Abstract
Objective
To find out whether maternal serum screening for fetal chromosomal aneuploidy predicts adverse pregnancy outcomes.
Methods
A two-year retrospective case–control study was conducted at a tertiary hospital. Pregnant women with a high-risk serum screen but with chromosomally normal fetuses (n = 189) were compared to those with low-risk screen (controls, n = 157) for adverse pregnancy outcomes.
Results
Women with high-risk double marker or combined screen were found to have higher prevalence of LBW [OR 2.56; 95 % CI (1.01–6.53), p < 0.05] and PT [OR 2.93; 95 % CI (1.11–7.65), p < 0.05], while women with high-risk triple screen had higher prevalence of PIH [OR 3.72; 95 % CI (1.23–11.18); p < 0.05], Oligo [OR 4.50; 95 % CI (1.30–15.64); p < 0.05], delivery by C-section [OR 2.51; 95 % CI (1.41–4.47); p < 0.005] as compared to low-risk women. PAPP-A was found to be a significant predictor of birth weight (R2 = 12.2 %, β ± SE = 0.224 ± 0.069; p < 0.005) and gestational age (R2 = 4.9 %, β ± SE = 0.613 ± 0.296; p < 0.05). Beta hCG in first and hCG in second trimester predicted oligohydramnios (R2 = 9.2 %, β ± SE = −0.077 ± 0.025; p < 0.005). The areas under the ROC curves of PAPP-A for LBW and PT were 0.70(p < 0.01) and 0.684 (p < 0.05), respectively.
Conclusion
A “high-risk” maternal serum screen with abnormal PAPP-A and/or beta hCG/HCG is associated with adverse pregnancy outcomes and may help identifying women requiring additional fetal surveillance.
Keywords: Maternal serum, PAPP-A, Beta hCG, Adverse pregnancy outcome
Introduction
Specific patterns of maternal serum pregnancy-associated plasma protein A (PAPP-A), beta unit of human chorionic gonadotropin (hCGb) in the first and hCG, alpha-fetoprotein (AFP) and unconjugated estriol (uE3) concentrations in the second trimester suggest an elevated risk of fetal aneuploidy. However, diagnosis of a normal fetal karyotype as a confirmatory test creates a management dilemma for obstetricians and doubts about fetal well-being in “high-risk” women.
We conducted a retrospective analysis to study whether maternal serum screen “high-risk” status and/or abnormal individual serum marker were associated with adverse pregnancy outcomes and whether they can be used to predict such outcomes in women with chromosomally normal fetuses.
Aim
To study whether maternal serum markers tested as a part of aneuploidy screening additionally help in predicting other pregnancy outcomes including low birth weight (LBW), prematurity (PT), pregnancy-induced hypertension (PIH), oligohydramnios (Oligo), operative delivery (C-section) and NICU admission.
Objectives
Whether a high-risk biochemical screen predicts adverse pregnancy outcomes.
Whether individual marker tested: PAPP-A, beta hCG in the first-trimester screens and AFP, hCG and uE3 in the second-trimester screens predicts adverse pregnancy outcomes.
Methods
Setting
Tertiary care private hospital with Fetal Medicine and Genetics units.
Type of study
Retrospective case–control study over 2 years.
Participants
Cases: pregnant women with a “high-risk” maternal serum screen but chromosomally normal fetuses (n = 189).
Controls: pregnant women with a “low-risk” maternal serum screen (n = 157).
Work flow
Out of the 422 samples (either chorine villous or amniotic fluid) received for karyotyping during the two-year study period for “high-risk” maternal serum screen, 21 samples with chromosomal anomalies were excluded.
Out of the remaining 401 women with high risk screen but normal fetal karyotype, 189 (53 with double and 136 with triple marker) whose details of serum screens and pregnancy outcomes could be traced were included as cases.
We enrolled 157 women who were maternal serum screen “low risk” and delivered at our hospital during the study period as controls (80 with double or combined and 77 with triple screen).
Data regarding individual marker and complete screen result, maternal age, parity and pregnancy outcomes including gestational age, birth weight (or in some cases LBW: birth weight <2.5 kg and PT: gestational age <37 weeks, respectively), PIH requiring treatment, oligohydramnios, C-section and NICU admission were recorded.
The concentrations of serum markers were considered as multiples of median (MoM), and standard cutoffs were used as follows:
First trimester: PAPP-A (<0.4 MoM) and beta hCG (<0.5 MoM)
Second trimester: AFP (<0.25 or >2.5 MoM), hCG (>3.0 MoM), uE3 (<0.5 MoM)
Statistical Analysis
Statistical analysis was carried out with the help of SPSS (version 20) for Windows package (SPSS Science, Chicago, IL, USA). The description of the data is done in the form of mean ± SD for quantitative data, while in the form of % proportion for qualitative (categorical) data. P values of <0.05 are considered significant. For quantitative data, unpaired Student’s t test was used to test statistical significance of difference between two independent group means. For comparison of categorical variables (i.e., to examine the associations between qualitative/quantitative variables), Chi-square test was used if the number of elements in each cell was 5 or higher and Fisher’s exact test, otherwise. To compare proportions between two independent groups, Z test of proportions was used. Correlation between quantitative variables was examined by calculating Pearson’s correlation coefficient. Using ROC procedure, appropriate cutoff (threshold) of the test variable was estimated for optimum sensitivity and specificity. AUC from ROC indicates power of the test. Regression analysis was carried out to find out various determining factors of the variable of interest.
This study was approved by the institutional ethics committee.
Results
The mean maternal ages of cases and controls were 31.5 ± 4.7 and 29.1 ± 3.9 years, and the difference was significant (p < 0.001). Hence, statistical analysis was done after adjusting for maternal age. Parity of women in both groups was comparable (1.86 ± 0.88 and 2.0 ± 0.98, p > 0.05).
Association of a High-Risk Double/Combined Screen/First-Trimester Screen and Adverse Pregnancy Outcomes
Cases were found to have higher prevalence of LBW [OR 2.56; 95 % CI (1.01–6.53), p < 0.05] and PT [OR 2.93; 95 % CI (1.11–7.65), p < 0.05] compared to controls. No such association was found between a high-risk screen and other outcomes such as PIH, Oligo, C-section or NICU admission (Table 1).
Table 1.
Adverse maternal and neonatal outcomes associated with first- and second-trimester maternal screens
| Outcome | First trimester (double/combined) screen | Second trimester (triple) screen | ||||||
|---|---|---|---|---|---|---|---|---|
| Case (%) | Control (%) | OR (95 % CI) | p | Case (%) | Control (%) | OR (95 % CI) | p | |
| Preterm | 13/53 (24.5) | 8/80 (10.0) | 2.93 (1.11–7.65) | 0.02 | 24/136 (17.6) | 12/77 (15.6) | 1.46 (0.64–3.35) | Ns |
| LBW | 13/53 (24.5) | 9/80 (11.3) | 2.56 (1.01–6.53) | 0.04 | 22/131 (16.8) | 7/77 (9.1) | 2.08 (0.82–4.97) | Ns |
| Oligo | 5/53 (9.4) | 4/80 (5.1) | 1.98 (0.51–7.74) | Ns | 21/136 (15.4) | 3/77 (3.8) | 4.50 (1.30–15.64) | 0.01 |
| C-section | 33/53 (62.3) | 45/80 (56.3) | 1.28 (0.63–2.61) | Ns | 95/136 (69.9) | 37/77 (48.1) | 2.51 (1.41–4.47) | 0.002 |
| PIH | 6/53 (11.3) | 8/80 (10.3) | 1.15 (0.38–3.52) | Ns | 23/136 (16.9) | 4/77 (5.2) | 3.72 (1.23–11.18) | 0.01 |
| NICU | 9/53 (17.0) | 16/80 (20.5) | 0.82 (0.33–2.02) | Ns | 19/130 (14.6) | 8/77 (10.4) | 1.48 (0.61–3.56) | Ns |
| Resp distress | 3/53 (5.7) | 5/80 (6.3) | 0.90 (0.21–3.94) | Ns | 4/136 (2.9) | 4/77 (5.2) | 0.55 (0.13–2.28) | Ns |
PAPP-A in the first trimester showed a significant positive correlation with birth weight (r = +0.286, p < 0.01), even after adjusting for PIH, indicating that higher values of PAPP-A are associated with higher values birth weight. First trimester beta hCG showed a significant negative correlation with birth weight and oligohydramnios (r = −0.233, p < 0.05 and r = −0.304, p < 0.01, respectively) suggesting that higher beta hCG levels are associated with lower birth weight and lower levels with oligohydramnios (Table 2).
Table 2.
Correlations between adverse outcomes and biochemical markers in first- and second-trimester screens
| First trimester | Second trimester | ||||
|---|---|---|---|---|---|
| PAPP-A | hCGb | AFP | HCG | uE3 | |
| OLIGO | .078 | −.304** | .148 | −.281** | .042 |
| C-section | .017 | −.029 | .086 | −.090 | .029 |
| PIH | −.021 | −.060 | .138 | −.225** | .035 |
| NICU | −.066 | −.010 | .045 | −.062 | .063 |
| Gestation | .194 | −.134 | −.046 | −.141 | .095 |
| Birth weight | .297** | −.241* | −.133 | −.034 | .093 |
| Gestationa | .193 | −.134 | −.028 | −.096 | .118 |
| Birth weighta | .286** | −.233* | −.118 | −.137 | .060 |
* p < 0.05; ** p < 0.01
aAfter controlling for age and PIH
Association of a High-Risk Triple/Second-Trimester Maternal Screen and Adverse Pregnancy Outcomes
Table 1 reveals that although cases did not show any association with LBW or PT, they had higher prevalence of PIH [OR 3.72; 95 % CI (1.23–11.18); p < 0.05], Oligo [OR 4.50; 95 % CI (1.30–15.64); p < 0.05], delivery by C-section [OR 2.51; 95 % CI (1.41–4.47); p < 0.005] as compared to controls.
Among the second-trimester markers estimated as a part of triple test, only maternal serum HCG was significantly associated and showed a negative correlation after adjusting for age with PIH (r = −0.227; p > 0.01) and Oligo (r = −0.281; p < 0.01), indicating that low maternal hCG is associated with PIH and oligohydramnios. None of the maternal serum AFP or UE3 showed any such correlation with any of the outcomes considered (Table 2).
Association of Individual Serum Markers with Adverse Pregnancy Outcomes
Univariate and multivariate regression analysis considering birth weight and gestation as dependent variables and biochemical parameters in first and second trimester as independent variables (Table 3) showed that PAPP-A in first trimester is the only significant predictor of gestation (R2 = 4.9 %, β ± SE = 0.613 ± 0.296; p < 0.05). In case of birth weight, although it is determined by PAPP-A as well as hCGb in first trimester using univariate regression analysis, PAPP-A remained to be the only significant predictor of birth weight (R2 = 12.2 %, β ± SE = 0.224 ± 0.069; p < 0.005) after multivariate regression analysis. Not a single biochemical parameter in second trimester was able to predict birth weight and gestation.
Table 3.
Univariate and multivariate regression analysis for birth weight and gestation
| Indep | R 2 (%) | β | SE | p | R 2 (%) | β | SE | p | |
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Dep.: birthweight | Dep.: gestation | |||||||
| First trimester | PAPP-A-MOM | 9.3 | .231 | .070 | 0.001 | 4.9 | .613 | .296 | 0.041 |
| hCGb-MOM | 3.4 | −.088 | .046 | 0.050 | 0.8 | −.227 | .279 | 0.417 | |
| Multivariate | Dep.: Birthweight | – | |||||||
| First trimester | PAPP-A-MOM | 12.2 | .224 | .069 | .002 | – | – | – | – |
| hCGb-MOM | −.081 | .044 | .066 | – | – | – | – | ||
| Univariate | Dep.: birthweight | Dep.: gestation | |||||||
| Second trimester | AFP-MoM | 0.7 | −.106 | .122 | 0.388 | 1.9 | −.750 | .579 | 0.199 |
| hCG-MoM | 0.1 | −.012 | .045 | 0.791 | 0.1 | .059 | .211 | 0.789 | |
| uE3-MoM | 1.7 | .095 | .068 | 0.167 | 0 | .036 | .459 | 0.938 | |
Univariate as well as multivariate regression analysis was carried out after considering PIH, oligohydramnios and C-section delivery as dependent variables and biochemical markers in first and second trimester as independent variables (Table 4). It was observed that only hCGb in first trimester and hCG in the second trimester were significant predictors of oligohydramnios (R2 = 13.6 %, β ± SE = −0.088 ± 0.021; p < 0.001 R2 = 6.7 %, β ± SE = −0.074 ± 0.024; p < 0.005, respectively). Univariate analysis also revealed that second-trimester hCG is a significant determinant of PIH (R2 = 6.5 %, β ± SE = −0.063 ± 0.024; p < 0.05). None of the biochemical parameters in first and second trimester was able to predict C-section delivery.
Table 4.
Univariate and multivariate regression analysis for PIH, OLIGO and C-section delivery
| Indep | R 2 (%) | β | SE | p | R 2 (%) | β | SE | p | R 2 (%) | β | SE | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Dep.: PIH | Dep.: OLIGO | Dep.: C-SECTION | ||||||||||
| First trimester | PAPP-A-MOM | 0.1 | .009 | .044 | 0.836 | 0.2 | .007 | .036 | 0.850 | 2.4 | .032 | .069 | 0.637 |
| hCGb-MOM | 1.5 | −.035 | .028 | 0.207 | 13.6 | −.088 | .021 | 0.000 | 2.4 | −.022 | .043 | 0.605 | |
| NT-MoM | 0.4 | −.029 | .111 | 0.792 | 0.9 | .071 | .084 | 0.403 | 4.7 | .301 | .170 | 0.080 | |
| Univariate | Dep.: PIH | Dep.: OLIGO | Dep.: C-SECTION | ||||||||||
| Second trimester | AFP-MoM | 1.7 | .002 | .065 | 0.977 | 2.8 | .130 | .065 | 0.048 | 9.2 | .079 | .108 | 0.464 |
| hCG-MoM | 6.5 | −.063 | .024 | 0.009 | 6.7 | −.074 | .024 | 0.002 | 10.8 | −.068 | .040 | 0.088 | |
| uE3-MoM | 2.2 | −.031 | .036 | 0.393 | 0.1 | −.008 | .036 | 0.819 | 8.9 | −.018 | .059 | 0.076 | |
| Multivariate | Dep.: PIH | Dep.: OLIGO | Dep.: C-SECTION | ||||||||||
| Second trimester | AFP-MoM | – | – | – | – | 9.2 | .113 | .064 | .077 | – | – | – | – |
| hCG-MoM | – | – | – | −.077 | .025 | .002 | – | – | – | ||||
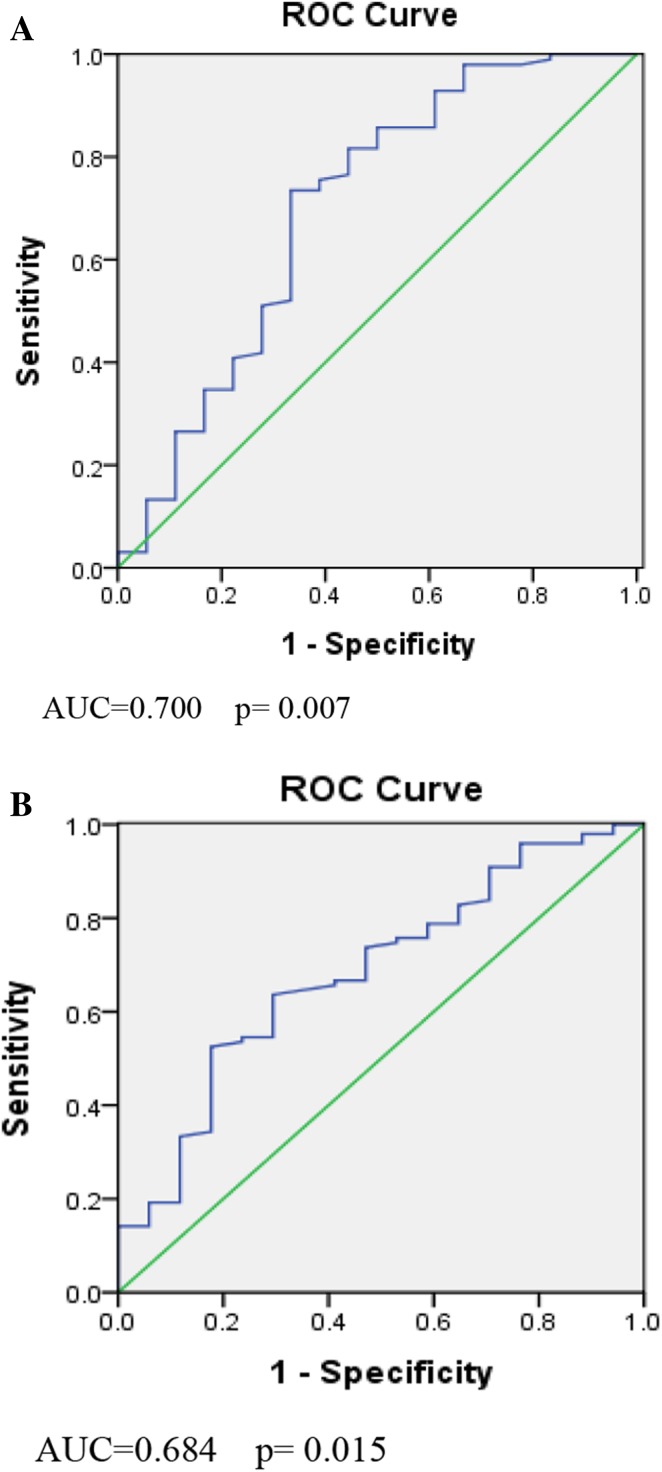
Finally, receiver operating characteristic (ROC) curve analysis was carried out to measure discriminative power of individual markers as a screening test for adverse outcomes. The areas under the ROC curves (AUC) of PAPP-A for LBW (Fig. 1a) and PT (Fig. 1b) were 0.70, p < 0.05 and 0.68, p < 0.05, respectively, indicating PAPP-A as a good marker for screening women at risk of LBW and PT.
Fig. 1.
a ROC curve for LBW using PAPP-A as test variable. AUC = 0.700, p = 0.007. b ROC curve for preterm using PAPP-A as test variable. AUC = 0.684, p = 0.015
The conventional cutoff for PAPP-A (<0.4) was associated with a very low sensitivity of 33.3 % but a high specificity of 94.9 % for LBW (data not shown). The optimum “cutoff” for PAPP-A MoM for LBW from this study was higher (<0.8) and provided 66.7 % sensitivity and 67.3 % specificity. This cutoff also had a sensitivity of 64.5 % and specificity of 64.6 % to predict PT, none very impressive as a screening test.
It was noted that for both first and second trimesters, a high-risk screen with an abnormal marker, especially PAPP-A in the first and hCG in the second trimester, best predicted adverse outcomes compared to an isolated abnormal marker but low-risk screen or a high-risk screen with normal individual markers (Table 5). This was evident from significant (p < 0.000) higher prevalence of LBW among women with both high-risk screen and abnormal marker as compared to that among women with either isolated abnormal marker or high risk screen alone.
Table 5.
Risk of adverse outcome (LBW or preterm) considering screen as well as biochemical markers in first and second trimester
| Total screen | Marker | First trimester | Second trimester | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAPP-A | Beta hCG | AFP | HCG | uE3 | ||||||||||||
| N | LBW (%) | Preterm (%) | N | LBW (%) | Preterm (%) | N | LBW (%) | Preterm (%) | N | LBW (%) | Preterm (%) | N | LBW (%) | Preterm (%) | ||
| High risk | Normal | 28 | 3 (10.7) | 5 (17.9) | 35 | 9 (25.7) | 8 (22.9) | 64 | 11 (17.2) | 11 (17.2) | 52 | 8 (15.4) | 7 (13.5) | 23 | 4 (17.4) | 3 (13.0) |
| High risk | Abnormal | 8 | 6 (75.0) | 4 (50.0) | 1 | 0 (0) | 1 (100) | 0 | – | – | 12 | 3 (25.0) | 4 (33.3) | 4 | 1 (25.0) | 1 (25.0) |
| Low risk | Normal | 3 | 0 (0) | 0 (0) | 4 | 1 (25.0) | 1 (25.0) | 0 | – | – | 0 | – | 41 | 7 (17.1) | 8 (19.5) | |
| Low risk | Abnormal | 77 | 9 (11.7) | 8 (10.4) | 76 | 8 (10.5) | 7 (9.2) | 77 | 7 (9.1) | 12 (15.6) | 77 | 7 (9.1) | 12 (15.6) | 73 | 6 (8.2) | 11 (15.1) |
| Total | 116 | 18 | 17 | 116 | 18 | 17 | 141 | 18 | 23 | 141 | 18 | 23 | 141 | 18 | 23 | |
Discussion
The data from this study suggest an association between a “high-risk” maternal serum screen and adverse pregnancy outcomes. PAPP-A was found to be a significant predictor of birth weight and gestational age, while second-trimester hCG predicted oligohydramnios and PIH.
Since PAPP-A and hCG contribute to trophoblast invasion and angiogenesis of uterine vasculature and uterine growth, respectively [1], there is a possibility of these two markers being interdependent and mechanistically associated with PIH and LBW due to failure of trophoblast invasion into the spiral arteries [2]. However, we found PAPP-A to be an independent and better predictor of LBW and PT than hCG, even in pregnant women without PIH. Dane et al. [3] similarly reported PAPP-A to be a predictor of PT delivery in normotensive pregnancies. A significant inverse association between maternal serum PAAP-A and the risk of LBW but not with free beta hCG was reported by others [4–6].
Although a high-risk triple screen in this study was associated with PIH, Oligo and operative delivery, none of the individual markers including AFP, HCG and UE3 showed any association with any of the adverse pregnancy/neonatal outcomes unlike earlier reports by others [7, 8].
The association between maternal serum analytes and abnormal pregnancy/neonatal outcomes is controversial [2, 4, 9, 10]. The use of maternal serum screening solely to predict and prevent these outcomes has not been supported due to overall low sensitivity and high false-positive rates [11, 12]. D’Antonio et al. [13] reported that maternal serum PAPP-A performs poorly as a screening test for PT, SGA and preeclampsia. The ROC analysis showed that the AUC under the ROC curve in this analysis for PAPP-A to predict LBW and PT was 0.7 and 0.68, respectively, indicating that it has a “fair” predictive accuracy [14]. Thus, our study also suggests that PAPP-A may not be used in isolation as a clinical marker to predict LBW/PT.
It was interesting to note that sensitivity of PAPP-A MoM to predict LBW doubled when a cutoff of 0.8 was used instead of the conventional cutoff of 0.4, though it reduced its specificity. Ardawi et al. [15] reported that MoM values of free beta hCG vary and are higher in Africans, Orientals and Arabian women compared to Asians. Wright et al. [16] commented that biases in the serum marker MoM levels can alter false-positive rates of double screen and in turn may alter overall performance of the screen if a fixed risk cutoff is used. However, the use of PAPP-A cutoff of below 0.3 at an earlier gestation did not improve the detection rate for adverse pregnancy outcomes as reported by Quattrocchi et al. [17]. It might be worth looking at optimal cutoffs, on a larger sample if these markers are to be considered as predictors of LBW/PT.
We also found that a high risk screen with an abnormal marker, rather than a low-risk screen with an abnormal individual marker best predicted LBW and might imply that an isolated abnormal marker need not be specially followed up.
There are few guidelines about follow-up of women with high-risk screen-normal fetal karyotype, due to lack of consensus about management strategies. Sharp et al. [18] and Halscot et al. [19] comment that early diagnosis of conditions such as PT, LBW does not equate to better maternal/neonatal outcomes due to limited treatment modalities. The suggested measures include additional clinical monitoring, uterine artery Doppler studies and use of low-dose aspirin with inconsistent results. Roberge [20] reported early (<16 weeks) administration of low-dose aspirin to be associated with a greater reduction of perinatal death and other adverse perinatal outcomes than when initiated >16 weeks. Villa et al. [21] conducted a meta-analysis of randomized controlled trials including data on 346 women which suggested that aspirin may reduce the incidence of preeclampsia. Moore et al. [22] reported that aspirin initiated before 17 weeks reduced the risk for late-onset preeclampsia by 29 % supporting the practice of early initiation of aspirin in high-risk women. Prophylaxis with low-dose aspirin (60–150 mg) beginning after the first trimester of pregnancy was found to reduce the risk of preeclampsia and modestly reduce risks of preterm birth, IUGR and PIH [23]. There was a limited evidence of harms associated with low-dose aspirin use during pregnancy, mainly risk of abruption, but no evidence for complications such as postpartum hemorrhage, maternal blood loss and neonatal intracranial or intraventricular bleeding was found. Ayala et al. [24] concluded that low dose aspirin regulated ambulatory BP and reduced the incidence of preeclampsia, gestational hypertension, preterm delivery and IUGR.
In spite of significant correlations between high-risk maternal screen and/or abnormal PAPP-A and adverse pregnancy outcome evident in this and other studies, low sensitivity and limited options to improve birth weight and gestational age make it difficult to routinely prescribe low-dose aspirin to all women with high-risk maternal screen and/or abnormal PAPP-A. Huynch et al. [25] recommend that pregnant patients with high-risk screen-normal fetal karyotype should be counseled that currently there is no strong evidence to justify an ongoing ultrasound surveillance program. Authors, on the other hand, support a surveillance plan specific to the increased maternal and fetal risks including patient education on signs and symptoms of the most common complications, increased frequency of antenatal visits and ultrasound/Doppler studies for assessing fetal biometry and biophysical profile, and cervical length assessment as recommended by Gagnon et al. [12].
Our study had some limitations including its retrospective nature. Limited data were available on relevant maternal characteristics such as weight, presence of other comorbidity, use of assisted reproduction. In some cases, we had rather crude than specific information: full term/preterm rather than exact gestation in weeks and low birth weight (<2.5 kg) rather than exact weight. PIH and oligohydramnios were considered as present/absent without further considering its severity, gestational age at onset, etc. We were unable to comment on association of maternal serum markers with miscarriages and/or pregnancy loss since only live births were included in the study. Screening tests were done in different laboratories for cases as they were referred to our hospital only for invasive testing and received antenatal care and delivered at different hospitals while controls were tested and delivered at our hospital.
In conclusion, the present study shows significant correlation of a “high-risk screen” and abnormal markers with adverse pregnancy outcomes mainly LBW, PT, PIH and oligohydramnios. An abnormal screen with at least one abnormal marker was more predictive of these outcomes compared to abnormal marker or high risk screen alone. Neither PAPP-A nor beta hCG can be recommended as stand-alone tests to predict, but they might provide additional inputs for better surveillance for women with “high-risk screen-normal fetal karyotype” status. Further larger randomized controlled trials are awaited before routinely prescribing low-dose aspirin to these women.
Funding
This study was funded by Deenanath Mangeshkar Hospital and Research Center.
Dr. Koumudi Godbole
is a fellow of Canadian College of Medical Geneticists and works as a Consultant Clinical Geneticist at Deenanath Mangeshkar Hospital and Research Center. She is involved in the prenatal medicine program at this hospital and coordinates Pune Birth Defects Registry affiliated to BDRI, Chennai. She has started “Garbha-Swasthya” helpline to answer queries related to pregnancy and related issues including complications. She has about 25 publications in national and international journals and has contributed chapters to books in Genetics, assisted reproduction and also written a Marathi book to introduce medical genetics to the society. Her research interests include reproductive genetics and developmental origins of adult disease. She has completed multicenter research projects related to neural tube defects, congenital hearing loss and recurrent miscarriages funded by DBT and CSIR.
Compliance with Ethical Standards
Conflicts of interest
None.
Human and Animals Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent waived off for retrospective study.
Footnotes
Koumudi Godbole MD, FCCMG, is Consultant Clinical Geneticist in Department of Genetic Medicine, Deenanath Mangeshkar Hospital and Research Center, Pune, India; Aparna Kulkarni MD, MRCOG, M Phil, is Consultant in Fetal Medicine, Deenanath Mangeshkar Hospital and Research Center, Pune, India; Asawari Kanade PhD, is Consultant Statistician in Department of Research, Deenanath Mangeshkar Hospital and Research Center, Pune, India; Shilpa Kulkarni BHMS, is Coordinator in Prenatal Medicine Program, Deenanath Mangeshkar Hospital and Research Center, Pune, India; Girish Godbole MD, is Consultant Obstetrician and Gynecologist in Deenanath Mangeshkar Hospital and Research Center, Pune, India; Anuradha Wakankar MS, MRCOG, is Consultant Obstetrician and Gynecologist in Deenanath Mangeshkar Hospital and Research Center, Pune, India.
Contributor Information
Koumudi Godbole, Phone: +91-20-40151680, Email: koumudig@gmail.com, http://www.dmhospital.org.
Aparna Kulkarni, Phone: +91-20-49153360.
Asawari Kanade, Phone: +91-20-40151152.
Shilpa Kulkarni, Phone: +91-20-40151368.
Girish Godbole, Phone: +91-20-49153358.
Anuradha Wakankar, Phone: +91-20-40151000.
References
- 1.Cole LA. Biological functions of hCG and hCG related molecules. Reprod Biol Endocrinol. 2010;8:102. doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris RK, Cnossen JS, Langejans M, et al. Serum screening with Down’s syndrome markers to predict pre-eclampsia and small for gestational age: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2008;8:33. doi: 10.1186/1471-2393-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dane B, Dane C, Batmaz G, et al. First trimester maternal serum pregnancy-associated plasma protein-A is a predictive factor for early preterm delivery in normotensive pregnancies. Gynecol Endocrinol. 2013;29(6):592–595. doi: 10.3109/09513590.2013.788626. [DOI] [PubMed] [Google Scholar]
- 4.Spencer K, Cowans NJ, Avgidou KA, et al. First trimester biochemical markers of aneuploidy and the prediction of small for gestational age fetuses. Ultrasound Obstet Gynecol. 2008;31:15–19. doi: 10.1002/uog.5165. [DOI] [PubMed] [Google Scholar]
- 5.Canini S, Prefumo F, Pastorino D, et al. Association between birth weight and first-trimester free beta-human chorionic gonadotropin and pregnancy-associated plasma protein A. Fertil Steril. 2008;89(1):174–178. doi: 10.1016/j.fertnstert.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Patil M, Panchanadikar TM, Wagh G. Variation of papp-a level in the first trimester of pregnancy and its clinical outcome. J Obstet Gynaecol India. 2014;64(2):116–119. doi: 10.1007/s13224-013-0481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh TT, Hung TH, Hsu JJ, et al. Prediction of adverse perinatal outcome by maternal serum screening for Down syndrome in an Asian population. Obstet Gynecol. 1997;89(6):937–940. doi: 10.1016/S0029-7844(97)00151-8. [DOI] [PubMed] [Google Scholar]
- 8.Duric K, Skrablin S, Lesin J, et al. Second trimester total human chorionic gonadotropin, alpha-fetoprotein and unconjugated estriol in predicting pregnancy complications other than fetal aneuploidy. Eur J Obstet Gynecol Reprod Biol. 2003;110(1):12–15. doi: 10.1016/S0301-2115(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 9.Sritippayawan S, Vachirasrisoontra C. Adverse pregnancy outcomes after a false-positive second trimester serum screen for Down syndrome in Thai pregnant women. J Med Assoc Thai. 2005;88(4):449–454. [PubMed] [Google Scholar]
- 10.Morssink LP, Kornman LH, Hallahan TW, et al. Maternal serum levels of free beta-hCG and PAPP-A in the first trimester of pregnancy are not associated with subsequent fetal growth retardation or preterm delivery. Prenat Diagn. 1998;18(2):147–152. doi: 10.1002/(SICI)1097-0223(199802)18:2<147::AID-PD231>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 11.Schnettler WT, Hacker MR, Barber RE, et al. Management of abnormal serum markers in the absence of aneuploidy and neural tube defects. J Matern Fetal Neonatal Med. 2012;25(10):1895–1898. doi: 10.3109/14767058.2012.668583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagnon A, Wilson RD, Audibert F, et al. Obstetrical complications associated with abnormal maternal serum markers analytes. J Obstet Gynaecol Can. 2008;30(10):918–949. doi: 10.1016/S1701-2163(16)32973-5. [DOI] [PubMed] [Google Scholar]
- 13.D’Antonio F, Rijo C, Thilaganathan B, et al. Association between first-trimester maternal serum pregnancy-associated plasma protein-A and obstetric complications. Prenat Diagn. 2013;33(9):839–847. doi: 10.1002/pd.4141. [DOI] [PubMed] [Google Scholar]
- 14.Tape TG. Interpreting diagnostic tests. http://gim.unmc.edu/dxtests/Default.htm (2014). Accessed 22 Dec 2014.
- 15.Ardawi MS, Nasrat HA, Rouzi AA, et al. Maternal serum free-beta-chorionic gonadotrophin, pregnancy-associated plasma protein-A and fetal nuchal translucency thickness at 10–13(+6) weeks in relation to co-variables in pregnant Saudi women. Prenat Diagn. 2007;27(4):303–311. doi: 10.1002/pd.1661. [DOI] [PubMed] [Google Scholar]
- 16.Wright D, Abele H, Baker A, et al. Impact of bias in serum free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A multiples of the median levels on first-trimester screening for trisomy 21. Ultrasound Obstet Gynecol. 2011;38(3):309–313. doi: 10.1002/uog.8987. [DOI] [PubMed] [Google Scholar]
- 17.Quattrocchi T, Baviera G, Pochiero T, et al. Maternal serum PAPP-A as an early marker of obstetric complications? Fetal Diagn Ther. 2015;37(1):33–36. doi: 10.1159/000365147. [DOI] [PubMed] [Google Scholar]
- 18.Sharp AN, Alfirevic Z. First trimester screening can predict adverse pregnancy outcomes. Prenat Diagn. 2014;34(7):660–667. doi: 10.1002/pd.4406. [DOI] [PubMed] [Google Scholar]
- 19.Halscott TL, Ramsey PS, Reddy UM. First trimester screening cannot predict adverse outcomes yet. Prenat Diagn. 2014;34(7):668–676. doi: 10.1002/pd.4407. [DOI] [PubMed] [Google Scholar]
- 20.Roberge S, Nicolaides KH, Demers S, et al. Prevention of perinatal death and adverse perinatal outcome using low dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491–499. doi: 10.1002/uog.12421. [DOI] [PubMed] [Google Scholar]
- 21.Villa PM, Kajantie E, Raikkonen K, et al. Aspirin in the prevention of pre-eclampsia in high-risk women: a randomized placebo-controlled PREDO trial and a meta-analysis of randomized trials. Br J Obstet Gynaecol. 2013;120(1):64–74. doi: 10.1111/j.1471-0528.2012.03493.x. [DOI] [PubMed] [Google Scholar]
- 22.Moore GS, Allshouse AA, Post AL, et al. Early initiation of low-dose aspirin for reduction in preeclampsia risk in high-risk women: a secondary analysis of the MFMU high-risk aspirin study. Tang J Perinatol. 2014 doi: 10.1038/jp.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson JT, O’Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia. Ann Intern Med. 2014;161(8):613–614. doi: 10.7326/L14-5020-5. [DOI] [PubMed] [Google Scholar]
- 24.Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013;30(1–2):260–279. doi: 10.3109/07420528.2012.717455. [DOI] [PubMed] [Google Scholar]
- 25.Huynh L, Kingdom J, Akhtar S. Low pregnancy-associated plasma protein A level in the first trimester. Can Fam Physician. 2014;60(10):899–903. [PMC free article] [PubMed] [Google Scholar]