellular uptake of PNA and in targeting beta-catenin [62] as well as E-cadherin genes [60] which play an important role in WnT pathway. However, PNA backbone modification requires complex chemical synthetic procedures, and will require further optimization for more widespread use.
3. PLGA based nanoparticles for PNA delivery
Poly (lactic co-glycolic acids) (PLGA) is a commonly used biodegradable polymer for drug delivery systems and medical devices. It has been approved by both the US Food and Drug administration (USFDA) and European medical agency [63] in a variety of clinical applications [64]. An appealing feature of PLGA is that it degrades by hydrolysis into endogenous non-toxic metabolites (lactic acid and glycolic acid), which enhances its biocompatibility for in vivo delivery [65]. Microparticles of PLGA have been used clinically for prostate cancer treatment in the form of Lupron ® and Trelstar ®. Another advantage of PLGA is that the properties of the material can be adjusted, to some extent, by variation of the copolymerization ratio. For example, PLGA 80:20 identifies a copolymer consisting of 80% lactic acid and 20% glycolic acid, which exhibits different physical properties and degradation rates, than homopolymers of poly(lactic acid) (PLA) or poly(glycolic acid) (PGA). Interestingly, PLGA nanoparticles have been shown to facilitate uptake of various macromolecules ranging from proteins, lipopeptides, peptides, viruses, cell lysates and the plasmid DNA into the cytoplasm [64]. PLGA nanoparticles can enhance the activity of vaccines, by providing controlled and targeted delivery of antigens [66]. In addition to delivering purified antigen, PLGA nanoparticles containing tumor lysates have been tested as anticancer vaccines based on tumor-associated antigens (TAAs) [67]. Numerous reports suggest that PLGA nanoparticles can be used to encapsulate antibiotics for more effective antibacterial applications [68].
PLGA-nanoparticles appear to permeate the cell membrane by a combination of fluid phase pinocytosis and clathrin-mediated endocytosis. Cells of the reticuloendothelial system (RES) can phagocytose PLGA nanoparticles and eliminate them from the blood stream, predominantly by uptake in the liver. To prevent this unwanted uptake, PLGA nanoparticles have been decorated with chemical functionalities such as PEG and certain peptides, to avoid elimination by the RES and prolong the half-life for nanoparticle circulation in the blood [69, 70]. In this regard, another important characteristic of PLGA nanoparticles is their surface charge density, which can be manipulated by chemical conjugation or fabrication in the presence of surfactants. It was well established that PLGA nanoparticles that possess positive charge undergo more cellular uptake, as well as more efficient endosomal release [71–73]. Recent work has explored the utility of PLGA nanoparticles to deliver PNAs for their antisense and antigene applications [29, 31].
3a. Targeting microRNAs
MicroRNAs are members of a class of small non-coding RNAs (22–23nt), which originate from the precursor pre-microRNA, and are known to regulate the translation and stability of mRNA during post-transcriptional events in the cytoplasm. miRNA-profiling experiments have revealed that miRNAs are widely over-expressed in several types of cancers. Hence, development of novel therapeutic molecules to inhibit miRs (miR therapeutics) is an intriguing approach to cancer therapy [74, 75].
Engineered antisense molecules, such as locked nucleic acids (LNA) and phosphorothioates, have been developed to target specific miRs, but they require extensive optimization and/or elaborate screening in terms of cellular delivery, off target toxicity, tumor selectivity and enzymatic resistance properties. As an alternate, PNAs have been shown to target miRs and exhibit antisense properties. The chemical properties of PNA make it an attractive agent for targeting miRs, but delivery of PNAs across the cell membrane is a challenge. In early work, Fabani, Gait and co-workers demonstrated that PNAs exhibit inhibitory activity against miR-122 in human and rat liver cells when delivered by electroporation, or when modified by appending cell-penetrating peptide (R6-Penetratin), or by adding four lysines to the PNA oligomer [76]. Other studies found that polyarginine–PNAs can be employed to target miR-210, which is associated with hypoxia and erythroid differentiation. Gait and co-workers have shown that lysine-derivatized PNA and cysteine-containing PNA can target miR-122, which is involved in lipid metabolism and hepatitis C virus replication [77].
We have shown that PNA-based anti-miRs encapsulated in PLGA nanoparticles can inhibit miR properties [30, 78]. In these studies, nanoparticles of PLGA, composed of a 50:50 monomer ratio with terminal ester groups, were loaded with anti-miR-155 PNAs using a double emulsion solvent evaporation method [30, 79]. In addition to enzymatic resistance, we discovered that the neutral PNA backbone also facilitates loading of anti-miR PNAs into PLGA nanoparticles, when compared to oligonucleotides with a charged phosphodiester backbone. In order to enhance accumulation of anti-miR-155 in target cells, CPP was also attached to the surface of nanoparticles. Loading was significantly higher for PNA (410 ± 55 pmoles of PNA/mg of nanoparticles) than single-stranded DNA (ssDNA, 25 ± 5 pmoles of DNA/mg of nanoparticles) [30]. It has been noticed that coating of nanoparticles with CPP adds a cationic component to the surface of the nanoparticles; however, the charged surface ligand did not appear to impact loading of nucleic acids. A dual luciferase reporter assay was employed to measure the miR-155 inhibition. In this assay, a target binding sequence for miR-155 was inserted into the 3′UTR of renilla luciferase and firefly luciferase was used for normalization. Cells treated with CPP-coated PLGA nanoparticles containing antimiR-155 PNA decreased miR-155 activity to ~60% of control values, whereas nanoparticles with no surface modification only reduced miR155 activity to ~80% of control.
The in vivo effect of nanoparticle delivered antimiR-155 PNAs was tested in an oncomir-addicted murine model NesCre8; mir-155LSLtTA [30]. miR-155 expression in this mouse leads to disseminated lymphoma characterized by a clonal, transplantable pre-B-cell population of neoplastic lymphocytes. Withdrawal of miR-155 results in rapid regression of lymphadenopathy, which shows that these tumors are dependent on miR-155 expression. Injection of PLGA nanoparticles loaded with antimiR-155 PNAs—either into the tumor or intravenously—provided significant delays in tumor growth. To facilitate nanoparticle uptake in tumor cells, the nanoparticles were surface modified with the peptide penetratin, a cell-penetrating peptide. The effectiveness of the nanoparticle carrier is further supported by histopathological analysis (Fig. 4). Enhanced permeability and retention (EPR) is an important feature of formulations such as nanoparticles, allowing preferential accumulation in tumor tissues as compared to normal tissues because of lack of normal architecture of tumor blood vessels. Presumably due to the EPR effect, penetratin-coated nanoparticles showed preferential targeting to tumor tissue as compared to other organs i.e lungs, liver, spleen, stomach, brain and heart. The presence of penetratin on their surface facilitated the uptake of nanoparticles and intracellular delivery of the antimiR PNA. We note that dependence on the EPR effect may limit the generalizability of these results, and have reviewed this issue in a recent commentary [80].
Fig. 4.
(A) Change in tumor volume after single local injection of ANTP coated nanoparticles delivering antimiR-155 PNA molecules. (B) H & E analysis of locally treated subcutaneous lymphoid tumors. Scale bar represents 100uM for both low and high magnification images [30]
3b. Targeting mRNAs
Improved generations of PNAs have been developed where different functional groups (guanidinium, alanine, etc) are inserted at the gamma position in certain monomers, providing enhanced properties. PLGA-based nanoparticles can be used to deliver chemically-modified gamma PNAs (γPNAs) possessing antisense properties. γPNAs are modified PNAs where a chiral center is introduced at gamma position (Fig. 5) [81]. The presence of chirality at the gamma position provides right-handed helical characteristics to the PNAs. Biophysical and crystallographic studies have been shown that γPNAs possess high binding affinity with complementary DNA and RNA strands because of their helicity [82, 83]. Incorporation of a single gamma monomer stabilized a PNA-DNA and PNA-RNA duplex by 3–5oC. [82] The extent of stabilization gradually increased with additional gamma monomers. In addition to conferring backbone preorganization, making it possible for PNAs to invade mixed-sequence B-DNA at physiological temperature, introduction of a chiral mini-PEG substituent at the γ-position significantly improves the water solubility and specificity of the system [84, 85].
Fig. 5.
New generation gamma PNAs
PLGA-based nanoparticle delivery systems were used to deliver antisense molecules based on γPNAs targeting CCR5 mRNA [29]. CCR5 is a membrane protein receptor, which is required by R5-tropic HIV-1 virus to gain entry into CD4+ T-cells [86]. When the CCR5 receptor is unavailable, HIV virus cannot engage with CD4+ T-cells to infect the cell. Using in vitro assays, it was demonstrated that PLGA nanoparticles loaded with γPNAs exhibit an antisense effect that reduces CCR5 expression. PLGA nanoparticles containing γPNAs possess uniform size distribution as evident from scanning electron microscopy images. In studies to date, PLGA nanoparticles were shown capable of delivering different γPNAs including miniPEG as well as guanidinium containing γPNAs. Using RT-PCR and western blot analysis, it was shown that antisense yPNA, delivered into cells by PLGA nanoparticles, decrease CCR5 mRNA levels by about 60%.
3c. Targeting genomic DNA for gene editing
Hematopoietic stem cells are capable of self-renewal and differentiation into diverse components of blood throughout an individual’s lifetime [87–89]. Genetic manipulation of hematopoietic stem and progenitor cells (HSPCs) could provide curative treatments for single-gene disorders of the blood such as beta (β) thalassemia. If successful, gene therapy could provide a cure for genetic diseases such as sickle cell anemia and thalassemia by generating blood stem cells carrying expression constructs for wild-type adult β-globin gene. However, gene modification of HSPCs remains a challenge due to their quiescent nature and relative resistance to both viral and non-viral methods to introduce foreign genetic material [90, 91]. In addition, nuclease-based technologies such as CRISPR/Cas9, ZFNs, and TALENs—which offer alternate methods for gene editing—present many challenges, particularly the risk of making unwanted changes in other parts of the genome (off-target effects) [92–94], which are not commonly found using PNAs.
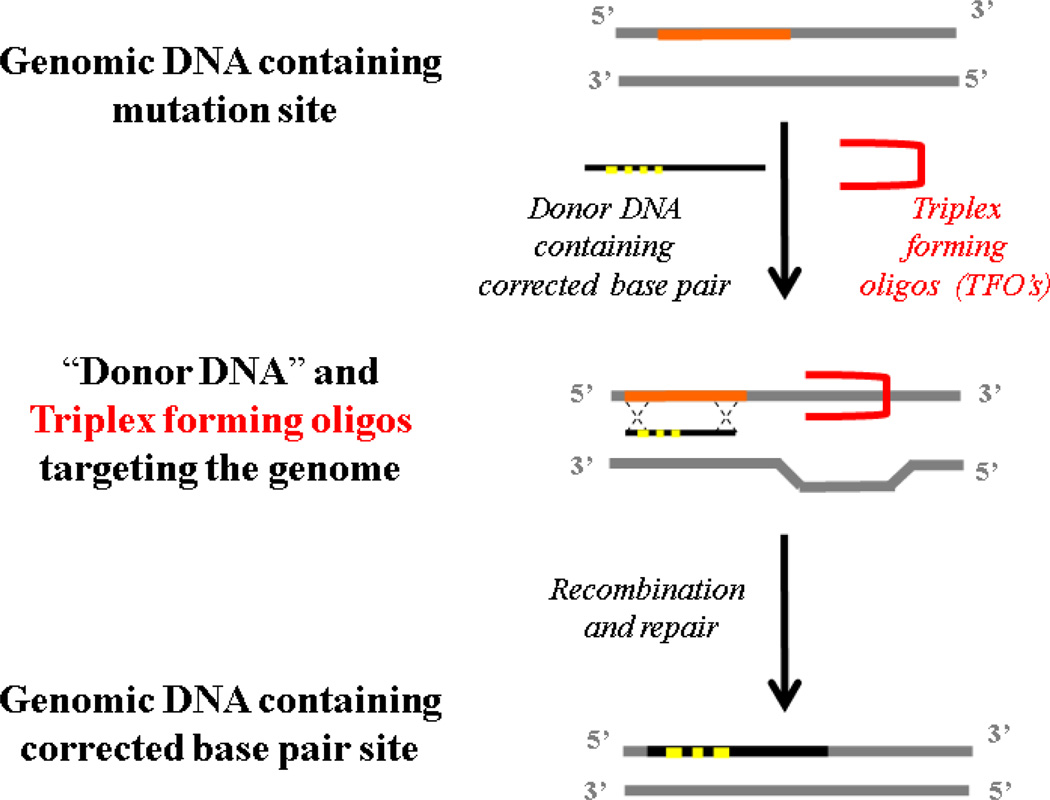
Triplex-forming PNAs (TFPs) have been used to stimulate the recombination of short 50–60 bp ―donor DNA‖ fragments into genomic DNA to site specifically correct a thalassemia-causing splice site mutation at the chromosomal DNA level [95–98]. TFPs can form PNA/DNA/PNA triplexes with displacement of one strand of the duplex DNA; by binding, the TFPs produce structures that are highly provocative of DNA repair via a mechanism initiated by the nucleotide excision repair (NER) pathway (Fig. 6) [99, 100].
Fig. 6.
Gene editing strategy using triplex forming oligonucleotides.
Most early PNAs for gene editing were designed based on using dimeric PNAs or ―bis-PNAs‖, in which the two PNA strands (one strand for Watson-Crick pairing and the other strand for Hoogsteen pairing) are joined by a linker [101, 102]. Further, another promising design has employed tail-clamp PNAs (tcPNAs) [103–105]. In tcPNAs, the Watson-Crick binding segment in a bis-PNA is extended so that it can bind beyond the homopurine run in order to bind a longer target site and thereby enhance the binding specificity. This creates an even larger helical distortion by increasing the length of the strand invasion and P-loop complex. In binding studies, tcPNAs show greater affinity and specificity compared to bis-PNAs [26, 98, 106]. Importantly, high-affinity binding to DNA by tcPNAs does not require a long homopurine run; in fact, homopurine stretches as short as 5 bp are sufficient [86]. This technology is known as ―minimally invasive‖ gene repair, as gene modification occurs in situ via recruitment of the cells’ own DNA repair machinery and without the need for viral vectors. We have shown that TFPs can be used to induce modification of the β-globin gene in HSPCs by stimulating recombination at the desired position in the β-globin locus with co-transfected donor DNAs that are homologous to the β-globin gene except for the base pairs to be changed [98].
PLGA nanoparticle-based delivery of TFPs and donor DNA can lead to site-specific gene editing of CD34+ HSPCs [26, 107]. Dye-loaded nanoparticles showed surprisingly efficient uptake in CD34+ HPSCs. TFPs and donor DNA were formulated into 150 nm spherical PLGA nanoparticles, with ample loading of nucleic acids (250–450 pmole/mg nanoparticles). Further, the PLGA nanoparticles loaded with TFP/donor DNA combinations stimulated genomic recombination to modify the IVS2-1 splice site within the β-globin gene. Allele-specific PCR confirmed that nanoparticle-delivered TFPs and donor DNA mediate site-specific modification in CD34+ cells. Importantly the PLGA nanoparticles with TFPs/DNA are not toxic to the progenitor cells. Progenitor cells that were genetically modified with nanoparticles were differentiated into both erythroid and neutrophil populations, without loss of the gene modification.
In another approach, PLGA nanoparticles were loaded with TFPs/donor DNA for site-specific gene editing in CCR5 gene [28]. As discussed above, CCR5 is required for HIV-1 entry into T cells. It has been reported that an HIV-1–positive individual treated by transplant of hematopoietic stem and progenitor cells from a CCR5-Δ32 homozygous donor was cured of HIV-AIDS, with no detectable HIV-1 even after discontinuation of antiretroviral therapy for more than 5 years. In addition, individuals containing heterozygous CCR5 alleles (with only one containing the protective mutation) also have a substantially reduced disease progression rates. PLGA nanoparticles loaded with TFPs/donor DNAs for recombination-mediated editing of the CCR5 gene were formulated for delivery into human peripheral blood mononuclear cells (PBMCs). The nanoparticles efficiently entered PBMCs without toxicity. Further, deep-sequencing revealed that a single treatment produced a ~1% gene editing frequency in the CCR5 gene and a low off-target frequency (0.004%) in the CCR2 gene. The nanoparticle treated PBMCs were efficiently engrafted into immunodeficient NOD-scid IL-2rγ (−/−) mice, and the targeted CCR5 modification was detected in splenic lymphocytes 4 weeks post transplantation. After infection with an R5-tropic strain of HIV-1, humanized mice containing CCR5-NP-treated PBMCs displayed significantly higher levels of CD4+ T cells and reduced plasma viral RNA loads compared with control mice engrafted with mock-treated PBMCs, which show drastic depletion of CD4+ T cells and high levels of viremia [107].
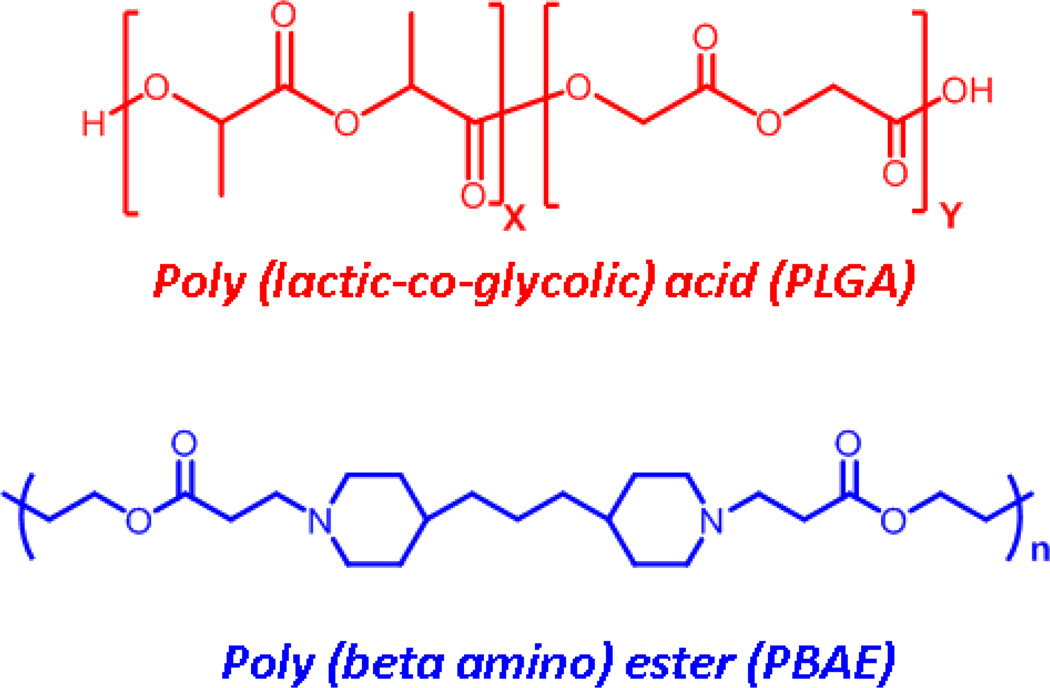
4. PBAE: PLGA based nanoparticles for PNA delivery
PLGA is a popular component for nanoparticles because of its biocompatibility and long history of use in medicine. But PLGA formulations have limitations; for example, it is a challenge to load high levels of plasmid DNA [108]. To improve loading of oligonucleotides, cationic polymers, such as poly(beta-amino-esters) (PBAE), have been explored (Fig. 7). PBAE is synthesized by Michael-like addition of bifunctional amines to diacrylate esters, producing a cationic polymer that is also biodegradable [109]. The cationic nature of PBAE polymers enables it to condense negatively-charged oligonucleotides more efficiently than PLGA. Additionally, the cationic surface density of PBAE materials also acts as a buffer in the low pH environment of endosomes, which further leads to its disruption, and release of the encapsulated molecules. However, because of its cationic character, PBAE is toxic to many cells. Blends of PLGA and PBAE can be used to produce nanoparticles that are less toxic than those with PBAE alone. For example, microparticle delivery systems composed of 15% to 25% PBAE with PLGA result in controlled release of plasmid DNA to antigen presenting cells (APC) in vivo.
Fig. 7.
Chemical structures of PLGA and PBAE units.
PLGA/PBAE based nanoparticles have been used to deliver small molecules like paclitaxel to MCF7 tumor bearing mice in a sustained manner [110]. In addition, it has been noticed that PLGA/PBAE nanoparticles increase the retention time of drug at tumor sites by reducing its clearance rate. PLGA/PBAE blends can also be used to administer two small molecules, paclitaxel and c6 ceramide, in a single formulation in a very effective manner.
4a. Targeting genomic DNA for gene editing
PBAE:PLGA nanoparticles have been employed for delivery of gamma PNAs for site specific gene editing. A transgenic murine model containing a β-globin/GFP fusion gene, consisting of intron 2 of human β-globin inserted within the GFP coding regions, was used to test these materials [27, 111]. The intron contains the IVS2–654 (C->T) mutation, which is a common cause of thalassemia in individuals of Southeast Asian heritage. The β-globin/GFP fusion gene in the mice contains a cryptic splice site that causes incorrect splicing of the intron and prevents GFP expression [8, 9, 112]. For ex vivo and in vivo delivery to mouse bone marrow cells, mini-PEG modified gamma PNAs and 60 nt donor DNAs were loaded into PLGA/PBAE nanoparticles. In comparison to the standard regular PNAs/donor DNA mixtures, mini-PEG modified gamma PNA/donor DNA mixtures showed substantial improvements in the encapsulation and subsequent release of nucleic acids. In gene targeting assays, miniPEG modified gamma PNA/donor DNA containing PBAE:PLGA nanoparticles showed site-specific genome editing of the IVS2–654 (C->T) mutation site in vivo in mouse bone marrow at a frequency of 0.1%, with an off target frequency at a partially homologous site more than 10,000 fold lower. In addition, no toxicity was observed with multiple injections of PBAE:PLGA nanoparticles. Moreover, ex vivo treatment of mouse bone marrow cells revealed a gene correction frequency of 0.8% with a single treatment of PBAE:PLGA nanoparticles.
When PBAE/PLGA and PLGA nanoparticles were compared for DNA delivery, it was found that 15% PBAE:PLGA system are the most effective [113]. To reduce the toxicity and increase uptake into target cells, PBAE:PLGA particles were surface-modified with various CPPs via a PEGylated phospholipid linker (DSPE-PEG2000). Out of a number of CPPs tested, MPG decorated nanoparticles provided the most improved loading and transfection efficiency in CFBE cells.
MPG coated PBAE/PLGA nanoparticles are also effective in the in vivo delivery of C6 coumarin molecules in the lungs of mice [111]. Using the transgenic reporter mouse described above, it was demonstrated that MPG coated PBAE:PLGA nanoparticles effectively deliver triplex PNAs/ donor DNA to airway cells in the lung: MPG-coated PBAE:PLGA nanoparticles delivered PNAs induce gene editing efficiency of up to 0.6 % in the alveolar epithelial cells and 0.3 % in the lung macrophages.
PBAE:PLGA nanoparticles were used to deliver tcPNA and donor DNA to the lung to correct a mutation associated with cystic fibrosis (CF) [31]. CF is a lethal genetic disorder most commonly caused by the F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. PNAs provide the opportunity for a different, safer strategy: tcPNAs and donor DNA molecules loaded into MPG-coated PBAE/PLGA based nanoparticles corrected the F508del CFTR mutation in vitro in human bronchial epithelial cells and in vivo in a CF mouse [31]. In vitro, after a single treatment, approximately 7% of CFBE cells treated with nanoparticles demonstrated Cl− efflux similar to control animals (without a CF gene defect). When these CFBE cells were treated repeatedly with nanoparticles, 25% of the cells demonstrated Cl− efflux equivalent to positive controls. In vivo, after intranasal delivery of tcPNAs and donor DNA loaded PBAE:PLGA nanoparticles, CF mice exhibited changes in the electrochemical potential of the nasal epithelium that indicated successful restoration of CFTR function.
5. Other Polymer based delivery of PNAs
In addition to PLGA and PBAE-based delivery systems, other nanoparticle formulation methods have been also tested for PNA delivery. Promising results have been shown in formulating and encapsulating PNAs, as well as delivery in vitro. In vivo delivery of nanoparticles remains to be tested, however.
5a. Avidin-labeled protein nanoparticles for PNA delivery
Kreuter and coworkers have shown that protein based nanoparticles can be engineered using sulfhydryl-based chemistry for the delivery of 15mer biotinylated antisense PNA targeting the pol gene of HIV type 1 and 2 [32]. The protein nanoparticles were produced by a double desolvation procedure, and then sulphydryl residues were introduced on the nanoparticle surface by reacting the free amines with Traut’s reagent (2-imino thiolate). Activated NeutrAvidin was coupled to the nanoparticles via a thioether linkage between the sulfhydryl groups and the sulfhydryl reactive maleinimide group of the activated NeutrAvidin. Further biotinylated PNAs were loaded by incubation with the avidin nanoparticles followed by ultracentrifugation. Hence, protein nanoparticles can be engineered using biotin-avidin interactions to generate stable and effective carriers for biotinylated PNAs.
5b. Zeolite –L-nanocrystal mediated PNA delivery
Cola and coworkers have explored inorganic materials to simultaneously deliver PNA and organic molecules into the cells. Porous zeolite-L-nanoparticles were used to deliver a model drug and a PNA to living cells [33]. The PNA used in the nanoparticles was complementary to a DNA sequence bearing a single-point mutation (W1282X) implicated in human CF. Briefly, the zeolite-L-crystals were filled with fluorescent guest molecules and the outer particle surface was functionalized with (3-aminopropyl)triethoxysilane (APTES). The amine groups were converted into carboxylic acid groups which were further converted into NHS esters, so that the PNA probes H-(AEEA) 2 -CTTTCCTTCACTGTT-NH 2 (AEEA = 2-(2-aminoethoxy) ethoxyacetyl spacer) could be covalently attached via acyl coupling reactions. Finally a thin coating with poly-L-lysine (PLL), a cationic polymer, was added, in order to favor cell uptake. The PNA retained its DNA/RNA-binding activity when attached to the zeolites. By imaging after exposure to HeLa cells, it was observed that the PNA-functionalized zeolites exhibited rapid uptake in the cells. Further it was evaluated that these PNA-zeolite particles did not exert any toxic effect on the cells. Hence, zeolite nanosystems can be used to deliver specific PNA sequences with a combined drug in living cells opening new possibilities for bioimaging and drug delivery
5c. Fluorescent Mesoporous Silica nanoparticles deliver antisense PNAs
Another interesting approach to deliver antisense PNAs is based on mesoporous silica nanoparticles (MSNP) [34]. In an attempt to silence the Bcl-2 protein, which decreases cell apoptotic cell death and causes resistance in chemotherapy, antisense PNA sequences complementary to the first six codons of Bcl-2 mRNA were designed. MSNP were formulated for intracellular delivery of this PNA. The PNA was covalently conjugated with the MSNP via amidation between the amino group of PNAs and carboxylic group on MSNP surface (Fig. 8). The release of PNA from the MSNP depended on the cleavage of disulphide bond embedded on the MSNP. Due to the presence of an intracellular reducing environment—provided by intracellular glutathione (GSH)—the disulfide bond was cleaved facilitating the release of PNA inside cancer cells. Using this system for redox-triggered PNA release, it was shown that intracellular uptake effectively silenced Bcl-2 expression in vitro.
Fig. 8.
Description of conjugation of PNA with FITC labeled MSNP and the release of Cy5 labeled PNA by redox-triggered mechanism inside the cells [34].
5d. Psi nanoparticles for PNA delivery
Duvall et al. reported an interesting strategy for intracellular delivery of an anti-miR PNA using porous silicon (Psi) [114]. Proof of concept was illustrated using a well-characterized anti-miR-122 PNA [76]; the 23-mer anti-miR-122 PNA targets a liver-specific miRNA, the suppression of which leads to decrease in hepatitis C viremia [115]. The PNA was synthesized in situ from Psi substrate leading to the formation of anti-miR-122 PNA loaded multilayer Psi films. For delivery into the cytoplasm, these films were ultrasonically fractured to produce PNA functionalized Psi nanoparticles (PSNP). The activity of this complex was tested using Huh 7 human hepatic carcinoma cells expressing increased levels of miR-122. Cells treated with PNA-PSNP exhibited higher intracellular delivery of PNA as compared to those treated with free PNA, with no reduction in cell viability. Using a luciferase based reporter assay [116], PSNP delivery of 2 uM PNA resulted in significant inhibition of miR 122 in Huh7 cells as evident from 44 % increase in luciferase activity.
5e. Cationic shell-cross-linked knedel-like (cSCK) nanoparticles for PNA delivery
Another PNA delivery approach uses cationic shell cross-linked knedel-like nanoparticles (cSCK) [35–38]. cSCK nanoparticles consist of a hydrophobic core and a positively charged highly functionalizable cross-linked shell. SCKs belong to a large family of cross-linked block copolymer micelles in which shape, composition, and functionality can be tailored to their particular utility for applications [117]. To formulate cSCK, amphiphlic block copolymers are synthesized: for example, polystyrene blocks linked to a poly(acrylic acid) segment were used in one study. Coupling the carboxylic acids to monoprotected diamine, followed by deprotection, generates primary amines. These amines are largely protonated at neutral pH 7, leading to the formation of a micelle which consists of a hydrophobic polystyrene core and a hydrophilic, positively charged shell. The micelle is stabilized by covalently cross-linking of the shell by amide formation between chains with an activated diester. cSCKs were complexed by electrostatic interactions with negatively charged PNA· ODN (DNA oligonucleotide) hybrid, and via covalent attachment of a PNA through a bioreductively cleavable disulfide bond. These PNA-conjugated cSCKs facilitated endocytosis and endosomal release of the PNAs into Hela cells. To test the activity of these constructs, a luciferase splice correction assay developed by Kole and coworkers [118] was used. In this assay, a luciferase gene (pLuc705) results in a loner, mis-spliced mRNA, which encodes a defective luciferase. The designed PNA was complementary to the aberrant splice site and upon binding of the PNA, splicing is restored leading to shorter mRNA and an active luciferase. In Hela cells, the cSCK-PNA.ODN conjugates resulted in three times higher bioactivity and two-fold lower toxicity than commercially available Lipofectamine 2000. Likewise, the bio-reductively cleavable cSCK-SS-PNA2 conjugates also showed superior properties demonstrating the utility of these nanoparticles.
The potential in vitro and in vivo applications of cSCK nanoparticles for PNA delivery were demonstrated using inducible nitric oxide synthase (iNOS) mRNA targeting for improved diagnostic and treatment strategies for acute lung injury (ALI) [35]. ALI is a life threatening medical condition characterized by widespread inflammation in the lungs [119]. iNOS, an enzyme abundant in activated lung macrophages, is one of the mediators commonly upregulated in ALI. Increased expression of iNOS in macrophages produces nitric oxide (NO), which results in tissue damage [120, 121]. Inhibition of iNOS reduces the accumulation of NO leading to improved outcome from ALI in experimental models[122]. An iNOS imaging probe was prepared by electrostatic complexation between a radiolabelled antisense PNA-YR9.. oligonucleotide (ODN) hybrid and cSCK nanoparticle [35]. The PNA was labeled with 123I and an R9 (arginine9) peptide was used to facilitate the exit of untargeted PNA from the cell. The radiolabelled, antisense PNA showed significant sequence-specific retention in both iNOS expressing cells in vitro and in mouse lung as compared with control mismatch PNA.
In another study, cSCK nanoparticles were used to deliver PNA-based strand displacement probes for live-cell imaging of iNOS mRNA [39]. Subsequently, this cSCK nanoparticle was used for delivery of antisense PNA.DNA binary FRET probes for both in vitro and in vivo imaging [38].
6. Conclusions
Since its discovery in 1991, PNA technology has been employed in diverse biomedical applications. Although PNA has shown promise in nucleic acid based therapeutics, intracellular delivery of PNA is a major issue that needs to be resolved for clinical applications. Numerous peptide based methods have been deployed for delivery of PNAs in cells, but this work remains hampered by toxicity and lack of specificity. Because of this, recent attention has focused on nanoparticle-based delivery methods, which offer the potential for targeted and sustained delivery of PNA-based therapeutics. PLGA and PBAE:PLGA nanoparticles have been shown to deliver PNAs into the cytoplasm and nucleus of cells, which enables antisence and gene editing by PNAs. In addition, PLGA and PBAE:PLGA nanoparticles are non-toxic after multiple injections systemically in mice. Recently, some promising results have been shown for other delivery materials such as zeolite and mesosporus silica for encapsulation of PNAs. Still comprehensive in vivo testing need be performed to evaluate the toxicity profile of these new materials. Hence, we believe that engineered nanocarriers, perhaps decorated with targeting ligands, will open new avenues for diagnostic and therapeutic applications of PNAs.
Acknowledgments
Our original work on PNA delivery systems was supported by grants from the Doris Duke Foundation, Brain Research Foundation (BRF SIA-2014-02), and the US National Institutes of Health (AI112443, HL125892, and EB00487).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cartwright IL, Hertzberg RP, Dervan PB, Elgin SC. Cleavage of chromatin with methidiumpropyl-EDTA. iron(II) Proc Natl Acad Sci U S A. 1983;80:3213–3217. doi: 10.1073/pnas.80.11.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Dyke MW, Hertzberg RP, Dervan PB. Map of distamycin netropsin and actinomycin binding sites on heterogeneous DNA: DNA cleavage-inhibition patterns with methidiumpropyl-EDTA. Fe(II) Proc Natl Acad Sci U S A. 1982;79:5470–5474. doi: 10.1073/pnas.79.18.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monroig Pdel C, Chen L, Zhang S, Calin GA. Small molecule compounds targeting miRNAs for cancer therapy. Advanced drug delivery reviews. 2015;81:104–116. doi: 10.1016/j.addr.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheng J, Gan J, Huang Z. Structure-based DNA-targeting strategies with small molecule ligands for drug discovery. Medicinal research reviews. 2013;33:1119–1173. doi: 10.1002/med.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckstein F. Nucleoside phosphorothioates. Journal of the American Chemical Society. 1970;92:4718–4723. doi: 10.1021/ja00718a039. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe EK, Cohn M. Diastereomers of the nucleoside phosphorothioates as probes of the structure of the metal nucleotide substrates and of the nucleotide binding site of yeast hexokinase. J Biol Chem. 1979;254:10839–10845. [PubMed] [Google Scholar]
- 7.Yuan S, Sun Z. Microinjection of mRNA and morpholino antisense oligonucleotides in zebrafish embryos. J Vis Exp. 2009 doi: 10.3791/1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sazani P, Gemignani F, Kang S-H, Maier MA, Manoharan M, Persmark M, Bortner D, Kole R. Systemically delivered antisense oligomers upregulate gene expression in mouse tissues. Nat. Biotechnol. 2002;20:1228–1233. doi: 10.1038/nbt759. [DOI] [PubMed] [Google Scholar]
- 9.Roberts J, Palma E, Sazani P, Orum H, Cho M, Kole R. Efficient and Persistent Splice Switching by Systemically Delivered LNA Oligonucleotides in Mice. Mol. Ther. 2006;14:471–475. doi: 10.1016/j.ymthe.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, Hansen HF, Koch T, Pappin D, Hannon GJ, Kauppinen S. Silencing of microRNA families by seed-targeting tiny LNAs. Nat. Genet. 2011;43:371–378. doi: 10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White S, Szewczyk JW, Turner JM, Baird EE, Dervan PB. Recognition of the four Watson-Crick base pairs in the DNA minor groove by synthetic ligands. Nature. 1998;391:468–471. doi: 10.1038/35106. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science (Washington, D. C., 1883-) 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 13.Demidov VV, Potaman VN, Frank-Kamenetskii MD, Egholm M, Buchard O, Sonnichsen SH, Nielsen PE. Stability of peptide nucleic acids in human serum and cellular extracts. Biochemical pharmacology. 1994;48:1310–1313. doi: 10.1016/0006-2952(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen PE. Applications of peptide nucleic acids. Current opinion in biotechnology. 1999;10:71–75. doi: 10.1016/s0958-1669(99)80013-5. [DOI] [PubMed] [Google Scholar]
- 15.Christensen L, Fitzpatrick R, Gildea B, Petersen KH, Hansen HF, Koch T, Egholm M, Buchardt O, Nielsen PE, et a. Solid-phase synthesis of peptide nucleic acids. J. Pept. Sci. 1995;1:185–183. doi: 10.1002/psc.310010304. [DOI] [PubMed] [Google Scholar]
- 16.Ratilainen T, Holmen A, Tuite E, Nielsen PE, Norden B. Thermodynamics of sequence-specific binding of PNA to DNA. Biochemistry. 2000;39:7781–7791. doi: 10.1021/bi000039g. [DOI] [PubMed] [Google Scholar]
- 17.Thomas SM, Sahu B, Rapireddy S, Bahal R, Wheeler SE, Procopio EM, Kim J, Joyce SC, Contrucci S, Wang Y, Chiosea SI, Lathrop KL, Watkins S, Grandis JR, Armitage BA, Ly DH. Antitumor Effects of EGFR Antisense Guanidine-Based Peptide Nucleic Acids in Cancer Models. ACS Chem. Biol. 2013;8:345–352. doi: 10.1021/cb3003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM, Saltzman WM, Slack FJ. MicroRNA silencing for cancer therapy targeted to the tumor microenvironment. Nature (London, U. K.) 2014 doi: 10.1038/nature13905. Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanvey JC, Peffer NJ, Bisi JE, Thomson SA, Cadilla R, Josey JA, Ricca DJ, Hassman CF, Bonham MA, Au KG, et al. Antisense and antigene properties of peptide nucleic acids. Science. 1992;258:1481–1485. doi: 10.1126/science.1279811. [DOI] [PubMed] [Google Scholar]
- 20.Koppelhus U, Awasthi SK, Zachar V, Holst HU, Ebbesen P, Nielsen PE. Cell-dependent differential cellular uptake of PNA peptides and PNA-peptide conjugates. Antisense & nucleic acid drug development. 2002;12:51–63. doi: 10.1089/108729002760070795. [DOI] [PubMed] [Google Scholar]
- 21.Shiraishi T, Nielsen PE. Peptide nucleic acid (PNA) cell penetrating peptide (CPP) conjugates as carriers for cellular delivery of antisense oligomers, Artificial DNA. PNA & XNA. 2011;2:90–99. doi: 10.4161/adna.18739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen PE. Addressing the challenges of cellular delivery and bioavailability of peptide nucleic acids (PNA) Quarterly reviews of biophysics. 2005;38:345–350. doi: 10.1017/S0033583506004148. [DOI] [PubMed] [Google Scholar]
- 23.Koppelhus U, Nielsen PE. Cellular delivery of peptide nucleic acid (PNA) Advanced drug delivery reviews. 2003;55:267–280. doi: 10.1016/s0169-409x(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 24.Noguchi E, Shigi N, Komiyama M. Intracellular localization of PNA in human cells upon its introduction by electroporation. Natural product communications. 2012;7:349–352. [PubMed] [Google Scholar]
- 25.Bendifallah N, Rasmussen FW, Zachar V, Ebbesen P, Nielsen PE, Koppelhus U. Evaluation of cell-penetrating peptides (CPPs) as vehicles for intracellular delivery of antisense peptide nucleic acid (PNA) Bioconjugate chemistry. 2006;17:750–758. doi: 10.1021/bc050283q. [DOI] [PubMed] [Google Scholar]
- 26.McNeer NA, Chin JY, Schleifman EB, Fields RJ, Glazer PM, Saltzman WM. Nanoparticles Deliver Triplex-forming PNAs for Site-specific Genomic Recombination in CD34+ Human Hematopoietic Progenitors. Mol. Ther. 2011;19:172–180. doi: 10.1038/mt.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahal R, Quijano E, McNeer NA, Liu Y, Bhunia DC, Lopez-Giraldez F, Fields RJ, Saltzman WM, Ly DH, Glazer PM. Single-Stranded γPNAs for In Vivo Site-Specific Genome Editing via Watson-Crick Recognition. Curr. Gene Ther. 2014;14:331–342. doi: 10.2174/1566523214666140825154158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNeer NA, Schleifman EB, Cuthbert A, Brehm M, Jackson A, Cheng C, Anandalingam K, Kumar P, Shultz LD, Greiner DL, Mark Saltzman W, Glazer PM. Systemic delivery of triplex-forming PNA and donor DNA by nanoparticles mediates site-specific genome editing of human hematopoietic cells in vivo. Gene therapy. 2013;20:658–669. doi: 10.1038/gt.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bahal R, McNeer NA, Ly DH, Saltzman WM, Glazer PM. Nanoparticle for delivery of antisense γPNA oligomers targeting CCR5. Artificial DNA, PNA & XNA. 2013;4:49–57. doi: 10.4161/adna.25628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babar IA, Cheng CJ, Booth CJ, Liang X, Weidhaas JB, Saltzman WM, Slack FJ. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Author summary. Proc. Natl. Acad. Sci. U. S. A. 2012;109:10140–10141. doi: 10.1073/pnas.1201516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNeer NA, Anandalingam K, Fields RJ, Caputo C, Kopic S, Gupta A, Quijano E, Polikoff L, Kong Y, Bahal R, Geibel JP, Glazer PM, Saltzman WM, Egan ME. Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nature communications. 2015;6:6952. doi: 10.1038/ncomms7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langer K, Coester C, Weber C, von Briesen H, Kreuter J. Preparation of avidin-labeled protein nanoparticles as carriers for biotinylated peptide nucleic acid. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2000;49:303–307. doi: 10.1016/s0939-6411(00)00068-0. [DOI] [PubMed] [Google Scholar]
- 33.Bertucci A, Lulf H, Septiadi D, Manicardi A, Corradini R, De Cola L. Intracellular delivery of peptide nucleic acid and organic molecules using zeolite-L nanocrystals. Adv Healthc Mater. 2014;3:1812–1817. doi: 10.1002/adhm.201400116. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Devi G, Qu Q, Toh DF, Chen G, Zhao Y. Intracellular delivery of antisense peptide nucleic acid by fluorescent mesoporous silica nanoparticles. Bioconjugate chemistry. 2014;25:1412–1420. doi: 10.1021/bc5002714. [DOI] [PubMed] [Google Scholar]
- 35.Shen Y, Shrestha R, Ibricevic A, Gunsten SP, Welch MJ, Wooley KL, Brody SL, Taylor JS, Liu Y. Antisense peptide nucleic acid-functionalized cationic nanocomplex for in vivo mRNA detection. Interface focus. 2013;3:20120059. doi: 10.1098/rsfs.2012.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrestha R, Shen Y, Pollack KA, Taylor JS, Wooley KL. Dual peptide nucleic acid-and peptide-functionalized shell cross-linked nanoparticles designed to target mRNA toward the diagnosis and treatment of acute lung injury. Bioconjugate chemistry. 2012;23:574–585. doi: 10.1021/bc200629f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang H, Zhang K, Shen G, Wooley KL, Taylor JS. Cationic shell-cross-linked knedel-like (cSCK) nanoparticles for highly efficient PNA delivery. Molecular pharmaceutics. 2009;6:615–626. doi: 10.1021/mp800199w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Zhang K, Shen Y, Smith J, Bloch S, Achilefu S, Wooley KL, Taylor JS. Imaging mRNA expression levels in living cells with PNA.DNA binary FRET probes delivered by cationic shell-crosslinked nanoparticles. Organic & biomolecular chemistry. 2013;11:3159–3167. doi: 10.1039/c3ob26923j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Zhang K, Wooley KL, Taylor JS. Imaging mRNA Expression in Live Cells via PNA.DNA Strand Displacement-Activated Probes. Journal of nucleic acids. 2012;2012:962652. doi: 10.1155/2012/962652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi VG, Chindera K, Singh AK, Sahoo AP, Dighe VD, Thakuria D, Tiwari AK, Kumar S. Rapid label-free visual assay for the detection and quantification of viral RNA using peptide nucleic acid (PNA) and gold nanoparticles (AuNPs) Analytica chimica acta. 2013;795:1–7. doi: 10.1016/j.aca.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 41.Pita M, Abad JM, Vaz-Dominguez C, Briones C, Mateo-Marti E, Martin-Gago JA, Morales Mdel P, Fernandez VM. Synthesis of cobalt ferrite core/metallic shell nanoparticles for the development of a specific PNA/DNA biosensor. Journal of colloid and interface science. 2008;321:484–492. doi: 10.1016/j.jcis.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Hirschman SZ, Chen CW. Peptide nucleic acids stimulate gamma interferon and inhibit the replication of the human immunodeficiency virus. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 1996;44:347–351. [PubMed] [Google Scholar]
- 43.Wang G, Xu X, Pace B, Dean DA, Glazer PM, Chan P, Goodman SR, Shokolenko I. Peptide nucleic acid (PNA) binding-mediated induction of human gamma-globin gene expression. Nucleic Acids Res. 1999;27:2806–2813. doi: 10.1093/nar/27.13.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doyle DF, Braasch DA, Simmons CG, Janowski BA, Corey DR. Inhibition of gene expression inside cells by peptide nucleic acids: effect of mRNA target sequence mismatched bases and PNA length. Biochemistry. 2001;40:53–64. doi: 10.1021/bi0020630. [DOI] [PubMed] [Google Scholar]
- 45.Corey DR. Recognition of chromosomal DNA in human cells by peptide nucleic acids and small duplex RNAs. Annals of the New York Academy of Sciences. 2005;1058:16–25. doi: 10.1196/annals.1359.003. [DOI] [PubMed] [Google Scholar]
- 46.Janowski BA, Kaihatsu K, Huffman KE, Schwartz JC, Ram R, Hardy D, Mendelson CR, Corey DR. Inhibiting transcription of chromosomal DNA with antigene peptide nucleic acids. Nat. Chem. Biol. 2005;1:210–215. doi: 10.1038/nchembio724. [DOI] [PubMed] [Google Scholar]
- 47.Hu J, Corey DR. Inhibiting gene expression with peptide nucleic acid (PNA)--peptide conjugates that target chromosomal DNA. Biochemistry. 2007;46:7581–7589. doi: 10.1021/bi700230a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faruqi AF, Egholm M, Glazer PM. Peptide nucleic acid-targeted mutagenesis of a chromosomal gene in mouse cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1398–1403. doi: 10.1073/pnas.95.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erazo-Oliveras A, Muthukrishnan N, Baker R, Wang T-Y, Pellois J-P. Improving the Endosomal Escape of Cell-Penetrating Peptides and Their Cargos: Strategies and Challenges. Pharmaceuticals. 2012;5:1177–1209. doi: 10.3390/ph5111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zoonens M, Reshetnyak YK, Engelman DM. Bilayer interactions of pHLIP, a peptide that can deliver drugs and target tumors. Biophys. J. 2008;95:225–235. doi: 10.1529/biophysj.107.124156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reshetnyak YK, Andreev OA, Segala M, Markin VS, Engelman DM. Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15340–15345. doi: 10.1073/pnas.0804746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ljungstrom T, Knudsen H, Nielsen PE. Cellular uptake of adamantyl conjugated peptide nucleic acids. Bioconjugate chemistry. 1999;10:965–972. doi: 10.1021/bc990053+. [DOI] [PubMed] [Google Scholar]
- 53.Muratovska A, Lightowlers RN, Taylor RW, Turnbull DM, Smith RA, Wilce JA, Martin SW, Murphy MP. Targeting peptide nucleic acid (PNA) oligomers to mitochondria within cells by conjugation to lipophilic cations: implications for mitochondrial DNA replication, expression and disease. Nucleic acids Research. 2001;29:1852–1863. doi: 10.1093/nar/29.9.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaubey B, Tripathi S, Pandey VN. Single acute-dose and repeat-doses toxicity of anti- HIV-1 PNA TAR-penetratin conjugate after intraperitoneal administration to mice. Oligonucleotides. 2008;18:9–20. doi: 10.1089/oli.2007.0088. [DOI] [PubMed] [Google Scholar]
- 55.Abes S, Turner JJ, Ivanova GD, Owen D, Williams D, Arzumanov A, Clair P, Gait MJ, Lebleu B. Efficient splicing correction by PNA conjugation to an R6-Penetratin delivery peptide. Nucleic acids Research. 2007;35:4495–4502. doi: 10.1093/nar/gkm418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaubey B, Tripathi S, Ganguly S, Harris D, Casale RA, Pandey VN. A PNA- transportan conjugate targeted to the TAR region of the HIV-1 genome exhibits both antiviral and virucidal properties. Virology. 2005;331:418–428. doi: 10.1016/j.virol.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 57.Cogoi S, Codognotto A, Rapozzi V, Xodo LE. Antigene property of PNA conjugated to the nuclear localization signal peptide. Nucleosides, nucleotides & nucleic acids. 2005;24:971–974. doi: 10.1081/ncn-200059333. [DOI] [PubMed] [Google Scholar]
- 58.Turner JJ, Ivanova GD, Verbeure B, Williams D, Arzumanov AA, Abes S, Lebleu B, Gait MJ. Cell-penetrating peptide conjugates of peptide nucleic acids (PNA) as inhibitors of HIV-1 Tat-dependent trans-activation in cells. Nucleic acids Research. 2005;33:6837–6849. doi: 10.1093/nar/gki991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dragulescu-Andrasi A, Rapireddy S, Frezza BM, Gayathri C, Gil RR, Ly DH. A Simple γ-Backbone Modification Preorganizes Peptide Nucleic Acid into a Helical Structure. J. Am. Chem. Soc. 2006;128:10258–10267. doi: 10.1021/ja0625576. [DOI] [PubMed] [Google Scholar]
- 60.Dragulescu-Andrasi A, Rapireddy S, He G, Bhattacharya B, Hyldig-Nielsen JJ, Zon G, Ly DH. Cell-Permeable Peptide Nucleic Acid Designed to Bind to the 5'-Untranslated Region of E-cadherin Transcript Induces Potent and Sequence-Specific Antisense Effects. J. Am. Chem. Soc. 2006;128:16104–16112. doi: 10.1021/ja063383v. [DOI] [PubMed] [Google Scholar]
- 61.Rapireddy S, Bahal R, Ly DH. Strand Invasion of Mixed-Sequence Double-Helical B-DNA by γ-Peptide Nucleic Acids Containing G-Clamp Nucleobases under Physiological Conditions. Biochemistry-Us. 2011;50:3913–3918. doi: 10.1021/bi2002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delgado E, Bahal R, Yang J, Lee JM, Ly DH, Monga SPS. β-Catenin Knockdown in Liver Tumor Cells by a Cell Permeable Gamma Guanidine-based Peptide Nucleic Acid. Curr. Cancer Drug Targets. 2013;13:867–878. doi: 10.2174/15680096113139990081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, Croce CM. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7024–7029. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danhier F, Ansorena E, Silva JM, Coco R, Le BA, Preat V. PLGA-based nanoparticles: An overview of biomedical applications. J. Controlled Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 65.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and surfaces. B, Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Fahmy TM, Demento SL, Caplan MJ, Mellman I, Saltzman WM. Design opportunities for actively targeted nanoparticle vaccines. Nanomedicine. 2008;3:343–355. doi: 10.2217/17435889.3.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solbrig CM, Saucier-Sawyer JK, Cody V, Saltzman WM, Hanlon DJ. Polymer nanoparticles for immunotherapy from encapsulated tumor-associated antigens and whole tumor cells. Molecular pharmaceutics. 2007;4:47–57. doi: 10.1021/mp060107e. [DOI] [PubMed] [Google Scholar]
- 68.Ungaro F, d'Angelo I, Coletta C, d'Emmanuele di Villa R, Sorrentino R, Perfetto B, Tufano MA, Miro A, La Rotonda MI, Quaglia F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. Journal of controlled release : official journal of the Controlled Release Society. 2012;157:149–159. doi: 10.1016/j.jconrel.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 70.Park J, Fong PM, Lu J, Russell KS, Booth CJ, Saltzman WM, Fahmy TM. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin, Nanomedicine : nanotechnology. biology, and medicine. 2009;5:410–418. doi: 10.1016/j.nano.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tahara K, Sakai T, Yamamoto H, Takeuchi H, Kawashima Y. Establishing chitosan coated PLGA nanosphere platform loaded with wide variety of nucleic acid by complexation with cationic compound for gene delivery. International journal of pharmaceutics. 2008;354:210–216. doi: 10.1016/j.ijpharm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31:358–365. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cun D, Jensen DK, Maltesen MJ, Bunker M, Whiteside P, Scurr D, Foged C, Nielsen HM. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: quality by design optimization and characterization. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2011;77:26–35. doi: 10.1016/j.ejpb.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Singh SR, Rameshwar P. MicroRNA in development and in the progression of cancer [Google Scholar]
- 75.Schmitz U, Wolkenhauer O, Vera J. MicroRNA cancer regulation : advanced concepts. bioinformatics and systems biology tools [Google Scholar]
- 76.Torres AG, Fabani MM, Vigorito E, Williams D, Al-Obaidi N, Wojciechowski F, Hudson RH, Seitz O, Gait MJ. Chemical structure requirements and cellular targeting of microRNA-122 by peptide nucleic acids anti-miRs. Nucleic acids Research. 2012;40:2152–2167. doi: 10.1093/nar/gkr885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Torres AG, Threlfall RN, Gait MJ. Potent and sustained cellular inhibition of miR-122 by lysine-derivatized peptide nucleic acids (PNA) and phosphorothioate locked nucleic acid (LNA)/2'-O-methyl (OMe) mixmer anti-miRs in the absence of transfection agents. Artif DNA PNA XNA. 2011;2:71–78. doi: 10.4161/adna.17731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng CJ, Saltzman WM. Polymer Nanoparticle-Mediated Delivery of MicroRNA Inhibition and Alternative Splicing. Mol. pharmaceutics. 2012;9:1481–1488. doi: 10.1021/mp300081s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nature materials. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tietjen GT, Saltzman WM. Nanomedicine gets personal. Science translational medicine. 2015;7:314fs347. doi: 10.1126/scitranslmed.aad6645. [DOI] [PubMed] [Google Scholar]
- 81.Yeh JI, Shivachev B, Rapireddy S, Crawford MJ, Gil RR, Du S, Madrid M, Ly DH. Crystal Structure of Chiral γPNA with Complementary DNA Strand: Insights into the Stability and Specificity of Recognition and Conformational Preorganization. J. Am. Chem. Soc. 2010;132:10717–10727. doi: 10.1021/ja907225d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahu B, Sacui I, Rapireddy S, Zanotti KJ, Bahal R, Armitage BA, Ly DH. Synthesis and Characterization of Conformationally Preorganized, (R)-Diethylene Glycol-Containing γ-Peptide Nucleic Acids with Superior Hybridization Properties and Water Solubility. J. Org. Chem. 2011;76:5614–5627. doi: 10.1021/jo200482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crawford MJ, Rapireddy S, Bahal R, Sacui I, Ly DH. Effect of steric constraint at the γ-backbone position on the conformations and hybridization properties of PNAs. J. Nucleic acids. 2011:652702–652710. doi: 10.4061/2011/652702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bahal R, Sahu B, Rapireddy S, Lee C-M, Ly DH. Sequence-Unrestricted, Watson-Crick Recognition of Double Helical B-DNA by (R)-MiniPEG-γPNAs. ChemBioChem. 2012;13:56–60. doi: 10.1002/cbic.201100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chenna V, Rapireddy S, Sahu B, Ausin C, Pedroso E, Ly DH. A simple cytosine to G-clamp nucleobase substitution enables chiral γ-PNAs to invade mixed-sequence double-helical B-form DNA. ChemBioChem. 2008;9:2388–2391. doi: 10.1002/cbic.200800441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schleifman EB, Bindra R, Leif J, del CJ, Rogers FA, Uchil P, Kutsch O, Shultz LD, Kumar P, Greiner DL, Glazer PM. Targeted Disruption of the CCR5 Gene in Human Hematopoietic Stem Cells Stimulated by Peptide Nucleic Acids. Chem. Biol. (Cambridge, MA, U. S.) 2011;18:1189–1198. doi: 10.1016/j.chembiol.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Forget BG. Molecular basis of hereditary persistence of fetal hemoglobin. Ann. N. Y. Acad. Sci. 1998;850:38–44. doi: 10.1111/j.1749-6632.1998.tb10460.x. [DOI] [PubMed] [Google Scholar]
- 88.Peters C, Steward CG. Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant. 2003;31:229–239. doi: 10.1038/sj.bmt.1703839. [DOI] [PubMed] [Google Scholar]
- 89.Saito AM, Zahrieh D, Cutler C, Ho VT, Antin JH, Soiffer RJ, Alyea EP, Lee SJ. Lower costs associated with hematopoietic cell transplantation using reduced intensity vs high-dose regimens for hematological malignancy. Bone Marrow Transplant. 2007;40:209–217. doi: 10.1038/sj.bmt.1705733. [DOI] [PubMed] [Google Scholar]
- 90.Arnould S, Delenda C, Grizot S, Desseaux C, Paques F, Silva GH, Smith J. The I-CreI meganuclease and its engineered derivatives: applications from cell modification to gene therapy. Protein Eng. Des. Sel. 2010;24:27–31. doi: 10.1093/protein/gzq083. [DOI] [PubMed] [Google Scholar]
- 91.Khan IF, Hirata RK, Wang P-R, Li Y, Kho J, Nelson A, Huo Y, Zavaljevski M, Ware C, Russell DW. Engineering of human pluripotent stem cells by AAV-mediated gene targeting. Mol. Ther. 2010;18:1192–1199. doi: 10.1038/mt.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palpant NJ, Dudzinski DM. Zinc-finger nucleases: looking toward translation. Gene Ther. 2013;20:121–127. doi: 10.1038/gt.2012.2. [DOI] [PubMed] [Google Scholar]
- 93.Scharenberg AM, Duchateau P, Smith J. Genome engineering with TAL-effector nucleases and alternative modular nuclease technologies. Curr. Gene Ther. 2013;13:291–303. doi: 10.2174/15665232113139990026. [DOI] [PubMed] [Google Scholar]
- 94.Cradick TJ, Fine EJ, Antico CJ, Bao G. CRISPR/Cas9 systems targeting β-globin and CCR5 genes have substantial off-target activity. Nucleic Acids Res. 2013;41:9584–9592. doi: 10.1093/nar/gkt714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang G, Seidman MM, Glazer PM. Mutagenesis in mammalian cells induced by triple helix formation and transcription-coupled repair. Science (Washington, D. C.) 1996;271:802–805. doi: 10.1126/science.271.5250.802. [DOI] [PubMed] [Google Scholar]
- 96.Vasquez KM, Narayanan L, Glazer PM. Specific mutations induced by triplex-forming oligonucleotides in mice. Science. 2000;290:530–533. doi: 10.1126/science.290.5491.530. [DOI] [PubMed] [Google Scholar]
- 97.Rogers FA, Vasquez KM, Egholm M, Glazer PM. Site-directed recombination via bifunctional PNA-DNA conjugates. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16695–16700. doi: 10.1073/pnas.262556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chin JY, Kuan JY, Lonkar PS, Krause DS, Seidman MM, Peterson KR, Nielsen PE, Kole R, Glazer PM. Correction of a splice-site mutation in the β-globin gene stimulated by triplex-forming peptide nucleic acids. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13514–13519. doi: 10.1073/pnas.0711793105. S13514/13511-S13514/13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vasquez KM, Christensen J, Li L, Finch RA, Glazer PM. Human XPA and RPA DNA repair proteins participate in specific recognition of triplex-induced helical distortions. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5848–5853. doi: 10.1073/pnas.082193799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Havre PA, Gunther EJ, Gasparro FP, Glazer PM. Targeted mutagenesis of DNA using triple helix-forming oligonucleotides linked to psoralen. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7879–7883. doi: 10.1073/pnas.90.16.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rogers FA, Lin SS, Hegan DC, Krause DS, Glazer PM. Targeted gene modification of hematopoietic progenitor cells in mice following systemic administration of a PNA-peptide conjugate. Mol Ther. 2012;20:109–118. doi: 10.1038/mt.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rogers FA, Hu R-H, Milstone LM. Local Delivery of Gene-Modifying Triplex-Forming Molecules to the Epidermis. J. Invest. Dermatol. 2013;133:685–691. doi: 10.1038/jid.2012.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yin HF, Betts C, Saleh AF, Ivanova GD, Lee H, Seow Y, Kim D, Gait MJ, Wood MJA. Optimization of Peptide Nucleic Acid Antisense Oligonucleotides for Local and Systemic Dystrophin Splice Correction in the mdx Mouse. Mol. Ther. 2010;18:819–827. doi: 10.1038/mt.2009.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaihatsu K, Shah RH, Zhao X, Corey DR. Extending Recognition by Peptide Nucleic Acids (PNAs): Binding to Duplex DNA and Inhibition of Transcription by Tail-Clamp PNA-Peptide Conjugates. Biochemistry. 2003;42:13996–14003. doi: 10.1021/bi035194k. [DOI] [PubMed] [Google Scholar]
- 105.Bentin T, Larsen HJ, Nielsen PE. Combined Triplex/Duplex Invasion of Double-Stranded DNA by "Tail-Clamp" Peptide Nucleic Acid. Biochemistry. 2003;42:13987–13995. doi: 10.1021/bi0351918. [DOI] [PubMed] [Google Scholar]
- 106.Kim K-H, Nielsen PE, Glazer PM. Site-Specific Gene Modification by PNAs Conjugated to Psoralen. Biochemistry-Us. 2006;45:314–323. doi: 10.1021/bi051379a. [DOI] [PubMed] [Google Scholar]
- 107.Schleifman EB, McNeer NA, Jackson A, Yamtich J, Brehm MA, Shultz LD, Greiner DL, Kumar P, Saltzman WM, Glazer PM. Site-specific Genome Editing in PBMCs With PLGA Nanoparticle-delivered PNAs Confers HIV-1 Resistance in Humanized Mice. Mol. Ther.--Nucleic acids. 2013;2:e135. doi: 10.1038/mtna.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blum JS, Saltzman WM. High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-L-lysine. Journal of controlled release : official journal of the Controlled Release Society. 2008;129:66–72. doi: 10.1016/j.jconrel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lynn DM, Langer R. Degradable Poly(β-amino esters): Synthesis Characterization and Self-Assembly with Plasmid DNA. J. Am. Chem. Soc. 2000;122:10761–10768. [Google Scholar]
- 110.van Vlerken LE, Duan Z, Little SR, Seiden MV, Amiji MM. Biodistribution and pharmacokinetic analysis of Paclitaxel and ceramide administered in multifunctional polymer-blend nanoparticles in drug resistant breast cancer model. Molecular pharmaceutics. 2008;5:516–526. doi: 10.1021/mp800030k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fields RJ, Quijano E, McNeer NA, Caputo C, Bahal R, Anandalingam K, Egan ME, Glazer PM, Saltzman WM. Modified Poly(lactic-co-glycolic Acid) Nanoparticles for Enhanced Cellular Uptake and Gene Editing in the Lung. Advanced healthcare materials. 2014 doi: 10.1002/adhm.201400355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Svasti S, Suwanmanee T, Fucharoen S, Moulton HM, Nelson MH, Maeda N, Smithies O, Kole R. RNA repair restores hemoglobin expression in IVS2-654 thalassemic mice. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1205–1210. doi: 10.1073/pnas.0812436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fields RJ, Cheng CJ, Quijano E, Weller C, Kristofik N, Duong N, Hoimes C, Egan ME, Saltzman WM. Surface modified poly(beta amino ester)-containing nanoparticles for plasmid DNA delivery. Journal of controlled release : official journal of the Controlled Release Society. 2012;164:41–48. doi: 10.1016/j.jconrel.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beavers KR, Mares JW, Swartz CM, Zhao Y, Weiss SM, Duvall CL. In situ synthesis of peptide nucleic acids in porous silicon for drug delivery and biosensing. Bioconjugate chemistry. 2014;25:1192–1197. doi: 10.1021/bc5001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 116.Connelly CM, Thomas M, Deiters A. High-throughput luciferase reporter assay for small-molecule inhibitors of microRNA function. Journal of biomolecular screening. 2012;17:822–828. doi: 10.1177/1087057112439606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.O'Reilly RK, Hawker CJ, Wooley KL. Cross-linked block copolymer micelles: functional nanostructures of great potential and versatility. Chemical Society reviews. 2006;35:1068–1083. doi: 10.1039/b514858h. [DOI] [PubMed] [Google Scholar]
- 118.Kang SH, Cho MJ, Kole R. Up-regulation of luciferase gene expression with antisense oligonucleotides: implications and applications in functional assay development. Biochemistry. 1998;37:6235–6239. doi: 10.1021/bi980300h. [DOI] [PubMed] [Google Scholar]
- 119.Johnson ER, Matthay MA. Acute lung injury: epidemiology pathogenesis and treatment. Journal of aerosol medicine and pulmonary drug delivery. 2010;23:243–252. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mehta S. The effects of nitric oxide in acute lung injury. Vascular pharmacology. 2005;43:390–403. doi: 10.1016/j.vph.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 121.Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7809–7813. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hosogi S, Iwasaki Y, Yamada T, Komatani-Tamiya N, Hiramatsu A, Kohno Y, Ueda M, Arimoto T, Marunaka Y. Effect of inducible nitric oxide synthase on apoptosis in Candida-induced acute lung injury. Biomedical Research. 2008;29:257–266. doi: 10.2220/biomedres.29.257. [DOI] [PubMed] [Google Scholar]