Abstract
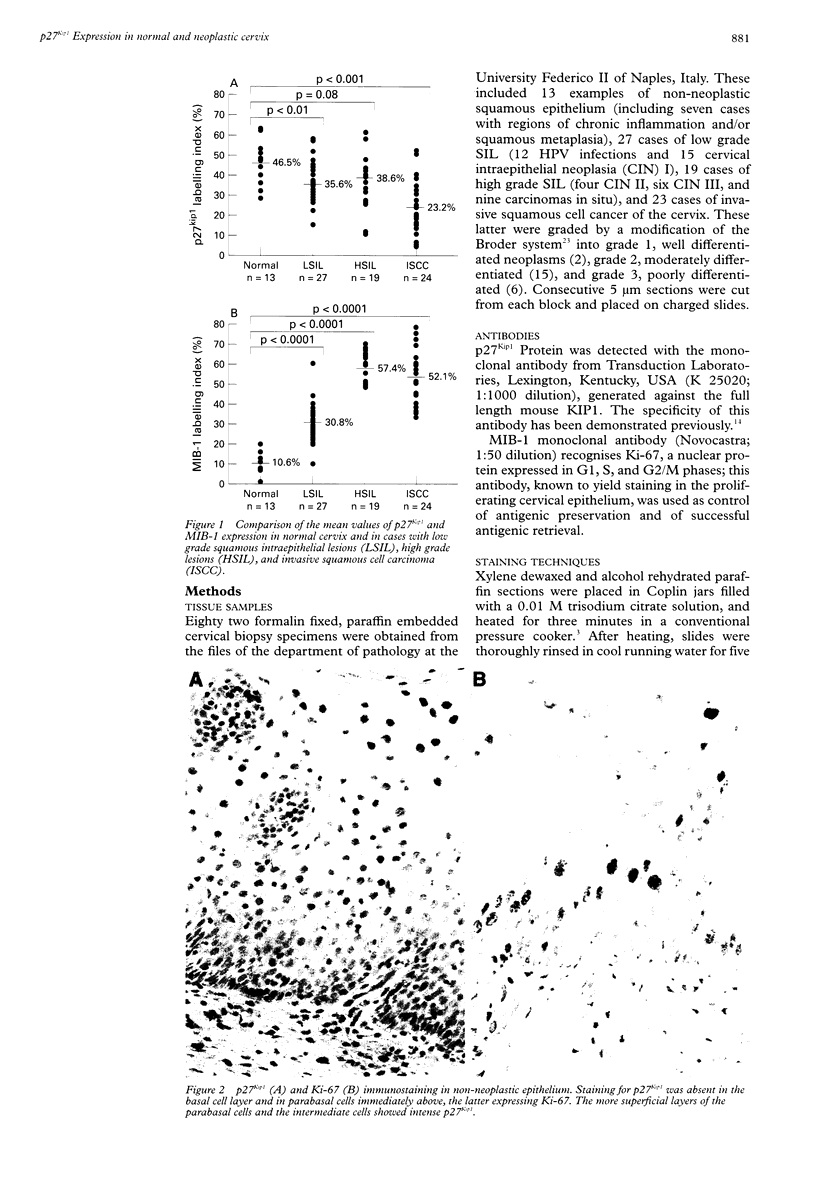
AIM: To investigate whether there is loss of the p27Kip1 protein in developing cervical cancer and whether p27Kip1 immunoreactivity has any relation to the proliferative indicator Ki-67. METHODS: The expression of p27Kip1 and Ki-67 was assessed by immunohistochemistry in serial sections from normal epithelium (13), low grade (27) and high grade (19) squamous intraepithelial lesions (LSIL, HSIL), and invasive cervical cancer (23). In the SIL cases the presence of human papillomavirus (HPV) genomic sequences was assessed by in situ hybridisation. The results were evaluated by image analysis, and reported as mean score of the percentage of p27Kip1 and of Ki-67 positive cells in each histological group. RESULTS: In general, p27Kip1 immunostaining was related to squamous differentation, and was intense in normal epithelium (47%), while it was reduced in SIL lesions as an effect of the decreased number of differentiating cells. However, decrease in the p27Kip1 expression was more evident in LSIL (36%) than in HSIL (39%); in the latter, p27Kip1 had a different intraepithelial distribution in that the staining extended to the basal cells. The average levels of p27Kip1 were similar in SIL lesions associated to low, intermediate, and high risk HPV types. Compared with normal epithelium and dysplasia, invasive cancer showed significantly lower p27Kip1 levels (23%). There was no relation between p27Kip1 and Ki-67 labelling indices in any of the histological groups examined. CONCLUSIONS: A reduction in p27Kip1 protein occurs in cervical cancer independently of the proliferative status. The changes in p27Kip1 expression may be related to the unregulated kinetics of developing cervical cancer.
Full text
PDF

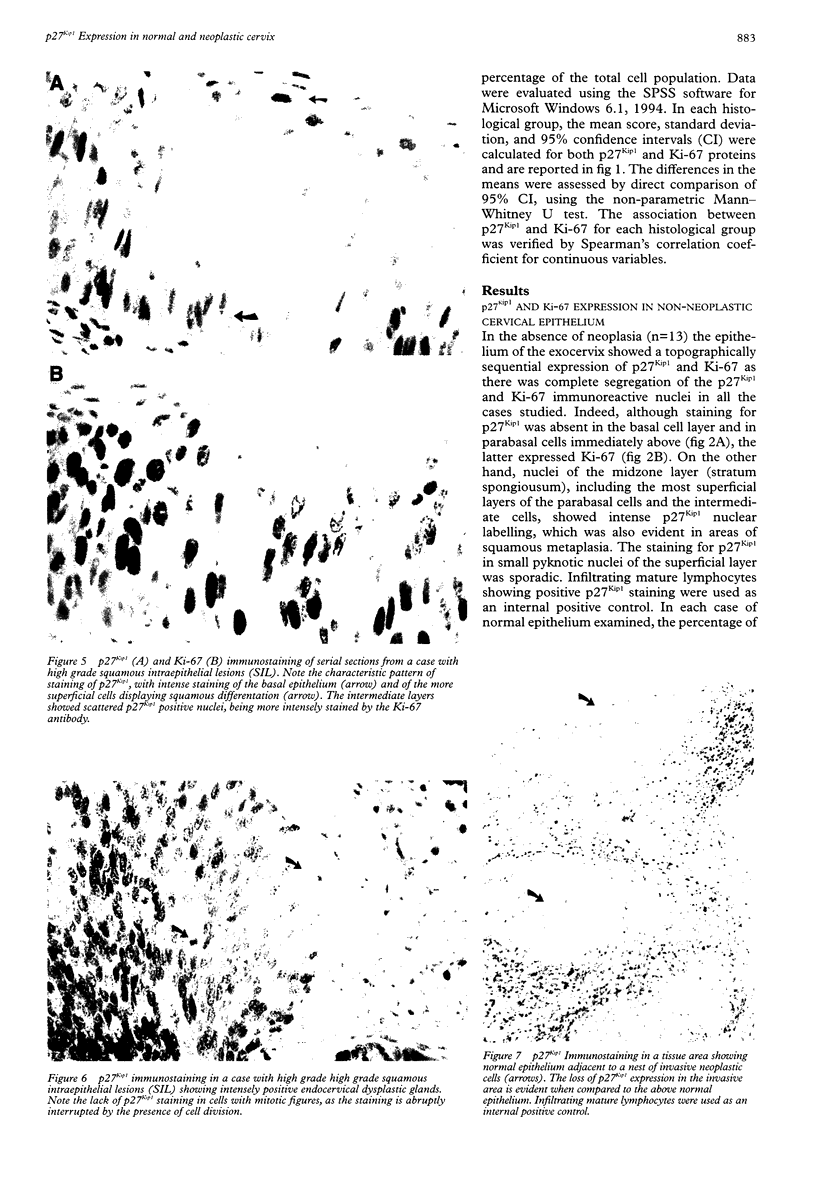

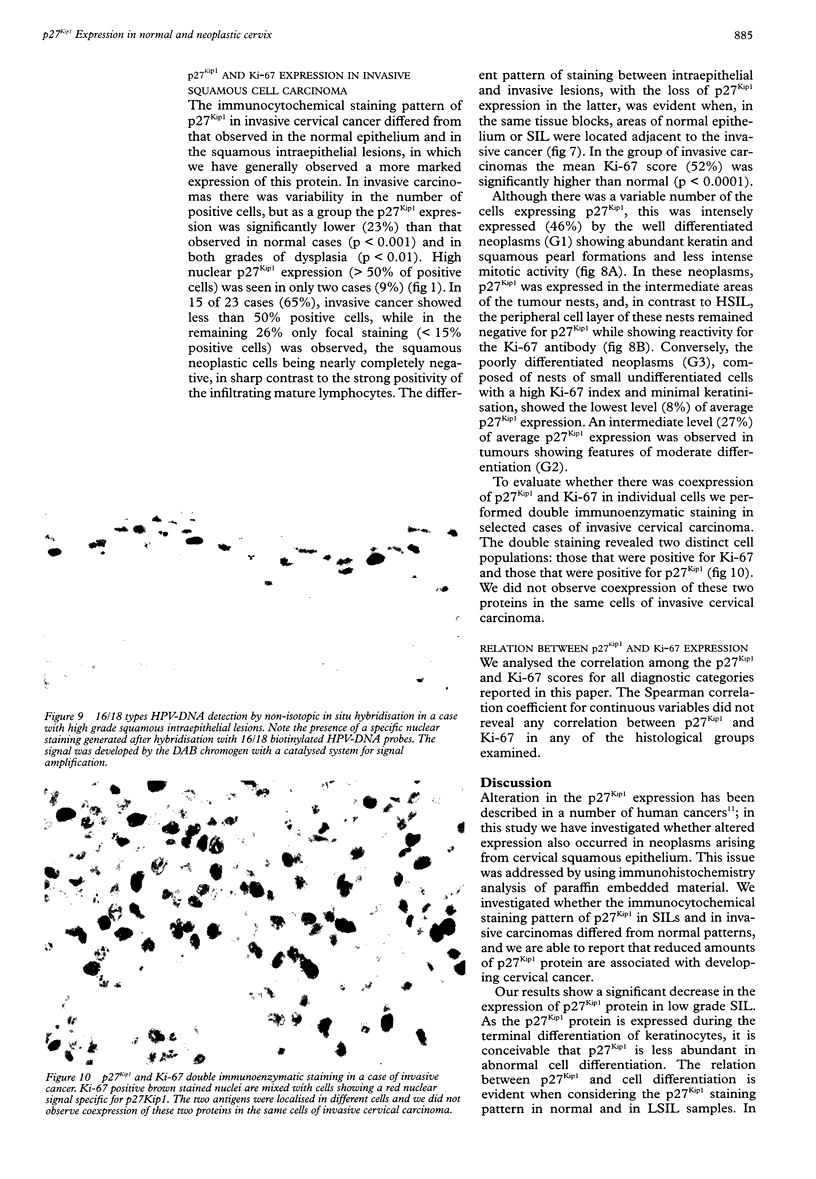


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheville J. C., Lloyd R. V., Sebo T. J., Cheng L., Erickson L., Bostwick D. G., Lohse C. M., Wollan P. Expression of p27kip1 in prostatic adenocarcinoma. Mod Pathol. 1998 Apr;11(4):324–328. [PubMed] [Google Scholar]
- Clurman B. E., Porter P. New insights into the tumor suppression function of P27(kip1) Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15158–15160. doi: 10.1073/pnas.95.26.15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats S., Flanagan W. M., Nourse J., Roberts J. M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996 May 10;272(5263):877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- Dellas A., Schultheiss E., Leivas M. R., Moch H., Torhorst J. Association of p27Kip1, cyclin E and c-myc expression with progression and prognosis in HPV-positive cervical neoplasms. Anticancer Res. 1998 Nov-Dec;18(6A):3991–3998. [PubMed] [Google Scholar]
- Erickson L. A., Jin L., Wollan P. C., Thompson G. B., van Heerden J., Lloyd R. V. Expression of p27kip1 and Ki-67 in benign and malignant thyroid tumors. Mod Pathol. 1998 Feb;11(2):169–174. [PubMed] [Google Scholar]
- Esposito V., Baldi A., De Luca A., Groger A. M., Loda M., Giordano G. G., Caputi M., Baldi F., Pagano M., Giordano A. Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res. 1997 Aug 15;57(16):3381–3385. [PubMed] [Google Scholar]
- Flørenes V. A., Maelandsmo G. M., Kerbel R. S., Slingerland J. M., Nesland J. M., Holm R. Protein expression of the cell-cycle inhibitor p27Kip1 in malignant melanoma: inverse correlation with disease-free survival. Am J Pathol. 1998 Jul;153(1):305–312. doi: 10.1016/S0002-9440(10)65572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst L., Reed S. I. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996 Mar 29;271(5257):1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- Herrington C. S. Human papillomaviruses and cervical neoplasia. II. Interaction of HPV with other factors. J Clin Pathol. 1995 Jan;48(1):1–6. doi: 10.1136/jcp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R. C., Bradley G., Slingerland J. Reduced levels of the cell-cycle inhibitor p27Kip1 in epithelial dysplasia and carcinoma of the oral cavity. Am J Pathol. 1998 Feb;152(2):585–590. [PMC free article] [PubMed] [Google Scholar]
- Kawana H., Tamaru J., Tanaka T., Hirai A., Saito Y., Kitagawa M., Mikata A., Harigaya K., Kuriyama T. Role of p27Kip1 and cyclin-dependent kinase 2 in the proliferation of non-small cell lung cancer. Am J Pathol. 1998 Aug;153(2):505–513. doi: 10.1016/s0002-9440(10)65593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Erickson L. A., Jin L., Kulig E., Qian X., Cheville J. C., Scheithauer B. W. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999 Feb;154(2):313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loda M., Cukor B., Tam S. W., Lavin P., Fiorentino M., Draetta G. F., Jessup J. M., Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997 Feb;3(2):231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- Mal A., Poon R. Y., Howe P. H., Toyoshima H., Hunter T., Harter M. L. Inactivation of p27Kip1 by the viral E1A oncoprotein in TGFbeta-treated cells. Nature. 1996 Mar 21;380(6571):262–265. doi: 10.1038/380262a0. [DOI] [PubMed] [Google Scholar]
- Mitchell M. F., Hittelman W. N., Hong W. K., Lotan R., Schottenfeld D. The natural history of cervical intraepithelial neoplasia: an argument for intermediate endpoint biomarkers. Cancer Epidemiol Biomarkers Prev. 1994 Oct-Nov;3(7):619–626. [PubMed] [Google Scholar]
- Newcomb E. W., Sosnow M., Demopoulos R. I., Zeleniuch-Jacquotte A., Sorich J., Speyer J. L. Expression of the cell cycle inhibitor p27KIP1 is a new prognostic marker associated with survival in epithelial ovarian tumors. Am J Pathol. 1999 Jan;154(1):119–125. doi: 10.1016/S0002-9440(10)65258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Tam S. W., Theodoras A. M., Beer-Romero P., Del Sal G., Chau V., Yew P. R., Draetta G. F., Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995 Aug 4;269(5224):682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Palazzo J. P., Mercer W. E., Kovatich A. J., McHugh M. Immunohistochemical localization of p21(WAF1/CIP1) in normal, hyperplastic, and neoplastic uterine tissues. Hum Pathol. 1997 Jan;28(1):60–66. doi: 10.1016/s0046-8177(97)90280-x. [DOI] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Qian X., Jin L., Kulig E., Lloyd R. V. DNA methylation regulates p27kip1 expression in rodent pituitary cell lines. Am J Pathol. 1998 Nov;153(5):1475–1482. doi: 10.1016/S0002-9440(10)65735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Grimminger D. C., Wu X., Jian Y., Broker T. R., Chow L. T. Post-transcriptional induction of p21cip1 protein in condylomata and dysplasias is inversely related to human papillomavirus activities. Am J Pathol. 1998 Apr;152(4):1015–1024. [PMC free article] [PubMed] [Google Scholar]
- Singh S. P., Lipman J., Goldman H., Ellis F. H., Jr, Aizenman L., Cangi M. G., Signoretti S., Chiaur D. S., Pagano M., Loda M. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 1998 Apr 15;58(8):1730–1735. [PubMed] [Google Scholar]
- Slingerland J. M., Hengst L., Pan C. H., Alexander D., Stampfer M. R., Reed S. I. A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor beta-arrested epithelial cells. Mol Cell Biol. 1994 Jun;14(6):3683–3694. doi: 10.1128/mcb.14.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Abrams J. S. Cancer prognostics: past, present and p27. Nat Med. 1997 Feb;3(2):152–154. doi: 10.1038/nm0297-152. [DOI] [PubMed] [Google Scholar]
- Sánchez-Beato M., Sáez A. I., Martínez-Montero J. C., Sol Mateo M., Sánchez-Verde L., Villuendas R., Troncone G., Piris M. A. Cyclin-dependent kinase inhibitor p27KIP1 in lymphoid tissue: p27KIP1 expression is inversely proportional to the proliferative index. Am J Pathol. 1997 Jul;151(1):151–160. [PMC free article] [PubMed] [Google Scholar]
- Tan P., Cady B., Wanner M., Worland P., Cukor B., Magi-Galluzzi C., Lavin P., Draetta G., Pagano M., Loda M. The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res. 1997 Apr 1;57(7):1259–1263. [PubMed] [Google Scholar]
- Troncone G., Martinez J. C., Palombini L., De Rosa G., Mugica C., Rodriguez J. A., Zeppa P., Di Vizio D., Lucariello A., Piris M. A. Immunohistochemical expression of mdm2 and p21WAF1 in invasive cervical cancer: correlation with p53 protein and high risk HPV infection. J Clin Pathol. 1998 Oct;51(10):754–760. doi: 10.1136/jcp.51.10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werness B. A., Wang H. Q., Chance J., Goldstein D. J. p53-independent expression of p21waf1/cip1 in preinvasive and invasive squamous neoplasms of the uterine cervix. Mod Pathol. 1997 Jun;10(6):578–584. [PubMed] [Google Scholar]
- Zehbe I., Rätsch A., Alunni-Fabbroni M., Burzlaff A., Bakos E., Dürst M., Wilander E., Tommasino M. Overriding of cyclin-dependent kinase inhibitors by high and low risk human papillomavirus types: evidence for an in vivo role in cervical lesions. Oncogene. 1999 Apr 1;18(13):2201–2211. doi: 10.1038/sj.onc.1202549. [DOI] [PubMed] [Google Scholar]