Abstract
Angiotensin II (AngII) has been strongly implicated in hypertension and its complications. Evidence suggests the mechanisms by which angiotensin II (AngII) elevates blood pressure and enhances cardiovascular remodeling and damage may be distinct. However, the signal transduction cascade by which AngII specifically initiates cardiovascular remodeling such as hypertrophy and fibrosis remains insufficiently understood. In vascular smooth muscle cells, a metalloproteinase ADAM17 mediates epidermal growth factor receptor (EGFR) transactivation, which may be responsible for cardiovascular remodeling but not hypertension induced by AngII. Thus, the objective of this study was to test the hypothesis that activation of vascular ADAM17 is indispensable for vascular remodeling but not for hypertension induced by AngII. Vascular ADAM17 deficient mice and control mice were infused with AngII for 2 weeks. Control mice infused with AngII showed cardiac hypertrophy, vascular medial hypertrophy and perivascular fibrosis. These phenotypes were prevented in vascular ADAM17 deficient mice independent of blood pressure alteration. AngII infusion enhanced ADAM17 expression, EGFR activation and ER stress in the vasculature, which were diminished in ADAM17 deficient mice. Treatment with a human cross-reactive ADAM17 inhibitory antibody also prevented cardiovascular remodeling and ER stress but not hypertension in C57Bl/6 mice infused with AngII. In vitro data further supported these findings. In conclusion, vascular ADAM17 mediates AngII-induced cardiovascular remodeling via EGFR activation independent of blood pressure regulation. ADAM17 seems to be a unique therapeutic target for the prevention of hypertensive complications.
Keywords: Hypertension, Fibrosis, End-organ damage, Renin angiotensin system, Signal transduction
Introduction
The prevalence and morbidity of hypertension is growing steadily 1. End-organ damage is the most important clinical consequence of hypertension causing cardiac failure, renal failure and stroke. Despite great achievements in blood pressure therapy, optimally treated hypertensive patients still have a 50% greater risk than untreated normotensive subjects 2 suggesting the urgent need of add-on therapy to specifically target hypertensive end-organ damage. Vascular remodeling has been strongly implicated in hypertensive end-organ damage and associated with poor cardiovascular outcomes. The remodeling predisposes to end-organ damage and pharmacological intervention in vascular remodeling should have special clinical efficacy for prevention of hypertensive organ damage 3. The renin angiotensin system has been strongly implicated in hypertension and its complications. Importantly, it has been suggested that the mechanisms by which angiotensin II (AngII) elevates blood pressure and enhances cardiovascular remodeling and end-organ damage may be distinct 4. Although many downstream signaling cascades and target genes/proteins of AngII have been identified, the proximal key event primarily responsible for vascular remodeling such as vascular hypertrophy and fibrosis, independent of blood pressure, remains largely unclear 5, 6.
AngII mediates vascular smooth muscle cell (VSMC) contraction via Gq-mediated intracellular Ca2+ elevation and G12/13-mediated Rho kinase activation 7. ADAM (a disintegrin and metalloprotainase) proteins belong to a family of membrane spanning metalloprotainases that cleave ectodomains of several substrates including epidermal growth factor receptor (EGFR) ligands 8. We have shown that ADAM17-mediated EGFR transactivation via heparin-binding EGF-like growth factor (HB-EGF) shedding is required for extracellular signal-regulated kinase (ERK) activation but not for intracellular Ca2+ elevation or Rho kinase activation 9-11. Also, ADAM17 expression is enhanced in neointima after angioplasty, and dominant-negative ADAM17 gene-transfer prevents neointimal hyperplasia 12. Others have shown that ADAM17 expression is enhanced in atherosclerosis 13 and in the left ventricle upon AngII infusion 14 and that an ADAM17 polymorphism is associated with cardiovascular mortality 15. However, whether vascular ADAM17 manipulation has therapeutic potential against hypertensive complications remains completely unknown. Therefore, utilizing mice lacking VSMC ADAM17 or treated with a human cross-reactive ADAM17 antibody as well as with in vitro fibrosis assessment, we have tested our hypothesis that vascular ADAM17 is indispensable for cardiovascular remodeling but not for hypertension induced by AngII, thus highlighting a unique therapeutic target in hypertension.
Methods
Animal Studies and the Tissue Analysis
Animal procedures were performed in accordance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and Temple University IACUC guidelines. 8-10 week old male ADAM17flox/flox sm22αCre+/− mice 16 and control ADAM17flox/flox sm22αCre−/− mice were infused with AngII (Bachem, 1 μg/kg/min) for 2 weeks via osmotic mini-pump 17. 8-10 week old male C57Bl6 mice (Jackson) were infused with AngII (Bachem, 1 μg/kg/min) and treated with human cross-reactive ADAM17 inhibitory antibody A9B8 18 or control human IgG2 (Athens Research & Technology) which was solubilized in PBS, 10 mg/kg/day intraperitoneal injection, at day 1 and day 7. Blood pressure and heart rate were evaluated in the conscious state by telemetry (DSI equipped with ADInstrument 6 software) via carotid catheter (PA-C10 transmitter). Cardiac function was measured using VisualSonics Velvo 2100 (M-mode). Plasma B type natriuretic peptide and blood urea nitrogen concentrations were determined by the EIA kits (RayBiotech Inc. and Stanbio Laboratories, respectively). Extracted hearts, kidneys and aortas were fixed and used for histological studies as described previously 19.
To evaluate vascular hypertrophy and perivascular fibrosis in hearts and kidneys, serial cross-sections (5 μm thick) were stained in Sirius Red (EMS, Hatfield PA). Briefly, after de-paraffinization and re-hydration, sections were stained in equal parts Weigert's Iron Hematoxylin A and B (EMS, Hatfield PA) for 10 min at room temperature. Sections were then washed twice in distilled water for 3 min per wash. Sirius Red was added for 1 h at room temperature. Slides were washed twice in 0.01N HCl for 3 min per wash. Sections were then dehydrated and penetrated using ethanol and xylene, respectively. Thoracic aortas were stained with Masson's trichrome protocol to distinguish medial area from adventitia. Briefly, after de-paraffinization and re-hydration, sections were incubated with Bouin's fluid for 1 h at 56°C. Sections were washed three times in distilled water for 3 min per wash and then incubated with Working HE solution for 7.5 min followed by washing in distilled water for 30 sec. Sections were then incubated with Biebrich Scarlet-Acid Fuchsin solution for 1 h at 56°C. After incubation with phosphotungstic-phosphomolybdic acid solution for 5 min, sections were stained with Aniline Blue stain solution for 5 min. Sections were washed in 1% acetic acid for 30 sec and distilled water for 30 sec. Sections were then dehydrated and penetrated using ethanol and xylene, respectively. Images were visualized on an Olympus IX81 inverted microscope using an Olympus SC30 high resolution camera and were acquired with Olympus cellSens Entry 1.11 software. Analysis was conducted using ImageJ 1.50f software (http://rsb.info.nih.gov/ij).
To calculate vascular hypertrophy in the heart and kidney, the value of medial area was divided by the true area of the vessel. True area was calculated by vessel outer perimeter2 divided by 4π. The value generated was the area of the vessel in true circular form. To calculate perivascular fibrosis, the value of fibrosis area was subtracted from vessel area and divided by the true area of the vessel. In total, 6-8 randomly selected samples per group were used for analysis. 3 representative vascular images were analyzed per sample. Medial hypertrophy of thoracic aorta was quantified by measurements of medial thickness in 4 randomly-selected locations per slide. 3 representative vascular images were analyzed per sample. Adventitia of the aorta was not quantified as the area was occasionally damaged or removed during the dissection.
For immunohistochemistry (IHC), serial cross-sections were deparaffinized and blocked in 5% goat serum and 1% BSA for 1h at room temperature, incubated with primary antibody in PBS containing 1% BSA and 0.1% Tween 20 for 18 h at 4 °C, followed by biotinylated secondary antibody for 90 min at room temperature. Slides were incubated with avidin–biotin peroxidase complex for 30 min at room temperature and staining was visualized with the substrate diaminobenzidine (Vector) producing a brown color and counterstained with haematoxylin. An equal concentration of control IgG was used side-by-side with each antibody to ensure staining specificity 19. Quantification of the antibody staining was performed as reported previously with subtraction of the IgG background staining 19. All images were visualized on Olympus SC30 high resolution camera and were acquired with Olympus cellSens Entry 1.11 software using the same exposure time. Images were loaded into the ImageJ program for analysis. A vascular region of interest was drawn around the coronary arteries with the freehand selection tool. Adventitia was excluded from the quantification, since the adventitial areas were quite limited in the arteries, except those with AngII infusion alone. All images were set to the same hue, saturation and brightness. The area and intensity (optical density) in the region of interest were then measured and analyzed. Data were obtained from 4 mice in each group with 3 to 4 non-overlapping high power fields for each antibody.
To evaluate ADAM17 mRNA expression, thoracic aortas and hearts were homogenized using BioMasher (Takara) and total RNA was extracted using TRIzol reagent (Invitrogen). cDNA was synthesized with RevertAid First Strand cDNA Synthesis Kit (Thermo). Quantitative real-time PCR (qPCR) was performed with SYBR Green qPCR Master Mix (Fermentas) as described previously 20. mRNA abundance was calculated by normalization to ribosome 18S. The primers used were ADAM17: Forward GGC GCG GGA GGG AGA AGT TT, Reverse CGC CGC CTC ATG TTC CCG TC, Ribosome 18S: Forward AGT TCC AGC ACA TTT TGC GAG, Reverse TCA TCC TCC GTG AGT TCT CCA.
Cell Culture and Experiments
VSMCs were prepared from thoracic aortas of male Sprague-Dawley rats by the explant method as described previously 21. VSMCs were subcultured in DMEM containing 10% fetal bovine serum, penicillin and streptomycin. Cells from passage 3 to 10 at 80~90% confluence were made quiescent by incubation with serum-free medium for 2-3 days. To avoid any potential phenotypic alteration, VSMCs were renewed every 2-3 months and VSMCs from frozen stock were never used. The results were confirmed in at least 2 distinct cell preparations.
Immunoblotting (IB) was performed as previously described 21. Quiescent VSMCs grown on 6-well plates were stimulated with 100 nM AngII (Sigma) for specified durations. The reaction was terminated by the replacement of medium with 100 μL of 1xSDS lysis buffer. 40 μL of the cell lysates were subjected to SDS-PAGE gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane. The membranes were then exposed to primary antibodies overnight at 4 °C. After incubation with the peroxidase linked secondary antibody for 1 h at room temperature, immunoreactive proteins were visualized using a chemiluminescence reaction kit.
To evaluate pro-fibrotic response, serum-starved VSMCs were stimulated with 100 nM AngII for 48 hours and extracellular cellular collagen content was quantified by Sirius Red collagen quantification kit (Chondrex) according to the manufacture's protocol. Recombinant adenoviral vector encoding rat ADAM17 siRNA was created and specificity and efficiency has been reported 11. VSMCs were infected with 100 m.o.i. adenovirus prior to the AngII stimulation as reported previously 11. ADAM17 selective inhibitor compound #21 (JG26) 8 was generated as reported. VSMCs were pretreated with 1 μmol/L JG26 or vehicle (0.1% DMSO in final) for 30 min prior to the AngII stimulation. 4-phenylbutyrate (PBA) was obtained from Scandinavian Formulas and solubilized in DMEM. VSMCs were pretreated with 10 mmol/L PBA for 30 min. Erlotinib (OSI Pharmaceuticals) was obtained from Genentech. VSMCs were pretreated with 1 μmol/L erlotinib for 30 min.
Antibodies
Antibodies against Tyr1068-phosphorylated EGFR for IHC (2234) and Ser51-phosphorylated eIF2α were purchased from Cell Signaling. Antibody against Tyr1068-phosphorylated EGFR for IB (44788G) was purchased from Invitrogen. Antibody against KDEL for detection of an ER stress marker GRP78 (ADI-SPA-827) was purchased from Enzo Life Sciences. Antibodies against ADAM17 for IB (sc-13973), EGFR (sc-03) and an ER stress marker, CHOP-10/GADD-153 (sc-575) were purchased from Santa Cruz Biotechnology. Antibodies against ADAM17 for IHC (ab39163) were purchased from Abcam. Antibodies against Cre recombinase (MAB3120) and GAPDH (MAB374) were purchased from Millipore.
Statistical Analysis
Data are presented as mean ± SEM or SD where appropriate. Differences between the multiple groups were analyzed by 1-way or 2-way ANOVA, followed by the Tukey's post hoc test. Statistical significance was set at p<0.05.
Results
Prevention of AngII-induced cardiovascular remodeling in mice lacking VSMC ADAM17
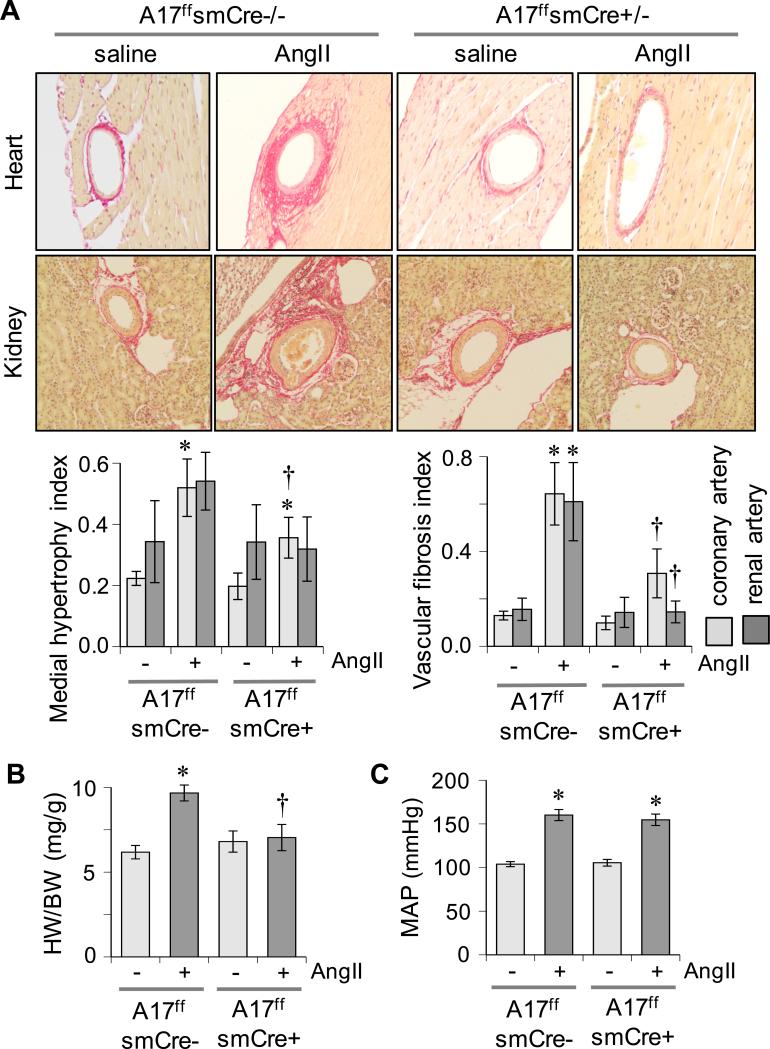
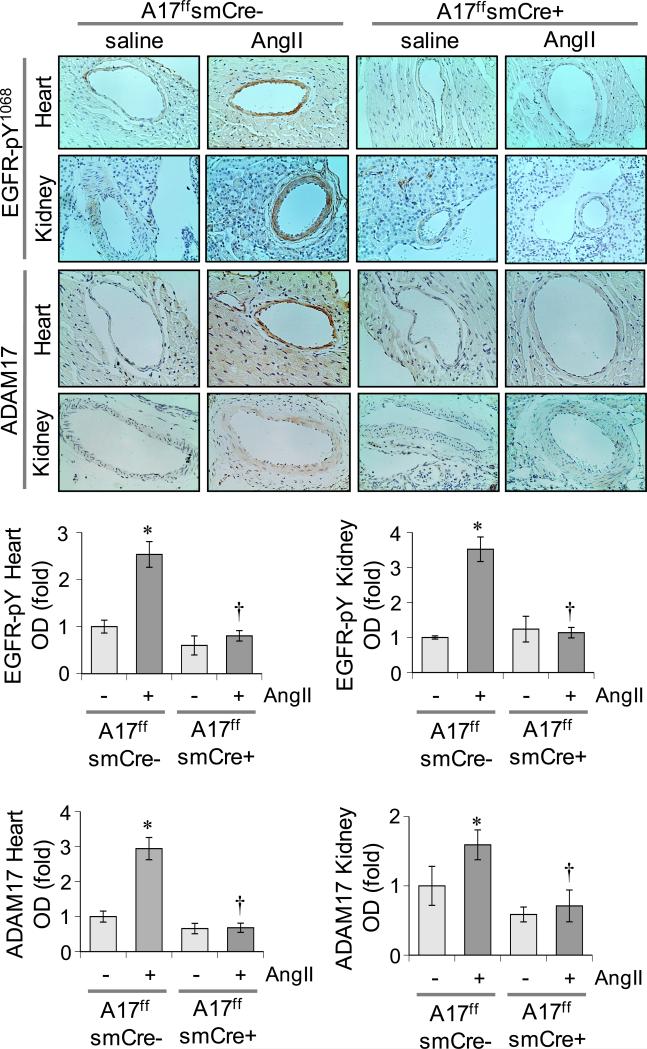
VSMC ADAM17 deficient ADAM17flox/flox sm22αCre+/− mice were generated 16 and vascular selective Cre expression was confirmed (Supplementary Figure S1A). VSMC ADAM17 deficient mice and littermate control ADAM17flox/flox sm22αCre−/− mice were infused with 1 μg/kg/min AngII for 2 weeks. In control littermate mice, 2 weeks of AngII infusion caused vascular hypertrophy in aorta and coronary arteries that was markedly prevented in VSMC ADAM17 deficient mice. Perivascular fibrosis induced by AngII infusion was also prevented in VSMC ADAM17 deficient mice (Figure 1A and Supplementary Figure S1B). In control mice, AngII infusion for 2 weeks induced cardiac hypertrophy assessed by heart weight to body weight ratio and echocardiogram (Figure 1B and Supplementary Table S1A). Serum B type natriuretic peptide and blood urea nitrogen concentrations were also elevated in these mice (Supplementary Figure S1C). These cardiac and renal alterations by AngII infusion were attenuated in VSMC ADAM17 deficient mice. In contrast, hypertension was induced in both groups infused with AngII at 2 weeks (Figure 1C and Supplementary Table S1B). AngII-induced vascular remodeling in control mice was associated with vascular-dominant EGFR activation and ER stress assessed by immunohistochemistry. These AngII responses were attenuated in VSMC ADAM17 deficient mice. ADAM17 expression was barely detectable in heart or kidney but was significantly induced upon AngII infusion in the vasculature. No such induction was observed in VSMC ADAM17 deficient mice (Figure 2 and Supplementary Figure S2). qPCR analysis of aortic mRNA confirmed ADAM17 induction by AngII as well as vascular ADAM17 silencing. ADAM17 mRNA was also increased in whole heart with AngII infusion in control mice but not in VSMC ADAM17 deficient mice (Supplementary Figure S3). In addition, while statistically insignificant, cardiac ADAM17 mRNA expression tends to be less in saline-infused VSMC ADAM17 deficient mice compared with saline-infused control mice.
Figure 1.
Prevention of cardiovascular remodeling in VSMC ADAM17 deficient mice. VSMC ADAM17 deficient mice (A17f/fsmCre+/−) and control littermate mice (A17f/fsmCre−/−) were infused with AngII or saline for 2 weeks. A: Tissues (heart and kidney) were stained with Sirius Red. Representative images are shown. Data are mean±SEM (n=6). B: Heart weight body weight ratio was calculated. Mean±SEM (n=8). C: Mean arterial blood pressure was evaluated by telemetry. Mean±SEM (n=8). *p<0.05 compared with saline control. †p<0.05 compared with AngII control.
Figure 2.
Suppression of vascular EGFR activation in VSMC ADAM17 deficient mice. VSMC ADAM17 deficient mice and control mice were infused with AngII or saline as in Fig 1. Tissues were immunostained with the antibodies indicated. Representative images are presented. Data are mean±SEM (n=4). *p<0.05 compared with saline control. †p<0.05 compared with AngII control.
A human cross-reactive ADAM17 antibody attenuates cardiovascular remodeling induced by AngII
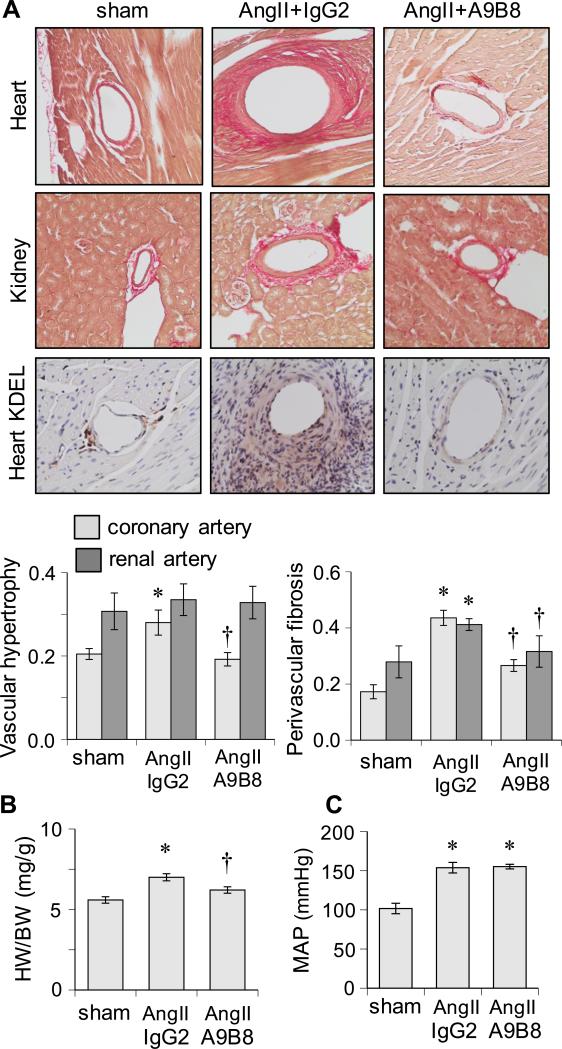
To ascertain that ADAM17 represents a novel therapeutic target contributing to target organ remodeling, AngII-infused C57Bl6 mice were treated with a human cross-reactive ADAM17 inhibitory antibody A9B8 18. C57Bl/6 mice were infused with 1 μg/kg/min AngII for 2 weeks with treatment of ADAM17 antibody or control IgG (10 mg/kg i.p. on day 1 and 7). A9B8 prevented AngII-induced cardiovascular hypertrophy and perivascular fibrosis but not hypertension or its development. These responses were associated with suppression of ER stress (Figure 3, Supplementary Figure S4 and Supplementary Table S2A and S2B).
Figure 3.
Effects of human cross-reactive ADAM17 inhibitory antibody, A9B8, on cardiovascular remodeling induced by AngII. A: C57Bl/6 mice were infused with AngII for 2 weeks with treatment of A9B8 or control human IgG2. Tissues were stained with Sirius Red (n=6) or the antibodies indicated (n=4). Representative staining images are presented. B: Heart weight body weight ratio was calculated. Mean±SEM (n=8). C: Mean arterial pressure was evaluated by telemetry (Mean±SEM, n=6). *p<0.05 compared with saline control. †p<0.05 compared with AngII control.
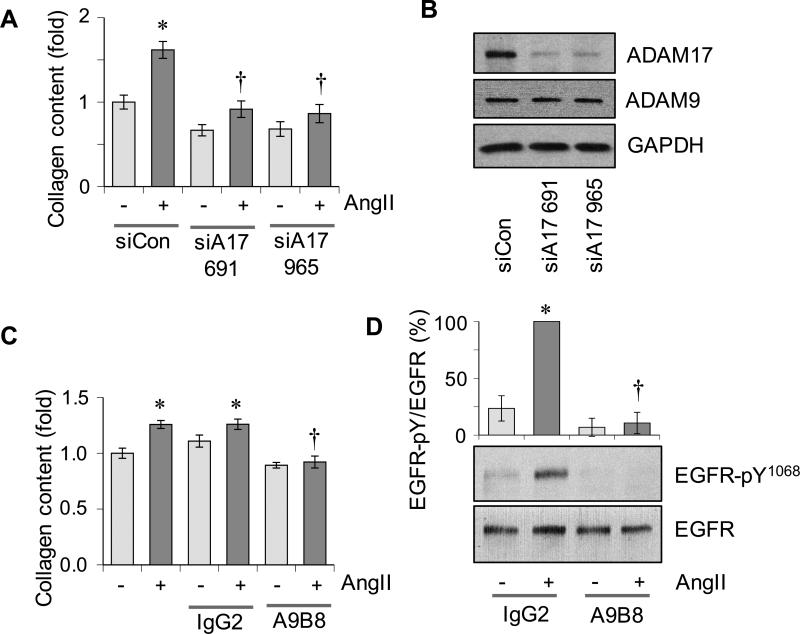
ADAM17 antibody, a small molecule ADAM17 inhibitor or adenovirus encoding ADAM17 siRNA attenuates a fibrotic response induced by AngII in vitro
To test for the role of ADAM17 in AngII-induced vascular fibrosis, extracellular collagen content was evaluated in VSMCs stimulated with AngII. Adenovirus encoding ADAM17 siRNA 11 attenuated the AngII-induced enhancement in collagen content (Figure 4A and 4B). Treatment of VSMCs with ADAM17 antibody A9B8, a highly-selective ADAM17 inhibitor JG26/compound 21 22, an EGFR kinase inhibitor erlotinib or a chemical ER chaperone PBA also attenuated AngII-induced enhancement in collagen content (Figure 4C and Supplementary Figure S5A-S5C). As shown with the ADAM17 siRNA 11, A9B8 as well as JG26 inhibited AngII-induced EGFR transactivation assessed with an auto-phosphorylation site antibody (Figure 4D and Supplementary Figure S5D).
Figure 4.
Effects of ADAM17 inhibition on pro-fibrosis response in VSMCs induced by AngII. A: Rat aortic VSMCs pretreated with adenovirus encoding ADAM17 siRNA or control non-silencing RNA were stimulated with 100 nmol/L AngII for 48 hours and extracellular collagen accumulation was quantified. B: ADAM17 expression was evaluated in VSMCs infected with adenoviruses encoding ADAM17 siRNAs or control non-silencing RNA for 48 hours. C: VSMCs pretreated with ADAM17 antibody A9B8 or control IgG (250 nmol/L) for 30 min were stimulated with 100 nmol/L AngII for 48 hours and extracellular collagen accumulation was quantified. Mean±SD (n=4). D: VSMCs pretreated with ADAM17 antibody A9B8 or control IgG (250 nmol/L) for 30 min were stimulated with 100 nmol/L AngII for 2 min and immunoblotting was performed with antibodies as indicated. Mean±SD (n=4). *p<0.05 compared with basal control. †p<0.05 compared with AngII control.
Discussion
The major finding of the present study is that AngII-induced cardiovascular hypertrophy and perivascular fibrosis but not hypertension were attenuated in mice lacking VSMC ADAM17 expression as well as in mice treated with an ADAM17 antibody. The suppression of AngII-induced EGFR activation and vascular hypertrophy in ADAM17 deficient mice is in line with our past in vitro observations that genetic ADAM17 inhibition or silencing prevents EGFR transactivation and subsequent hypertrophic responses in cultured VSMCs 9, 11. It is intriguing that VSMC silencing of ADAM17 or pharmacological inhibition of ADAM17 further prevented vascular fibrosis induced by AngII in vivo and in vitro. Faint expression of ADAM17 under normal conditions and enhanced expression in areas of interstitial fibrosis in damaged kidneys in humans has been reported 23. Additionally, cardiac specific HB-EGF transgenic mice develop cardiac fibrosis 24. AngII-induced renal interstitial fibrosis is attenuated in proximal tubule EGFR deficient mice 25. In our 2 week AngII infusion model, interstitial fibrosis in the heart or kidney was too marginal to be evaluated. However, it is likely that the paracrine production of HB-EGF and activation of EGFR via induction and activation of ADAM17 in VSMCs may be critical for development of perivascular fibrosis associated with hypertension 17, 26.
In the present study with AngII infused mice treated with an ADAM17 inhibitory antibody, hypertension was induced within a few days with blood pressure values comparable to the control mice. The values are also in agreement with reported values in C57Bl6 background mice with AngII infusion 27. While these data suggest that ADAM17 inhibition or silencing has no alteration of the development of hypertension in response to AngII, lack of continued blood pressure recording is a limitation of the present study. This study could also include an additionally important ADAM17wild/wild sm22Cre+/− control mouse group since endogenous lox-like sites are known to cause Cre-dependent chromosomal rearrangement in a Cre-transgenic mouse in the absence of loxP sequences 28. Although the protocol is different, control sm22αCre+/− mice develop hypertension, cardiac hypertrophy and vascular fibrosis in response to AngII infusion 29.
We have confirmed vascular dominant expression of the Cre transgene and intact presser responses to AngII in VSMC ADAM17 deficient mice or ADAM17 antibody-treated mice. However, contribution of VSMC ADAM17 to AngII-induced cardiac hypertrophy may require additional confirmation. Although lesser than smooth muscle, the sm22α promoter driver could show transgene expression in cardiac myocytes 30. There is a tendency of cardiac ADAM17 reduction in ADAM17flox/floxsm22αCre+/− mice, which could be due to a combination of VSMC and cardiac myocyte silencing in the heart according to literature 31. While the present study suggests that vascular-dominant ADAM17 and EGFR activation may mediate cardiac hypertrophy induced by AngII, cardiac myocyte-targeted expression of dominant-negative EGFR inhibits cardiac hypertrophy induced by AngII 32. Therefore, future experiments in cardiac myocyte specific ADAM17 deficient mice should be conducted to test the role of cardiac myocyte ADAM17 in cardiac hypertrophy induced by AngII.
ER stress has been implicated in cardiovascular diseases 33, whereas limited information is available for its role in hypertension 34. Our recent study suggests a potential prevention of hypertensive organ damage but not hypertension by PBA, which seems to involve VSMC ADAM17 as well as EGFR 17. Inhibition of the fibrotic response in VSMCs with ER stress inhibition further suggests the presence of ER stress-responsible downstream signal transduction leading to vascular fibrosis, which likely involves transcriptional up-regulation of several distinct genes 17, 20.
Perspectives
The vascular ADAM17/EGFR signal transduction axis appears to be essential for cardiovascular remodeling associated with ER stress but not for hypertension in mice with AngII infusion. The signal seems to include a feed forward mechanism involving vascular ADAM17 induction, which enhances EGFR ligand production and subsequent EGFR activation and vascular remodeling. While the intervention with ADAM17 antibody in mice appears promising, additional research in large animals and humans will be necessary to seek for an add-on treatment against hypertensive complications.
Supplementary Material
Novelty and Significance.
What is new?
Analysis of blood pressure and vascular pathology in the heart, kidney and aorta with VSMC ADAM17 deletion or ADAM17 inhibition by an antibody established a role for this metalloproteinase in AngII-induced pathological vascular remodeling independent of hypertension in mice.
The concept of the feed-forward induction of vascular ADAM17 to amplify the EGFR pathway and subsequent ER stress.
What is relevant?
Vascular restricted ADAM17 signal transduction highlights the importance of vascular pathology for subsequent tissue dysfunction in hypertension.
Results indicating prevention of vascular remodeling but not hypertension by human cross-reactive ADAM17 antibody provide a foundation to seek a potential add-on therapy to current pressure lowering treatments for hypertension.
Summary
In vascular smooth muscle ADAM17 deficient mice or mice treated with ADAM17 inhibitory antibody, vascular hypertrophy and perivascular fibrosis but not hypertension were prevented. AngII infusion showed vascular ADAM17 induction, EGFR activation and ER stress, which were attenuated in these mice. Cultured vascular smooth muscle cells were utilized to confirm the involvement of the ADAM17/EGFR signaling axis in induction of vascular fibrosis.
Acknowledgement
We thank Dr. Gillian Murphy for A9B8 preparation.
Sources of Funding: This work was supported by National Institute of Health grants, HL128324 (S.E. and V.R.), HL133248 (S.E.), F31HL127971 (S.J.F.) and DK09652 (R.S.), American Heart Association grants 13GRNT17060036 (S.E.), 16GRNT30410007 (S.E.), 16GRNT30130013 (V.R.), 16POST3051004 (T.K.), 15POST25550083 (K.J.E.), University of Macau Research grants, SRG201400006FHS and MYRG201500025FHS (HF.K.), Italian Ministry of Education grants, 2007JERJPC_005 (A.R.) and # 20109MXHMR_007 (E.N.) and University of Pisa, 2014-03Area (E.N. and A.R.).
Footnotes
Disclosures: None.
References
- 1.Egan BM, Li J, Hutchison FN, Ferdinand KC. Hypertension in the United States, 1999 to 2012: progress toward healthy people 2020 goals. Circulation. 2014;130:1692–1699. doi: 10.1161/CIRCULATIONAHA.114.010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Struthers AD, George J. High B-Type Natriuretic Peptide Hypertensives at Target Blood Pressure: Potential Role of beta-Blockers to Reduce Their Elevated Risk. Hypertension. 2015;66:927–932. doi: 10.1161/HYPERTENSIONAHA.115.06270. [DOI] [PubMed] [Google Scholar]
- 3.Briet M, Schiffrin EL. Treatment of arterial remodeling in essential hypertension. Curr Hypertens Rep. 2013;15:3–9. doi: 10.1007/s11906-012-0325-0. [DOI] [PubMed] [Google Scholar]
- 4.Weir MR. Effects of renin-angiotensin system inhibition on end-organ protection: can we do better? Clin Ther. 2007;29:1803–1824. doi: 10.1016/j.clinthera.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM. Angiotensin II and vascular injury. Curr Hypertens Rep. 2014;16:431. doi: 10.1007/s11906-014-0431-2. [DOI] [PubMed] [Google Scholar]
- 6.Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 7.Kanaide H, Ichiki T, Nishimura J, Hirano K. Cellular mechanism of vasoconstriction induced by angiotensin II: it remains to be determined. Circ Res. 2003;93:1015–1017. doi: 10.1161/01.RES.0000105920.33926.60. [DOI] [PubMed] [Google Scholar]
- 8.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133–137. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsu H, Higuchi S, Shirai H, Eguchi K, Suzuki H, Hinoki A, Brailoiu E, Eckhart AD, Frank GD, Eguchi S. Central role of Gq in the hypertrophic signal transduction of angiotensin II in vascular smooth muscle cells. Endocrinology. 2008;149:3569–3575. doi: 10.1210/en.2007-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott KJ, Bourne AM, Takayanagi T, Takaguri A, Kobayashi T, Eguchi K, Eguchi S. ADAM17 silencing by adenovirus encoding miRNA-embedded siRNA revealed essential signal transduction by angiotensin II in vascular smooth muscle cells. J Mol Cell Cardiol. 2013;62:1–7. doi: 10.1016/j.yjmcc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takaguri A, Kimura K, Hinoki A, Bourne AM, Autieri MV, Eguchi S. A disintegrin and metalloprotease 17 mediates neointimal hyperplasia in vasculature. Hypertension. 2011;57:841–845. doi: 10.1161/HYPERTENSIONAHA.110.166892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canault M, Peiretti F, Kopp F, Bonardo B, Bonzi MF, Coudeyre JC, Alessi MC, Juhan-Vague I, Nalbone G. The TNF alpha converting enzyme (TACE/ADAM17) is expressed in the atherosclerotic lesions of apolipoprotein E-deficient mice: possible contribution to elevated plasma levels of soluble TNF alpha receptors. Atherosclerosis. 2006;187:82–91. doi: 10.1016/j.atherosclerosis.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ, Oudit GY. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: A positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Morange PE, Tregouet DA, Godefroy T, Saut N, Bickel C, Rupprecht HJ, Lackner K, Barbaux S, Poirier O, Peiretti F, Nalbone G, Juhan-Vague I, Blankenberg S, Tiret L. Polymorphisms of the tumor necrosis factor-alpha (TNF) and the TNF-alpha converting enzyme (TACE/ADAM17) genes in relation to cardiovascular mortality: the AtheroGene study. J Mol Med (Berl) 2008;86:1153–1161. doi: 10.1007/s00109-008-0375-6. [DOI] [PubMed] [Google Scholar]
- 16.Weskamp G, Mendelson K, Swendeman S, Le Gall S, Ma Y, Lyman S, Hinoki A, Eguchi S, Guaiquil V, Horiuchi K, Blobel CP. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res. 2010;106:932–940. doi: 10.1161/CIRCRESAHA.109.207415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takayanagi T, Kawai T, Forrester SJ, Obama T, Tsuji T, Fukuda Y, Elliott KJ, Tilley DG, Davisson RL, Park JY, Eguchi S. Role of Epidermal Growth Factor Receptor and Endoplasmic Reticulum Stress in Vascular Remodeling Induced by Angiotensin II. Hypertension. 2015;65:1349–1355. doi: 10.1161/HYPERTENSIONAHA.115.05344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwok HF, Botkjaer KA, Tape CJ, Huang Y, McCafferty J, Murphy G. Development of a 'mouse and human cross-reactive' affinity-matured exosite inhibitory human antibody specific to TACE (ADAM17) for cancer immunotherapy. Protein Eng Des Sel. 2014;27:179–190. doi: 10.1093/protein/gzu010. [DOI] [PubMed] [Google Scholar]
- 19.Takayanagi T, Crawford KJ, Kobayashi T, Obama T, Tsuji T, Elliott KJ, Hashimoto T, Rizzo V, Eguchi S. Caveolin-1 is critical for abdominal aortic aneurysm formation induced by angiotensin II and inhibition of lysyl oxidase. Clin Sci. 2014;126:785–794. doi: 10.1042/CS20130660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obama T, Tsuji T, Kobayashi T, Fukuda Y, Takayanagi T, Taro Y, Kawai T, Forrester SJ, Elliott KJ, Choi E, Daugherty A, Rizzo V, Eguchi S. Epidermal growth factor receptor inhibitor protects against abdominal aortic aneurysm in a mouse model. Clin Sci (Lond) 2015;128:559–565. doi: 10.1042/CS20140696. [DOI] [PubMed] [Google Scholar]
- 21.Takaguri A, Shirai H, Kimura K, Hinoki A, Eguchi K, Carlile-Klusacek M, Yang B, Rizzo V, Eguchi S. Caveolin-1 negatively regulates a metalloprotease-dependent epidermal growth factor receptor transactivation by angiotensin II. J Mol Cell Cardiol. 2011;50:545–551. doi: 10.1016/j.yjmcc.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuti E, Casalini F, Santamaria S, Fabbi M, Carbotti G, Ferrini S, Marinelli L, La Pietra V, Novellino E, Camodeca C, Orlandini E, Nencetti S, Rossello A. Selective arylsulfonamide inhibitors of ADAM-17: hit optimization and activity in ovarian cancer cell models. J Med Chem. 2013;56:8089–8103. doi: 10.1021/jm4011753. [DOI] [PubMed] [Google Scholar]
- 23.Melenhorst WB, Visser L, Timmer A, van den Heuvel MC, Stegeman CA, van Goor H. ADAM17 upregulation in human renal disease: a role in modulating TGF-alpha availability? Am J Physiol Renal Physiol. 2009;297:F781–790. doi: 10.1152/ajprenal.90610.2008. [DOI] [PubMed] [Google Scholar]
- 24.Lian H, Ma Y, Feng J, Dong W, Yang Q, Lu D, Zhang L. Heparin-binding EGF-like growth factor induces heart interstitial fibrosis via an Akt/mTor/p70s6k pathway. PLoS One. 2012;7:e44946. doi: 10.1371/journal.pone.0044946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol. 2012;23:215–224. doi: 10.1681/ASN.2011070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forrester SJ, Kawai T, O'Brien S, Thomas W, Harris RC, Eguchi S. Epidermal Growth Factor Receptor Transactivation: Mechanisms, Pathophysiology, and Potential Therapies in the Cardiovascular System. Annu Rev Pharmacol Toxicol. 2016;56:627–653. doi: 10.1146/annurev-pharmtox-070115-095427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P, Wilcox CS. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension. 2006;48:934–941. doi: 10.1161/01.HYP.0000242928.57344.92. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt EE, Taylor DS, Prigge JR, Barnett S, Capecchi MR. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc Natl Acad Sci U S A. 2000;97:13702–13707. doi: 10.1073/pnas.240471297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imanishi M, Tomita S, Ishizawa K, Kihira Y, Ueno M, Izawa-Ishizawa Y, Ikeda Y, Yamano N, Tsuchiya K, Tamaki T. Smooth muscle cell-specific Hif-1alpha deficiency suppresses angiotensin II-induced vascular remodelling in mice. Cardiovasc Res. 2014;102:460–468. doi: 10.1093/cvr/cvu061. [DOI] [PubMed] [Google Scholar]
- 30.Lepore JJ, Cheng L, Min Lu M, Mericko PA, Morrisey EE, Parmacek MS. High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22alpha-Cre transgenic mice. Genesis. 2005;41:179–184. doi: 10.1002/gene.20112. [DOI] [PubMed] [Google Scholar]
- 31.Schreier B, Rabe S, Schneider B, Bretschneider M, Rupp S, Ruhs S, Neumann J, Rueckschloss U, Sibilia M, Gotthardt M, Grossmann C, Gekle M. Loss of epidermal growth factor receptor in vascular smooth muscle cells and cardiomyocytes causes arterial hypotension and cardiac hypertrophy. Hypertension. 2013;61:333–340. doi: 10.1161/HYPERTENSIONAHA.112.196543. [DOI] [PubMed] [Google Scholar]
- 32.Zhai P, Galeotti J, Liu J, Holle E, Yu X, Wagner T, Sadoshima J. An angiotensin II type 1 receptor mutant lacking epidermal growth factor receptor transactivation does not induce angiotensin II-mediated cardiac hypertrophy. Circ Res. 2006;99:528–536. doi: 10.1161/01.RES.0000240147.49390.61. [DOI] [PubMed] [Google Scholar]
- 33.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitler KM, Webb RC. Endoplasmic Reticulum Stress Contributes to Aortic Stiffening via Proapoptotic and Fibrotic Signaling Mechanisms. Hypertension. 2013;63:e40–e45. doi: 10.1161/HYPERTENSIONAHA.113.02558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.