Abstract
Chemically programmed bispecific antibodies (biAbs) endow target cell-binding small molecules with the ability to recruit and activate effector cells of the immune system. Here we report a platform of chemically programmed biAbs aimed at redirecting cytotoxic T cells to eliminate cancer cells. Two different antibody technologies were merged together to make a novel chemically programmed biAb. This was achieved by combining the humanized anti-hapten monoclonal antibody (mAb) h38C2 with the humanized anti-human CD3 mAb v9 in a clinically investigated diabody format known as Dual-Affinity Re-Targeting (DART). We show that h38C2 × v9 DARTs can readily be equipped with tumor-targeting hapten-derivatized small molecules without causing a systemic response harming healthy tissues. As a proof of concept, we chemically programmed h38C2 × v9 with hapten-folate and demonstrated its selectivity and potency against folate receptor 1 (FOLR1)-expressing ovarian cancer cells in vitro and in vivo. Unlike conventional biAbs, chemically programmed biAbs in DART format are highly modular with broad utility in terms of both target and effector cell engagement. Most importantly, they provide tumor-targeting compounds access to the power of cancer immunotherapy.
Keywords: antibody engineering, cancer therapy, chemical modification, folate, immunotherapy, ovarian cancer, T-cell
Introduction
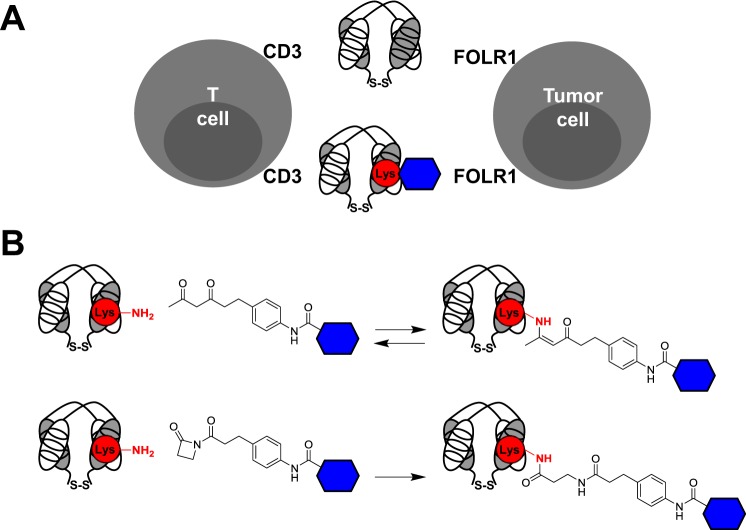
Chemically programmed bispecific antibodies (biAbs) 6 that exert cytotoxicity by binding to tumor cells with one arm and by simultaneously recruiting and activating tumor cell-lysing T cells with the other arm are an emerging category of next-generation antibody drugs for cancer therapy (1–4). Merging this promising platform with the concept of chemically programmed antibodies (5), we and others recently developed chemically programmed biAbs that recognize tumor cells with a variable small molecule component and that recruit and activate T cells with a generic antibody component (6–8). Chemically programmed biAbs are more versatile than conventional biAbs as they only require the cloning, expression, and purification of a single protein (Fig. 1A). Further, to target a variety of different tumor cell surface antigens, chemically programmed biAbs can make use of a wealth of small molecules derived from chemical libraries or from structure-based design campaigns, linking advances in both immunology and chemistry for the benefit of cancer patients.
FIGURE 1.
Conventional versus chemically programmed biAbs in DART format. A, biAbs in DART format are comprised of two polypeptides that are linked at their C termini via a disulfide bridge, where each polypeptide contains one of two cognate variable light (white) and heavy chain (gray) domains that form the antigen or hapten binding site. Conventional DARTs described in this study (top) combine a CD3-engaging with a FOLR1-engaging Fv module to bring T cells and tumor cells in close contact and enable the formation of cytolytic synapses. Chemically programmed DARTs described in this study engage the same two antigens; however, FOLR1 binding is mediated by a small molecule (blue hexagon) that is site-specifically and covalently conjugated to the reactive lysine residue (red circle) in the Fv module of humanized anti-β-diketone hapten mAb h38C2. B, mAb h38C2 harbors a reactive Lys residue (red circle) with an unusually low pKa of ∼6.0 at the bottom of its hydrophobic hapten binding site. The nucleophilic ϵ-amino group of this Lys residue can be covalently conjugated to the β-diketone group of the hapten and compounds that incorporate the hapten and a targeting moiety (blue hexagon). This reversible covalent conjugation (top) is stabilized by imine-enamine tautomerism. An irreversible covalent conjugation (bottom) is achieved by replacing the β-diketone group with a β-lactam group.
Here we introduce a molecular format for chemically programmed biAbs that is based on humanized anti-β-diketone hapten mAb h38C2 (9) derived from mouse mAb 38C2 (10, 11). As depicted in Fig. 1B, mAb h38C2 bears a reactive lysine residue in its hapten binding site which, via a β-diketone or β-lactam functionality, can be covalently conjugated to small molecules that bind to antigens of interest. In fact, chemical programming (12–14) of mAb h38C2 with β-lactam derivatives of peptides targeting angiopoetin-2, vascular endothelial growth factor, and thrombospondin-1 in cancer patients as well as glucagon-like peptide-1 in diabetes mellitus type II patients has been investigated in phase I and II clinical trials (5). However, our current report is the first to utilize h38C2 as an antibody component in chemically programmed biAbs designed to recruit and activate endogenous T cells in cancer patients. To do so, we combined mAb h38C2 with humanized anti-human CD3 mAb v9, which is derived from mouse mAb UCHT1 (15, 16) and has high efficiency for redirecting cytotoxic T cells (6, 17, 18).
For combination of h38C2 and v9, a number of biAb formats exist (2). Among these, the BiTE (for Bispecific T-cell Engager) format, which combines two single chain Fv (scFv) modules linked by a polypeptide linker, is of particular interest. Specifically, the CD19 × CD3 BiTE blinatumomab has revealed impressive clinical activity at doses several orders of magnitude below those administered in conventional mAb therapy (19, 20) and was approved by the Food and Drug Administration (FDA) for the therapy of relapsed and refractory acute lymphoblastic leukemia in 2014 (21). In addition to bypassing MHC/peptide recognition or the need for ex vivo pre-stimulation or in vivo co-stimulation, T cells recruited via BiTEs only depend on the presence of biAb-decorated tumor cells for activation. These favorable features of the BiTE format are attributed to: (i) its small size (∼50 kDa), which brings target and effector cells into close proximity to enable cytolytic synapses; and (ii) the monovalent engagement of the T-cell receptor (TCR) complex, which prevents systemic activation of effector cells in the absence of target cells (22).
The success of the BiTE format triggered the search for intellectual property space among biAb formats of similar size and valence (23). For example, a potentially competing format coined DART (for Dual-Affinity Re-Targeting) is based on the so called diabody format that separates cognate variable domains of heavy and light chains of the two antigen or hapten binding specificities on two separate polypeptide chains (24). Whereas the two polypeptide chains associate non-covalently in the diabody format, the DART format provides additional stabilization via a C-terminal disulfide bridge (Figs. 1 and 2). DARTs can be produced in high quantity and quality and have exceptional stability in both formulation buffer and human serum (25). Further, side-by-side comparisons of the in vitro performance of CD19 × CD3 DART and BiTE molecules showed that the DART format is superior in provoking tumor cell lysis and in inducing T-cell activation markers (26). The more rigid configuration of the DART format, where there is limited flexibility between the two antigen or hapten binding specificities, likely accounts for these improved features (23, 26). In addition to the CD19 × CD3 DART, several additional DARTs are currently being investigated in phase I clinical trials.
FIGURE 2.
Chemically programmable DARTs. Two configurations, hv-L (A) and hv-H (B), of h38C2 × v9 DARTs were generated. Each consisted of two polypeptides that are linked at their C termini via a disulfide bridge and that each contain one of two cognate variable light (white) and heavy chain (gray) domains that form the antigen (CD3 for v9) or hapten (β-diketone for h38C2) binding site. The variable domains on each polypeptide are fused with a short polypeptide (G3SG4) that favors diabody over scFv formation. The two polypeptide expression cassettes were cloned under the control of a CMV promoter into mammalian expression vector pCEP4 for transient co-transfection into HEK 293 cells. A C-terminal His6 tag (H6) was included in one of the paired polypeptides to facilitate purification by IMAC. The reactive Lys residue of h38C2 is indicated in red. Note that the hv-L configuration has free light chain N termini, whereas configuration hv-H displays free heavy chain N termini. SP, signal peptide. All amino acid sequences are given in the supplemental information.
Here we describe the generation, chemical programmability, and utility of h38C2 × v9 DARTs. Following chemical programming with hapten-folate, the h38C2 × v9 DARTs acquired FOLR1 binding capability and were systematically compared with conventional FOLR1 × CD3 DARTs we generated in parallel. Folate was used as a representative tumor-cell targeting small molecule as FOLR1 is a clinically investigated target for both mAbs and small molecules in cancer therapy in general and in the treatment of ovarian cancer in particular (27–29). Both chemically programmed and conventional FOLR1 × CD3 DARTs selectively and potently killed FOLR1-expressing human ovarian cancer cell lines in vitro and in vivo. Our findings support the notion of broad therapeutic utility of chemically programmed biAbs at the interface of immunology and chemistry.
Results
BiAbs in DART format are comprised of two polypeptides that are linked at their C termini via a disulfide bridge, where each polypeptide contains one of two cognate variable light and heavy chain domains that form the antigen or hapten binding site (25) (Fig. 1A). We engineered the chemically programmable h38C2 × v9 DART in two possible configurations, hv-L and hv-H, either with the variable domain of the kappa light chains (Vκ) on the N terminus (Vκ1-VH2/Vκ2-VH1) or with the variable domain of the heavy chains (VH) on the N terminus (VH1-Vκ2/VH2-Vκ1). Specifically, hv-L (Fig. 2A) paired an h38C2Vκ-G3SG4-v9VH-VEPKSC-H6 and a v9Vκ-G3SG4-h38C2VH-FNRGEC polypeptide, using the previously established G3SG4 linker to fuse the humanized variable domains on each polypeptide and VEPKSC/FNRGEC from the upper hinge region of human IgG1 and the C terminus of human constant domain Cκ, respectively, to introduce a native disulfide bridge between the two polypeptides. A His tag (H6) was included on one of the polypeptides to facilitate purification and detection. Accordingly, hv-H (Fig. 2B) paired an h38C2VH-G3SG4-v9Vκ-FNRGEC-H6 and a v9VH-G3SG4-h38C2Vκ-VEPKSC polypeptide. The amino acid sequences of hv-L and hv-H are given in the supplemental information with the reactive lysine residue of h38C2 highlighted. These two different configurations were initially pursued in parallel as they may have different antigen or hapten binding properties depending on the involvement of their N termini. The four polypeptide encoding sequences were generated by custom synthesis and cloned into mammalian expression vector pCEP4 under control of a CMV promoter. Cognate plasmid pairs encoding the h38C2 × v9 DARTs hv-L and hv-H were transiently co-transfected into human embryonic kidney (HEK) 293 cells and the DARTs were purified from concentrated supernatants by immobilized metal ion affinity chromatography (IMAC) followed by size exclusion chromatography (SEC).
In addition to the chemically programmable DARTs, we cloned, expressed, and purified conventional DARTs consisting of the antigen binding sites of humanized anti-human FOLR1 mAb farletuzumab, also known as MORAb-003 or hLK26 (30), and v9. These conventional farletuzumab × v9 DARTs were also generated in configuration 1 (VL1-VH2/VL2-VH1; designated fv-L) and configuration 2 (VH1-VL2/VH2-VL1; designated fv-H). The amino acid sequences of fv-L and fv-H are given in the supplemental information. Note that farletuzumab recognizes a FOLR1 epitope that does not overlap with the folate binding site (31).
Using SDS-PAGE and SEC, we showed that the purified chemically programmable and conventional DARTs were correctly assembled and pure (Fig. 3, A and B). In addition, the DARTs showed similar binding to CD3-expressing human T-cell line Jurkat by flow cytometry, confirming the integrity of their v9 antigen binding site (Fig. 4). Correct assembly of the chemically programmable DART was further corroborated by detecting catalytic activity of the chemically programmable DARTs but not the conventional DARTs, indicating the functional formation of the h38C2 hapten binding site which involves both variable domains (Fig. 3C) (11, 32).
FIGURE 3.
Biochemical characterization of chemically programmed and conventional FOLR1 × CD3 DARTs. A, Coomassie Blue-stained SDS-PAGE gel with 2 μg/lane purified representative DARTs (hv-L and fv-L) in reducing (red) and nonreducing (nonred) conditions, showing the expected bands at ∼27.5 and 55 kDa, respectively. A protein marker (in kDa) was run in the center lane. B, size-exclusion chromatography profile of a purified representative DART (hv-L). C, catalytic activity of purified DARTs hv-L (top; open red squares) and hv-H (bottom; open red squares) measured with the fluorogenic retro-aldol substrate methodol. Chemical programming with compound 1 eradicated the catalytic activity of both DARTs (solid red squares). Conventional DART fv-L served as negative control (solid green squares). D, efficacy of the conjugation of compound 1 to DART hv-L as measured by the EZ Biotin Quantitation kit. Conventional DART fv-L following attempted conjugation of compound 1 served as negative control.
FIGURE 4.
Cell surface binding of chemically programmed and conventional FOLR1 × CD3 DARTs. The indicated DARTs were analyzed for binding to human CD3+ T-cell line Jurkat and human FOLR1+ ovarian cancer cell lines IGROV1, OVCAR3, and SKOV3 by flow cytometry at a concentration of 2 μg/ml, using a mouse anti-His6 mAb followed by Alexa Fluor 488-conjugated goat anti-mouse IgG pAbs. The background signal of the secondary reagents alone is shown in pale blue.
To chemically program hv-L and hv-H and directly compare them to fv-L and fv-H, we synthesized trifunctional compounds β-lactam-biotin-folate 1 and β-diketone-biotin-folate 2 as well as control compound β-diketone-biotin 3 for hapten-driven covalent conjugation to the reactive lysine residue in the hapten binding site of h38C2 (Fig. 5). Following chemical programming, hv-L/1, hv-H/1, hv-L/2, and hv-H/2, but not hv-L/3 and hv-H/3 revealed strong binding to FOLR1-expressing human ovarian cancer cell line IGROV1, demonstrating folate-mediated recognition (Fig. 6A and data not shown). Similar although consistently stronger binding was observed for conventional DARTs fv-L and fv-H (Fig. 6A). This same pattern, albeit to a lesser extent, was also seen with two additional human ovarian cancer cell lines from the NCI-60 panel, OVCAR3 and SKOV3, which have a lower FOLR1 cell surface density compared with IGROV1 (Fig. 4).
FIGURE 5.
Folate derivatives. Trifunctional β-lactam-biotin-folate 1 and β-diketone-biotin-folate 2 contain a folate (a.k.a. pteroyl-glutamate) moiety derivatized at its γ-carboxyl group with a branched linker that incorporates a biotin moiety and an electrophilic hapten functionality enabling covalent conjugation to the nucleophilic ϵ-amino group of the reactive Lys residue in the hapten binding site of mAb h38C2. Bifunctional β-diketone-biotin 3 was used as negative control.
FIGURE 6.
Cell surface binding and crosslinking of chemically programmed and conventional FOLR1 × CD3 DARTs. A, binding of chemically programmed hv-L and hv-H DARTs and conventional fv-L and fv-H DARTs to FOLR1-expressing IGROV1 cells. All DARTs were analyzed by flow cytometry at a concentration of 2 μg/ml (∼40 nm), using a mouse anti-His tag mAb followed by Alexa Fluor 488-conjugated goat anti-mouse IgG pAbs. hv-L and hv-H DARTs were chemically programmed by incubation with 400 nm β-lactam-biotin-folate compound 1 (red) or β-diketone-biotin compound 3 (blue), incubated for 1 h at room temperature, and purified by ultrafiltration. The background signal of the secondary reagents alone is shown in gray. B, crosslinking of CD3+ primary human T cells and FOLR1-expressing OVCAR3 cells in the presence of FOLR1 × CD3 DARTs. hv-L and hv-H DARTs were chemically programmed as noted above. The various DARTs were added at 2 μg/ml to 5 × 104 T cells stained with CellTrace Far Red DDAO-SE. Following incubation for 1 h at room temperature and washing, 5 × 104 OVCAR3 cells stained with CellTracker Blue CMAC were added. The mixtures were then incubated for 1 h at room temperature, gently washed, and fixed with 1% (w/v) paraformaldehyde. The formation of blue/far red cell aggregates was detected by flow cytometry and plotted as percentage of double positive events among all events. The results are representative of three experiments.
Because of its irreversible covalent conjugation to the reactive lysine residue of h38C2 and the successful clinical translation of adducts of h38C2 IgG1 and β-lactam derivatives (5), we subsequently focused on β-lactam-biotin-folate 1. Conjugation of 1 to hv-L and hv-H completely eradicated their catalytic activity (Fig. 3C), revealing near quantitative covalent conjugation to the reactive lysine residue of h38C2. As shown in Fig. 3D, the biotin moiety of 1 allowed to determine a conjugation yield of 93 ± 15% and 0 ± 0% to the chemically programmable and conventional DART, respectively, further demonstrating the high efficacy and specificity of hapten-driven covalent conjugation to h38C2.
With both their CD3 and FOLR1 binding capabilities shown, we next compared the two different sets of DARTs for crosslinking CD3+ and FOLR1+ cells by flow cytometry. For this, primary human T cells were stained red and mixed with blue stained OVCAR3 cells in the presence of the DARTs. The formation of red- and blue-stained cell aggregates was quantified by flow cytometry. Whereas hv-L/3 and hv-H/3, which do not contain a folate moiety, did not reveal mixed colored aggregate formation above background, hv-L/1 and hv-H/1 were as potent as fv-L and fv-H in cross-linking CD3+ and FOLR1+ cells (Fig. 6B). As expected, the higher FOLR1 cell surface density on IGROV1 cells led to more crosslinking events with primary human T cells or Jurkat cells (Fig. 7). Consistent with the weaker IGROV1 cell binding of the chemically programmed compared with the conventional DART (Fig. 4), we observed an analogous difference in crosslinking capability (Fig. 7).
FIGURE 7.
Crosslinking of chemically programmed and conventional FOLR1 × CD3 DARTs. A, IGROV1 cells were stained with CellTracker Blue CMAC and T cells or Jurkat cells with CellTrace Far Red DDAO-SE. The indicated DARTs were added at 2 μg/ml to 5 × 104 T cells or Jurkat cells. Following incubation for 1 h at room temperature and washing, 5 × 104 IGROV1 cells were added. The mixtures were then incubated for 1 h at room temperature, gently washed, and fixed with 1% (w/v) paraformaldehyde. The formation of blue/far red cell aggregates was detected by flow cytometry, using a forward scatter/side scatter gate that excluded the smaller non-crosslinked T cells and Jurkat cells from the analysis. The results are representative of three experiments. B, results from all three experiments were plotted as percentage of double positive events among all events following subtraction of the background observed with unprogrammed DART hv-L. Shown are mean values ± S.D.
Next, we compared the chemically programmed and conventional DARTs with respect to mediating cytotoxicity in the presence of ex vivo expanded primary human T cells. As shown in Fig. 8A, hv-L/1 and hv-H/1 were found to selectively and potently kill OVCAR3 cells in vitro without significant difference and in a dose-dependent manner. By contrast, hv-L/3 and hv-H/3 were indistinguishable from unprogrammed hv-L and hv-H in not revealing cytotoxicity above background levels detected in the absence of DARTs (Fig. 8A and data not shown). Unlike their equivalent potency toward OVCAR3 cells and consistent with the noted differences in cell binding and crosslinking capability, we detected significantly lower in vitro cytotoxicity of the chemically programmed compared with the conventional DART toward IGROV1 cells. Nonetheless, significant activity of hv-L/1 over background defined by unprogrammed hv-L was measured down to a concentration of 6 ng/ml (∼0.1 nm) (Fig. 8B). The potent in vitro activity of hv-L following chemical programming with 1 was also apparent from an interferon-γ release assay (Fig. 8C).
FIGURE 8.
In vitro activity of chemically programmed and conventional FOLR1 × CD3 DARTs. A, hv-L and hv-H DARTs were chemically programmed with biotin-β-diketone (compound 3) or folate-biotin-β-lactam (compound 1) as above. The cytotoxicity of chemically programmed DARTs hv-L and hv-H and conventional DARTs fv-L and fv-H was tested at 2 μg/ml (black), 0.6 μg/ml (gray), and 0.2 μg/ml (white) with ex vivo expanded primary human T cells (E) and OVCAR3 cells (T; 2.5 × 104/well in a 96-well tissue culture plate) at an E:T ratio of 10:1. In all experiments cytotoxicity was measured with the CytoTox-Glo Cytotoxicity Assay (Promega, Inc.) after 16-h incubation at 37 °C in folate-deficient cell culture medium supplemented with 5% (v/v) human serum. Luminescence was measured in a SpectraMax M5 microplate reader with SoftMax Pro software. Shown are mean values of triplicates ± S.D. B, cytotoxicity of conventional DART fv-L (black), chemically programmed DART hv-L/1 (white), and unprogrammed DART hv-L (gray) was assayed as described in A over a concentration range of 2 ng/ml to 2 μg/ml at half-log intervals with ex vivo expanded primary human T cells and IGROV1 cells at an E:T ratio of 10:1. Luminescence measured after incubation of effector and target cells in the absence of DARTs was subtracted. Shown are mean values of triplicates ± S.D. C, supernatants from B at the 2 μg/ml DART concentration were used to measure interferon-γ release by ELISA. Shown are mean values of triplicates ± S.D.
Encouraged by their strong in vitro activity, we next sought to compare the chemically programmed and conventional DARTs with respect to mediating cytotoxicity in vivo using NOD/SCID/IL-2Rγnull (NSG) mice xenotransplanted with IGROV1 cells in their peritoneal cavity. IGROV1 xenografts are established models of ovarian cancer with peritoneal carcinomatosis and ascites mimicking the human disease (33, 34). To allow in vivo bioluminescence imaging, we first stably transduced IGROV1 cells with a lentiviral vector encoding firefly luciferase (ffluc). We then injected 1 × 106 IGROV1/ffluc cells i.p. into each of 30 NSG mice in six cages and divided them into five cohorts consisting of six mice each (Fig. 9A). Prior to treatment, the human tumor xenograft was allowed to establish and grow for 6 days. This is in contrast to a previous study with a FOLR1-targeting chemically programmed biAb, where tumor cells and T cells (at 1:10 ratio) were implanted as mixture and treatment started on the same day (8). On day 6, four cohorts received 1 × 107 ex vivo expanded primary human T cells (∼30% CD8+ and ∼70% CD4+; Fig. 9B) by i.p. injection followed one hour later by an i.p injection of 10 μg of either fv-L, unprogrammed hv-L, or hv-L/1. The fourth cohort received PBS alone. The cohorts were treated with a total of 10 daily i.p. injections of 10 μg DARTs or PBS alone and tumor growth was monitored by in vivo bioluminescence imaging twice a week until day 23 when the tumor burden in the untreated cohorts required euthanasia. The three control groups, i.e. IGROV1 cells alone, IGROV1 cells with T cells, and IGROV1 cells with T cells and unprogrammed hv-L, revealed aggressive tumor growth without significant difference between the cohorts. By contrast, treatment with hv-L/1 and fv-L robustly decreased tumor burden in the first week and significantly slowed (hv-L/1) or stalled (fv-L) further tumor growth (Figs. 9A and 10A). No weight loss or other obvious signs of toxicity were observed during the treatment with the DARTs (Fig. 10B).
FIGURE 9.
In vivo activity of chemically programmed and conventional FOLR1 × CD3 DARTs. A, IGROV1/ffluc-cell-engrafted NSG mice in 6 cages were divided into 5 cohorts and treated as described in Fig. 10. Shown are bioluminescence images from day 23 for all 30 animals. B, flow cytometry analysis of ex vivo expanded T cells injected on day 6, using 2 μg/ml mouse anti-CD3, CD4, CD8, or CD28 mAbs followed by Alexa Fluor 488-conjugated goat anti-mouse IgG pAbs.
FIGURE 10.
In vivo activity and safety of chemically programmed and conventional FOLR1 × CD3 DARTs. A total of 30 NSG mice were i.p. injected with 1 × 106 IGROV1/ffluc cells on day 0 and divided into 5 cohorts, each consisting of 6 animals. Following human tumor xenograft establishment and growth, the animals in the 4 treatment cohorts received 1 × 107 ex vivo expanded T cells by i.p. injection and 1 h later 10 μg of the indicated DARTs or PBS alone by i.p. injection on day 6. The animals received a total of 10 daily (day 6 to 15) i.p. injections of DARTs or PBS alone. A, starting on day 2, the animals were imaged every 3–4 days as indicated. The graph shows the mean ± S.D. luminescence signal for the 5 cohorts. Significant differences between cohorts treated with unprogrammed hv-L DART (open red squares) and chemically programmed hv-L/1 DART (solid red squares) were calculated using Microsoft Excel software (two-tailed and heteroscedastic t test; *, p < 0.05; **, p < 0.01). Treatment time points are indicated by black arrows. B, starting on day 0, the animals were weighted on the indicated days. The graph shows the mean ± S.D. weights for the 5 cohorts using the same color code as in A.
Discussion
BiAbs are an emerging class of antibody drugs for cancer therapy. Of particular interest are biAbs that kill tumor cells by recruiting and activating endogenous T cells of the cancer patient. As such, these biAbs are comprised of a variable antibody module with specificity for a tumor cell surface antigen and a generic antibody module that has specificity for an activating T-cell receptor, typically CD3. Examples include the FDA- and European Medicines Agency (EMA)-approved CD19 × CD3 biAb blinatumomab and the EMA-approved EPCAM × CD3 biAb catumaxomab (35). In addition, several T-cell recruiting and activating biAbs in various formats have recently entered clinical trials, including the CD19 × CD3 biAb JNJ-64052781 (26), the CD123 × CD3 biAb MGD006 (36), the CD20 × CD3 biAb REGN1979 (37), and the CEA × CD3 biAb RO6958688 (38). Among these, JNJ-64052781, MGD006, and several additional clinically investigated biAbs are based on the DART format which, like the BiTE format used for blinatumomab, is approximately one third the size of IgG and does not include any light and heavy chain constant domains. Its favorable preclinical data along with its successful clinical translation, prompted us to explore the DART format for the generation of chemically programmed biAbs. For this concept, we paired two generic antibody modules, one with a covalent hapten binding site and the other with specificity for CD3. The antibody module with the covalent hapten binding site, h38C2, replaces the variable antibody module targeting tumor cell surface antigens. Through covalent conjugation of hapten derivatized small molecules that target tumor cell surface antigens to a unique reactive lysine residue in the h38C2 antibody module, our DART can be chemically programmed to acquire virtually any specificity mediated by a small molecule. In fact, h38C2 IgG1 has been previously utilized for chemical programming (9, 12) and translated as so called CovX-Bodies (39) to phase I and II clinical trials enrolling hundreds of cancer and type II diabetes mellitus patients (5). Reasoning that a combination of the DART and the CovX-Body platform, i.e. a rearranged set of clinically validated antibody molecules, antibody format, and antibody conjugation technology, would facilitate fast track translation for chemically programmed biAbs, we conducted the current proof-of-concept study.
To directly compare chemically programmed and conventional biAbs in DART format, we focused on FOLR1 as tumor cell surface antigen. FOLR1, also known as folate receptor α, is a clinically investigated target for both small molecules and mAbs (40, 41). It functions as a high affinity cellular entry receptor for folate (a.k.a. vitamin B9), which is essential for DNA synthesis, repair, and methylation, and thus for cell proliferation and survival. Tumor cells, activated macrophages, and proximal tubule epithelial cells of the kidney express FOLR1 to direct effective uptake of folate, whereas most other cell types obtain folate via the low affinity reduced folate carrier SLC19A1 or the proton-coupled folate transporter SLC46A1. Folate conjugates, which bind FOLR1 with nanomolar affinity and enter FOLR1-expressing cells by receptor-mediated endocytosis, do not bind SLC19A1 and SLC46A1, and thus can be used to selectively deliver drugs (28). Notably, FOLR1 is overexpressed in 82% of ovarian carcinoma, 66% of non-small-cell lung cancer (NSCLC), 64% of endometrial carcinoma, 64% of renal cell carcinoma, and at lower frequencies in many other solid malignancies (28). On the apical surface of the proximal tubule of the kidney FOLR1 serves as a salvage receptor for folate transport from the nascent urine back to the blood. Nonetheless, despite rigorous evaluation, kidney toxicity was not observed in phase I clinical trials with FOLR1-targeting small molecules (42) and mAbs (43), likely due to rapid transcytosis and inaccessibility, respectively. The two most advanced FOLR1-targeting drugs are the small molecule vintafolide, which is a conjugate of folate and vinblastine, and the humanized mouse anti-human FOLR1 mAb farletuzumab. After proving generally safe and well tolerated in phase I and II clinical trials, vintafolide and farletuzumab entered phase III clinical trials for the therapy of ovarian cancer. However, both failed to statistically improve progression free survival. This disappointing outcome provides a strong incentive for the development of more potent FOLR1-targeting therapeutics. Particularly attractive are T-cell recruiting and activating biAbs that target FOLR1 (44). In fact, several previous studies have explored strategies for initially random (45–47) and eventually site-specific (6, 8) conjugation of folate derivatives to CD3- or TCR-targeting mAbs. In addition, conventional FOLR1 × CD3 biAbs were among the first clinically investigated biAbs utilizing hybrid-hybridoma technology (48, 49) and continue to be pursued by contemporary antibody engineering strategies (50).
Taking advantage of the fact that both small molecules and mAbs bind to FOLR1 with high affinity, we generated and compared FOLR1 × CD3 biAbs in conventional DART format sporting a farletuzumab arm with FOLR1 × CD3 biAbs in chemically programmed DART format displaying a folate moiety. Our in vitro and in vivo data show that both potently kill ovarian cancer cells through recruitment and activation of human T cells. Comparable activity was noted for the ovarian cancer cell line OVCAR3. By contrast, the conventional DART performed moderately but consistently better than the chemically programmed DART when targeting the ovarian cancer cell line IGROV1 with higher FOLR1 expression levels. This higher activity appeared to be directly related to the stronger binding and cross-linking ability of the conventional DART and can be attributed to their distinct affinities, epitopes, and internalizations. Importantly, it is conceivable that modifications to the small molecule component of our chemically programmed DART will further improve its performance. For example, synthetic folate derivatives with two folate moieties spaced by a flexible linker would afford an avidity gain through bivalent binding of FOLR1. Indeed, we previously showed that a chemically programmed biAb with a bivalent small molecule component was outperforming its counterpart with a monovalent small molecule component (6). Notably, our generic design enables rapid lead optimization as a series of synthetic folate derivatives can be utilized to chemically program the same protein without any need for further cloning, expression, and purification.
The small size of BiTEs and DARTs (∼50 kDa) when compared with mAbs in IgG1 format (∼150 kDa), brings target and effector cells into close proximity, augmenting the formation of cytolytic synapses. However, this small size (along with the absence of an Fc domain that permits IgG recycling via binding to the neonatal Fc receptor) results in a relatively short circulatory half-life (t½). In fact, clinical trials with blinatumomab revealed a t½ of ∼2 h (51), requiring continuous i.v. infusion by portable minipumps to ensure prolonged exposure and enable close regulation (22). Although it is highly desirable to reduce treatment to weekly or biweekly i.v. injections, extending t½ without compromising cytolytic synapse formation and monovalent engagement of CD3 is challenging. On the other hand, chemically programmed biAbs provide unique solutions to extend t½ by means of derivatizing the small molecule component. For example, the biotin moiety in our trifunctional β-lactam-biotin-folate compound could easily be replaced with a polyethylene glycol polymer or with small molecules that bind to human serum albumin and thus extend t½ of the chemically programmed DART without substantially increasing its molecular weight. Collectively, the ability to tailor valence, specificity, and t½ via chemical synthesis are distinctive assets of chemically programmed biAbs and highly rely on site-specific as opposed to random conjugation (5).
A key advantage of chemically programmed antibodies and biAbs is their generic design that not only enables to confine lead optimization to the small molecule component but also permits targeting a virtually unlimited number and variety of antigens with a single protein (5). Our chemically programmable DART is compatible with a number of existing β-lactam derivatives of peptides, peptidomimetics, and other small molecules generated for the CovX-Body platform (5, 52). Several of these have already been investigated in phase I and II clinical trials, including a bispecific peptide (53). In preliminary studies, we chemically programmed the h38C2 × v9 DART with a β-lactam derivative of the human integrin α4β1-targeting peptidomimetic LLP2A (54) and successfully redirected cytotoxic T cells to integrin α4β1-expressing tumor cells. Beyond small molecules, β-lactam derivatives of RNA aptamers have also been successfully used for chemically programming mAb 38C2 (55). Collectively, our chemically programmable DARTs afford a versatile plug-and-play platform with broad utility in cancer immunotherapy.
Experimental Procedures
Chemical Compounds
Methodol was synthesized as described (56). For the synthesis of β-lactam-biotin-folate 1, β-diketone-biotin-folate 2, and β-diketone-biotin 3, see supplemental Experimental Procedures.
Cell Lines and Primary Cells
NCI-60 panel ovarian cancer cell lines IGROV1, OVCAR3, and SKOV3 cells were obtained from The Scripps Research Institute's Cell-based Screening Core and cultured in RPMI 1640 without folate supplemented with l-glutamine, 100 units/ml penicillin-streptomycin, and 10% fetal calf serum (all from Thermo Fisher Scientific). HEK 293 cells (American Type Culture Collection) were grown in DMEM supplemented with l-glutamine, 100 units/ml penicillin-streptomycin, and 10% fetal calf serum. Jurkat cells (American Type Culture Collection) were grown in RPMI 1640 supplemented with l-glutamine, 100 units/ml penicillin-streptomycin, and 10% fetal calf serum. Peripheral blood mononuclear cells (PBMC) were isolated from healthy donor buffy coats using Lymphoprep (Axis-Shield) and cultured in X-VIVO 20 medium (Lonza) with 5% off-the-clot human serum AB (Gemini Bio-Products) and 100 units/ml IL-2 (Cell Sciences). Primary T cells were expanded from PBMC as previously described (57) using Dynabeads® ClinExVivo™ CD3/CD28 (Thermo Fisher Scientific). IGROV1 cells used in the xenograft studies were stably transduced with ffluc using Lentifect™ Purified Lentivirus (GeneCopoeia).
Cloning, Expression, and Purification of DARTs
Sequences of the constructs were based on published or patented amino acid sequences. The variable domain encoding DNA sequences of kappa light chain (Vκ) and heavy chain (VH) with N-terminal signal peptide (MDWTWRILFLVAAATGAHS) and C-terminal upper hinge and His6 tag encoding sequences as indicated in Fig. 2 were synthesized as gBlocks (Integrated DNA Technologies). The G3SG4 linker encoding sequence was used to fuse the gBlocks by overlap extension PCR. The DART expression cassettes were NheI/XhoI-cloned into mammalian expression vector pCEP4 and transiently transfected into HEK 293 cells for production. The supernatants were collected 3 times over a 9-day period followed by filtration and concentration prior to purification by IMAC using 1-ml HisTrap HP columns (GE Healthcare Life Sciences) in conjunction with an ÄKTA FPLC instrument (GE Healthcare Life Sciences). Subsequent preparative and analytic SEC was performed with a Superdex 200 10/300 GL column (GE Healthcare) in conjunction with an ÄKTA FPLC instrument. The purity of DARTs was confirmed by SDS-PAGE followed by Coomassie Blue staining, and the concentration was determined by measuring the absorbance at 280 nm.
Chemical Programming of DARTs
Purified DARTs at 2 μg/ml (∼40 nm) were chemically programmed by incubation with 10 equivalents (400 nm) β-lactam or β-diketone derivatives, incubated for 1–2 h at room temperature, and purified with 15-ml Amicon Ultra Centrifugal Filter Devices equipped with 10-kDa MWCO membranes (Millipore). Quantitation of the chemical programming efficacy, i.e. the β-lactam-biotin-folate-to-DART ratio of hv-L/1, was measured using the EZ Biotin Quantitation kit (Thermo Fisher Scientific) using 4′-hydroxyazobenzene-2-carboxylic acid (HABA) dye and avidin as described in the manufacturer's protocol.
Catalytic Activity Assay
Catalytic activity was analyzed using methodol (56) as described previously (58). DARTs were diluted to 1 μm in PBS (pH 7.4) and dispensed in 98-μl aliquots into a 96-well plate in triplicate. Then, 2 μl of 10 mm methodol in ethanol was added and the fluorescence was assessed immediately using a SpectraMax M5 instrument (Molecular Devices) with SoftMax Pro software, a wavelength of excitation (λext) set to 330 nm, a wavelength of emission (λem) set to 452 nm, and starting at 0 min using 5-min time points. The signal was determined by normalizing to 98 μl PBS with 2 μl of the methodol preparation added. To analyze loss of catalytic activity after chemical programming, DARTs hv-L and hv-H were diluted to 3.4 μm in 118 μl PBS (pH 7.4), incubated with 4 μl 1 mm (10 equivalents) β-lactam-biotin-folate 1 in DMSO for 10 h at room temperature, and diluted for the catalytic assay as described above.
Flow Cytometry
Flow cytometry was performed on a LSR II flow cytometer (BD Biosciences) and data were analyzed with FlowJo software (Tree Star). Mouse anti-human CD3, CD4, CD8, and CD28 mAbs were purchased from BioLegend, PE-conjugated and APC-conjugated streptavidin from BD Biosciences, mouse anti-human FOLR1 from R&D Systems, Alexa Fluor 488-conjugated goat anti-mouse IgG pAbs from Jackson ImmunoResearch Laboratories, and mouse anti-His tag mAb Penta·His from Qiagen. For the cross-linking assay, target and effector cells were labeled with CellTracker Blue CMAC and CellTrace Far Red DDAO-SE (Thermo Fisher Scientific), respectively, according to the manufacturer's protocol. The labeled effector cells were then incubated with 2 μg/ml DARTs and washed before the target cells were added at a 1:1 ratio in 100 μl final volume. Following incubation for 1 h at room temperature, the cells were gently washed, fixed with 1% (w/v) paraformaldehyde, and analyzed by flow cytometry as described above.
Cytotoxicity Assay
Cytotoxicity was measured using CytoTox-Glo (Promega) following the manufacturer's protocol with minor modifications. Primary T cells expanded from healthy donor PBMC as described above were used as effector and IGROV1 or OVCAR3 were used as target cells. The cells were incubated at an effector-to-target (E:T) ratio of 10:1 in RPMI 1640 without folate supplemented with l-glutamine, 100 units/ml penicillin-streptomycin, and 5% off-the-clot human serum AB. The effector cells (2.5 × 105) were first incubated with the DARTs and washed prior to the addition of the targets cells (2.5 × 104) in a final volume of 100 μl/well in a 96-well tissue culture plate. The plates were incubated for 16 h at 37 °C with DART concentrations ranging from 2 ng/ml to 2 μg/ml. After centrifugation, 50 μl of the supernatant was transferred into a 96-well clear bottom white-walled plate (Costar 3610; Corning) containing 25 μl/well CytoTox-Glo. After 15 min at room temperature, the plate was read using a SpectraMax M5 instrument with SoftMax Pro software. The same supernatants (diluted 50-fold) used for the CytoTox-Glo assay were also used to determine IFN-γ secretion with the Human IFN gamma ELISA Ready-SET-Go! reagent set (eBioscience) following the manufacturer's protocol.
Mouse Xenograft Studies
Thirty 5-week-old NSG mice (The Jackson Laboratory) were each given 1 × 106 IGROV1-ffluc intraperitoneally (i.p.) on day 0. On day 2, the animals were i.p. injected with 150 mg/kg d-luciferin (15 mg/ml stock solution; Biosynth) and divided into 5 groups of 6 animals each by average bioluminescence. On day 6, each animal (except the no treatment cohort) were i.p. injected with 1 × 107 primary T cells expanded from healthy donor PBMC as described above, and 1 h later, with 10 μg of DARTs or PBS alone. This was repeated for a total of 10 daily (day 6 to 15) i.p. injections. Every 3–4 days, tumor growth was monitored by bioluminescent imaging 5 min after i.p. injections with 150 mg/kg d-luciferin. The weight of the mice was measured every 1–4 days.
Author Contributions
C. R. conceived, designed, supervised, and analyzed experiments; E. W. conceived, designed, conducted, and analyzed biological syntheses, biochemical conjugations, and in vitro and in vivo experiments; T. R. B. Jr. conceived, designed, analyzed, and supervised chemical syntheses; C. G. N. conceived, designed, conducted, and analyzed chemical syntheses; J. Q. conducted and analyzed in vitro experiments; A. R. N. conducted and analyzed chemical syntheses and in vitro experiments; R. K. G. conducted and analyzed chemical syntheses; W. R. R. and S. C. S. supervised and analyzed chemical syntheses; E. W. and C. R. wrote the manuscript.
Supplementary Material
Acknowledgments
We thank Scott Troutman for help with the mouse xenograft studies and Else Marit Inderberg for kindly providing PBMC. This is manuscript 29359 from The Scripps Research Institute.
This work was supported by National Institutes of Health Grant R01 CA181258 (to C. R.) and the Intramural Research Program, Center for Cancer Research, NCI, National Institutes of Health (to T. R. B., Jr.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental information.
- biAbs
- bispecific antibodies
- mAb
- monoclonal antibody
- DART
- dual-affinity re-targeting
- FOLR1
- folate receptor 1
- BiTE
- bispecific T-cell engager
- scFv
- single-chain Fv
- TCR
- T-cell receptor
- VL
- variable domain of the light chain
- Vκ
- variable domain of the kappa light chain
- VH
- variable domain of the heavy chain
- HEK
- human embryonic kidney
- IMAC
- immobilized metal affinity chromatography
- SEC
- size exclusion chromatography
- NSG
- NOD/SCID/IL-2Rγnull
- ffluc
- firefly luciferase
- PBMC
- peripheral blood mononuclear cells.
References
- 1. Zhukovsky E. A., Morse R. J., and Maus M. V. (2016) Bispecific antibodies and CARs: generalized immunotherapeutics harnessing T cell redirection. Curr. Opin. Immunol. 40, 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kontermann R. E., and Brinkmann U. (2015) Bispecific antibodies. Drug Discov. Today 20, 838–847 [DOI] [PubMed] [Google Scholar]
- 3. Frankel S. R., and Baeuerle P. A. (2013) Targeting T cells to tumor cells using bispecific antibodies. Curr. Opin. Chem. Biol. 17, 385–392 [DOI] [PubMed] [Google Scholar]
- 4. Perez P., Hoffman R. W., Shaw S., Bluestone J. A., and Segal D. M. (1985) Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature 316, 354–356 [DOI] [PubMed] [Google Scholar]
- 5. Rader C. (2014) Chemically programmed antibodies. Trends Biotechnol. 32, 186–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui H., Thomas J. D., Burke T. R. Jr, and Rader C. (2012) Chemically programmed bispecific antibodies that recruit and activate T cells. J. Biol. Chem. 287, 28206–28214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim C. H., Axup J. Y., Lawson B. R., Yun H., Tardif V., Choi S. H., Zhou Q., Dubrovska A., Biroc S. L., Marsden R., Pinstaff J., Smider V. V., and Schultz P. G. (2013) Bispecific small molecule-antibody conjugate targeting prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 17796–17801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kularatne S. A., Deshmukh V., Gymnopoulos M., Biroc S. L., Xia J., Srinagesh S., Sun Y., Zou N., Shimazu M., Pinkstaff J., Ensari S., Knudsen N., Manibusan A., Axup J. Y., Kim C. H., Smider V. V., Javahishvili T., and Schultz P. G. (2013) Recruiting cytotoxic T cells to folate-receptor-positive cancer cells. Angew. Chem. Int. Ed. Engl. 52, 12101–12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rader C., Turner J. M., Heine A., Shabat D., Sinha S. C., Wilson I. A., Lerner R. A., and Barbas C. F. (2003) A humanized aldolase antibody for selective chemotherapy and adaptor immunotherapy. J. Mol. Biol. 332, 889–899 [DOI] [PubMed] [Google Scholar]
- 10. Wagner J., Lerner R. A., and Barbas C. F. 3rd (1995) Efficient aldolase catalytic antibodies that use the enamine mechanism of natural enzymes. Science 270, 1797–1800 [DOI] [PubMed] [Google Scholar]
- 11. Barbas C. F. 3rd, Heine A., Zhong G., Hoffmann T., Gramatikova S., Björnestedt R., List B., Anderson J., Stura E. A., Wilson I. A., and Lerner R. A. (1997) Immune versus natural selection: antibody aldolases with enzymic rates but broader scope. Science 278, 2085–2092 [DOI] [PubMed] [Google Scholar]
- 12. Rader C., Sinha S. C., Popkov M., Lerner R. A., and Barbas C. F. 3rd (2003) Chemically programmed monoclonal antibodies for cancer therapy: adaptor immunotherapy based on a covalent antibody catalyst. Proc. Natl. Acad. Sci. U.S.A. 100, 5396–5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li L. S., Rader C., Matsushita M., Das S., Barbas C. F. 3rd, Lerner R. A., and Sinha S. C. (2004) Chemical adaptor immunotherapy: design, synthesis, and evaluation of novel integrin-targeting devices. J. Med. Chem. 47, 5630–5640 [DOI] [PubMed] [Google Scholar]
- 14. Popkov M., Rader C., Gonzalez B., Sinha S. C., and Barbas C. F. 3rd (2006) Small molecule drug activity in melanoma models may be dramatically enhanced with an antibody effector. Int. J. Cancer 119, 1194–1207 [DOI] [PubMed] [Google Scholar]
- 15. Beverley P. C., and Callard R. E. (1981) Distinctive functional characteristics of human “T” lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur. J. Immunol. 11, 329–334 [DOI] [PubMed] [Google Scholar]
- 16. Arnett K. L., Harrison S. C., and Wiley D. C. (2004) Crystal structure of a human CD3-epsilon/delta dimer in complex with a UCHT1 single-chain antibody fragment. Proc. Natl. Acad. Sci. U.S.A. 101, 16268–16273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodrigues M. L., Shalaby M. R., Werther W., Presta L., and Carter P. (1992) Engineering a humanized bispecific F(ab′)2 fragment for improved binding to T cells. Int. J. Cancer Suppl. 7, 45–50 [PubMed] [Google Scholar]
- 18. Zhu Z., Lewis G. D., and Carter P. (1995) Engineering high affinity humanized anti-p185HER2/anti-CD3 bispecific F(ab′)2 for efficient lysis of p185HER2 overexpressing tumor cells. Int. J. Cancer 62, 319–324 [DOI] [PubMed] [Google Scholar]
- 19. Bargou R., Leo E., Zugmaier G., Klinger M., Goebeler M., Knop S., Noppeney R., Viardot A., Hess G., Schuler M., Einsele H., Brandl C., Wolf A., Kirchinger P., Klappers P., Schmidt M., Riethmüller G., Reinhardt C., Baeuerle P. A., and Kufer P. (2008) Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321, 974–977 [DOI] [PubMed] [Google Scholar]
- 20. Topp M. S., Kufer P., Gökbuget N., Goebeler M., Klinger M., Neumann S., Horst H. A., Raff T., Viardot A., Schmid M., Stelljes M., Schaich M., Degenhard E., Köhne-Volland R., Brüggemann M., Ottmann O., Pfeifer H., Burmeister T., Nagorsen D., Schmidt M., Lutterbuese R., Reinhardt C., Baeuerle P. A., Kneba M., Einsele H., Riethmüller G., Hoelzer D., Zugmaier G., and Bargou R. C. (2011) Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 29, 2493–2498 [DOI] [PubMed] [Google Scholar]
- 21. Przepiorka D., Ko C. W., Deisseroth A., Yancey C. L., Candau-Chacon R., Chiu H. J., Gehrke B. J., Gomez-Broughton C., Kane R. C., Kirshner S., Mehrotra N., Ricks T. K., Schmiel D., Song P., Zhao P., Zhou Q., Farrell A. T., and Pazdur R. (2015) FDA Approval: Blinatumomab. Clin. Cancer Res. 21, 4035–4039 [DOI] [PubMed] [Google Scholar]
- 22. Baeuerle P. A., and Reinhardt C. (2009) Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 69, 4941–4944 [DOI] [PubMed] [Google Scholar]
- 23. Rader C. (2011) DARTs take aim at BiTEs. Blood 117, 4403–4404 [DOI] [PubMed] [Google Scholar]
- 24. Holliger P., Prospero T., and Winter G. (1993) “Diabodies”: small bivalent and bispecific antibody fragments. Proc. Natl. Acad. Sci. U.S.A. 90, 6444–6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson S., Burke S., Huang L., Gorlatov S., Li H., Wang W., Zhang W., Tuaillon N., Rainey J., Barat B., Yang Y., Jin L., Ciccarone V., Moore P. A., Koenig S., and Bonvini E. (2010) Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J. Mol. Biol. 399, 436–449 [DOI] [PubMed] [Google Scholar]
- 26. Moore P. A., Zhang W., Rainey G. J., Burke S., Li H., Huang L., Gorlatov S., Veri M. C., Aggarwal S., Yang Y., Shah K., Jin L., Zhang S., He L., Zhang T., Ciccarone V., Koenig S., Bonvini E., and Johnson S. (2011) Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 117, 4542–4551 [DOI] [PubMed] [Google Scholar]
- 27. Teng L., Xie J., and Lee R. J. (2012) Clinical translation of folate receptor-targeted therapeutics. Expert Opin. Drug Deliv. 9, 901–908 [DOI] [PubMed] [Google Scholar]
- 28. Xia W., and Low P. S. (2010) Folate-targeted therapies for cancer. J. Med. Chem. 53, 6811–6824 [DOI] [PubMed] [Google Scholar]
- 29. Vergote I. B., Marth C., and Coleman R. L. (2015) Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications. Cancer Metastasis Rev. 34, 41–52 [DOI] [PubMed] [Google Scholar]
- 30. Ebel W., Routhier E. L., Foley B., Jacob S., McDonough J. M., Patel R. K., Turchin H. A., Chao Q., Kline J. B., Old L. J., Phillips M. D., Nicolaides N. C., Sass P. M., and Grasso L. (2007) Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-α. Cancer Immun. 7, 6. [PMC free article] [PubMed] [Google Scholar]
- 31. Kamen B. A., and Smith A. K. (2012) Farletuzumab, an anti-folate receptor α antibody, does not block binding of folate or anti-folates to receptor nor does it alter the potency of anti-folates in vitro. Cancer Chemother. Pharmacol. 70, 113–120 [DOI] [PubMed] [Google Scholar]
- 32. Zhu X., Tanaka F., Lerner R. A., Barbas C. F. 3rd, and Wilson I. A. (2009) Direct observation of an enamine intermediate in amine catalysis. J. Am. Chem. Soc. 131, 18206–18207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Cesare M., Calcaterra C., Pratesi G., Gatti L., Zunino F., Mènard S., and Balsari A. (2008) Eradication of ovarian tumor xenografts by locoregional administration of targeted immunotherapy. Clin. Cancer Res. 14, 5512–5518 [DOI] [PubMed] [Google Scholar]
- 34. Sommariva M., De Cecco L., De Cesare M., Sfondrini L., Ménard S., Melani C., Delia D., Zaffaroni N., Pratesi G., Uva V., Tagliabue E., and Balsari A. (2011) TLR9 agonists oppositely modulate DNA repair genes in tumor versus immune cells and enhance chemotherapy effects. Cancer Res. 71, 6382–6390 [DOI] [PubMed] [Google Scholar]
- 35. Linke R., Klein A., and Seimetz D. (2010) Catumaxomab: clinical development and future directions. mAbs 2, 129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chichili G. R., Huang L., Li H., Burke S., He L., Tang Q., Jin L., Gorlatov S., Ciccarone V., Chen F., Koenig S., Shannon M., Alderson R., Moore P. A., Johnson S., and Bonvini E. (2015) A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates. Sci. Transl. Med. 7, 289ra282. [DOI] [PubMed] [Google Scholar]
- 37. Smith E. J., Olson K., Haber L. J., Varghese B., Duramad P., Tustian A. D., Oyejide A., Kirshner J. R., Canova L., Menon J., Principio J., MacDonald D., Kantrowitz J., Papadopoulos N., Stahl N., Yancopoulos G. D., Thurston G., and Davis S. (2015) A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci. Rep. 5, 17943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bacac M., Fauti T., Sam J., Colombetti S., Weinzierl T., Ouaret D., Bodmer W., Lehmann S., Hofer T., Hosse R. J., Moessner E., Ast O., Bruenker P., Grau-Richards S., Schaller T., et al. (2016) A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors. Clin. Cancer Res. 22, 3286–3297 [DOI] [PubMed] [Google Scholar]
- 39. Huang H., Lai J. Y., Do J., Liu D., Li L., Del Rosario J., Doppalapudi V. R., Pirie-Shepherd S., Levin N., Bradshaw C., Woodnutt G., Lappe R., and Bhat A. (2011) Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin. Cancer Res. 17, 1001–1011 [DOI] [PubMed] [Google Scholar]
- 40. Low P. S., and Kularatne S. A. (2009) Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 13, 256–262 [DOI] [PubMed] [Google Scholar]
- 41. Spannuth W. A., Sood A. K., and Coleman R. L. (2010) Farletuzumab in epithelial ovarian carcinoma. Expert Opin. Biol. Ther. 10, 431–437 [DOI] [PubMed] [Google Scholar]
- 42. Lorusso P. M., Edelman M. J., Bever S. L., Forman K. M., Pilat M., Quinn M. F., Li J., Heath E. I., Malburg L. M., Klein P. J., Leamon C. P., Messmann R. A., and Sausville E. A. (2012) Phase I study of folate conjugate EC145 (Vintafolide) in patients with refractory solid tumors. J. Clin. Oncol. 30, 4011–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Konner J. A., Bell-McGuinn K. M., Sabbatini P., Hensley M. L., Tew W. P., Pandit-Taskar N., Vander Els N., Phillips M. D., Schweizer C., Weil S. C., Larson S. M., and Old L. J. (2010) Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin. Cancer Res. 16, 5288–5295 [DOI] [PubMed] [Google Scholar]
- 44. Roy E. J., Gawlick U., Orr B. A., and Kranz D. M. (2004) Folate-mediated targeting of T cells to tumors. Adv. Drug Deliv. Rev. 56, 1219–1231 [DOI] [PubMed] [Google Scholar]
- 45. Kranz D. M., Patrick T. A., Brigle K. E., Spinella M. J., and Roy E. J. (1995) Conjugates of folate and anti-T-cell-receptor antibodies specifically target folate-receptor-positive tumor cells for lysis. Proc. Natl. Acad. Sci. U.S.A. 92, 9057–9061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kranz D. M., Manning T. C., Rund L. A., Cho B. K., Gruber M. M., and Roy E. J. (1998) Targeting tumor cells with bispecific antibodies and T cells. J. Control. Release 53, 77–84 [DOI] [PubMed] [Google Scholar]
- 47. Thompson S., Dessi J., and Self C. H. (2009) Preclinical evaluation of light-activatable, bispecific anti-human CD3 antibody conjugates as anti-ovarian cancer therapeutics. mAbs 1, 348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Ravenswaay Claasen H. H., van de Griend R. J., Mezzanzanica D., Bolhuis R. L., Warnaar S. O., and Fleuren G. J. (1993) Analysis of production, purification, and cytolytic potential of bi-specific antibodies reactive with ovarian-carcinoma-associated antigens and the T-cell antigen CD3. Int. J. Cancer 55, 128–136 [DOI] [PubMed] [Google Scholar]
- 49. Tibben J. G., Boerman O. C., Massuger L. F., Schijf C. P., Claessens R. A., and Corstens F. H. (1996) Pharmacokinetics, biodistribution and biological effects of intravenously administered bispecific monoclonal antibody OC/TR F(ab′)2 in ovarian carcinoma patients. Int. J. Cancer 66, 477–483 [DOI] [PubMed] [Google Scholar]
- 50. Schreiner J., Thommen D. S., Herzig P., Bacac M., Klein C., Roller A., Belousov A., Levitsky V., Savic S., Moersig W., Uhlenbrock F., Heinzelmann-Schwarz V. A., Umana P., Pisa P., Lardinois D., Müller P., Karanikas V., and Zippelius A. (2016) Expression of inhibitory receptors on intratumoral T cells modulates the activity of a T cell-bispecific antibody targeting folate receptor. Oncoimmunology 5, e1062969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu M., Wu B., Brandl C., Johnson J., Wolf A., Chow A., and Doshi S. (2016) Blinatumomab, a Bispecific T-cell Engager (BiTE) for CD-19 Targeted cancer immunotherapy: Clinical pharmacology and its implications. Clin. Pharmacokinet. May 21 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 52. Liu Y., Goswami R. K., Liu C., and Sinha S. C. (2015) Chemically programmed bispecific antibody targeting Legumain protease and αvβ3 integrin mediates strong antitumor effects. Mol. Pharm. 12, 2544–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Doppalapudi V. R., Huang J., Liu D., Jin P., Liu B., Li L., Desharnais J., Hagen C., Levin N. J., Shields M. J., Parish M., Murphy R. E., Del Rosario J., Oates B. D., Lai J. Y., Matin M. J., Ainekulu Z., Bhat A., Bradshaw C. W., Woodnutt G., Lerner R. A., and Lappe R. W. (2010) Chemical generation of bispecific antibodies. Proc. Natl. Acad. Sci. U.S.A. 107, 22611–22616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peng L., Liu R., Marik J., Wang X., Takada Y., and Lam K. S. (2006) Combinatorial chemistry identifies high-affinity peptidomimetics against α4β1 integrin for in vivo tumor imaging. Nat. Chem. Biol. 2, 381–389 [DOI] [PubMed] [Google Scholar]
- 55. Wuellner U., Gavrilyuk J. I., and Barbas C. F. 3rd (2010) Expanding the concept of chemically programmable antibodies to RNA aptamers: chemically programmed biotherapeutics. Angew. Chem. Int. Ed. Engl. 49, 5934–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. List B., Barbas C. F. 3rd, and Lerner R. A. (1998) Aldol sensors for the rapid generation of tunable fluorescence by antibody catalysis. Proc. Natl. Acad. Sci. U.S.A. 95, 15351–15355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Almåsbak H., Rian E., Hoel H. J., Pulè M., Wälchli S., Kvalheim G., Gaudernack G., and Rasmussen A. M. (2011) Transiently redirected T cells for adoptive transfer. Cytotherapy 13, 629–640 [DOI] [PubMed] [Google Scholar]
- 58. Sinha S. C., Das S., Li L. S., Lerner R. A., and Barbas C. F. 3rd (2007) Preparation of integrin α(v)β3-targeting Ab 38C2 constructs. Nat. Protoc. 2, 449–456 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.