Abstract
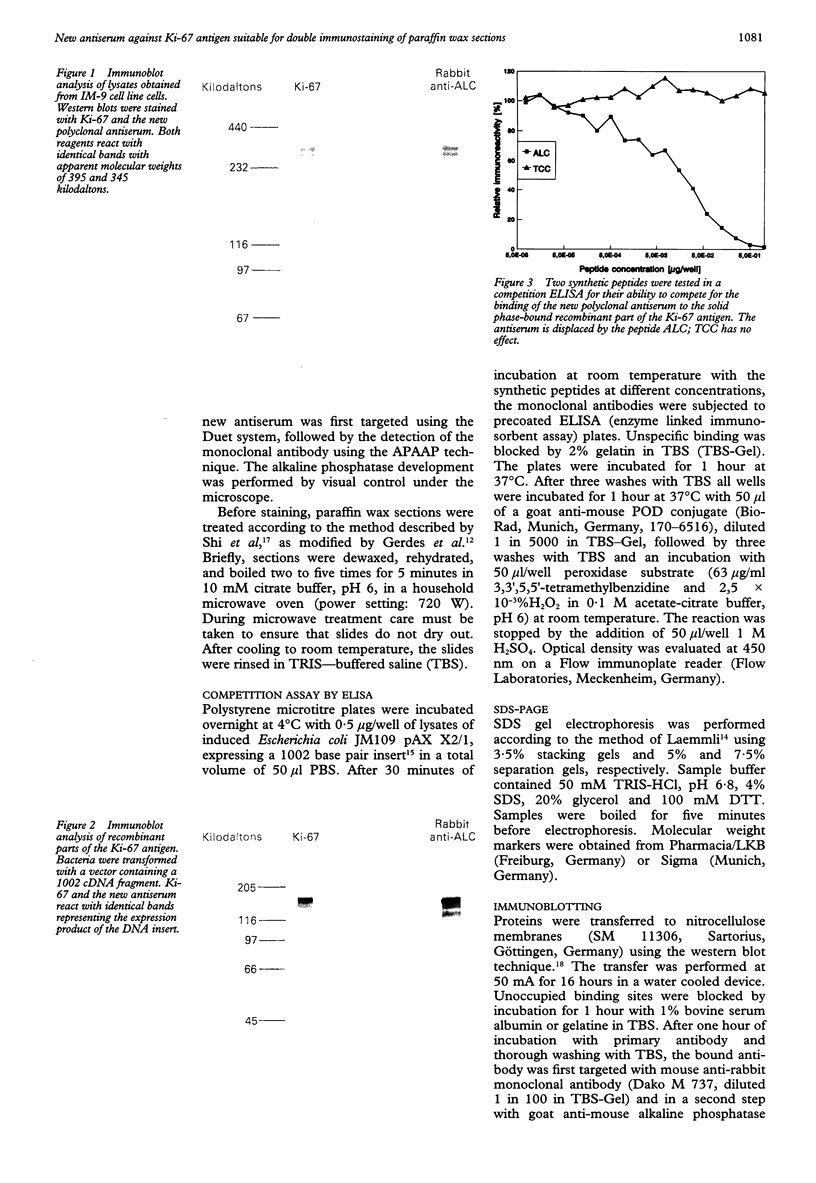
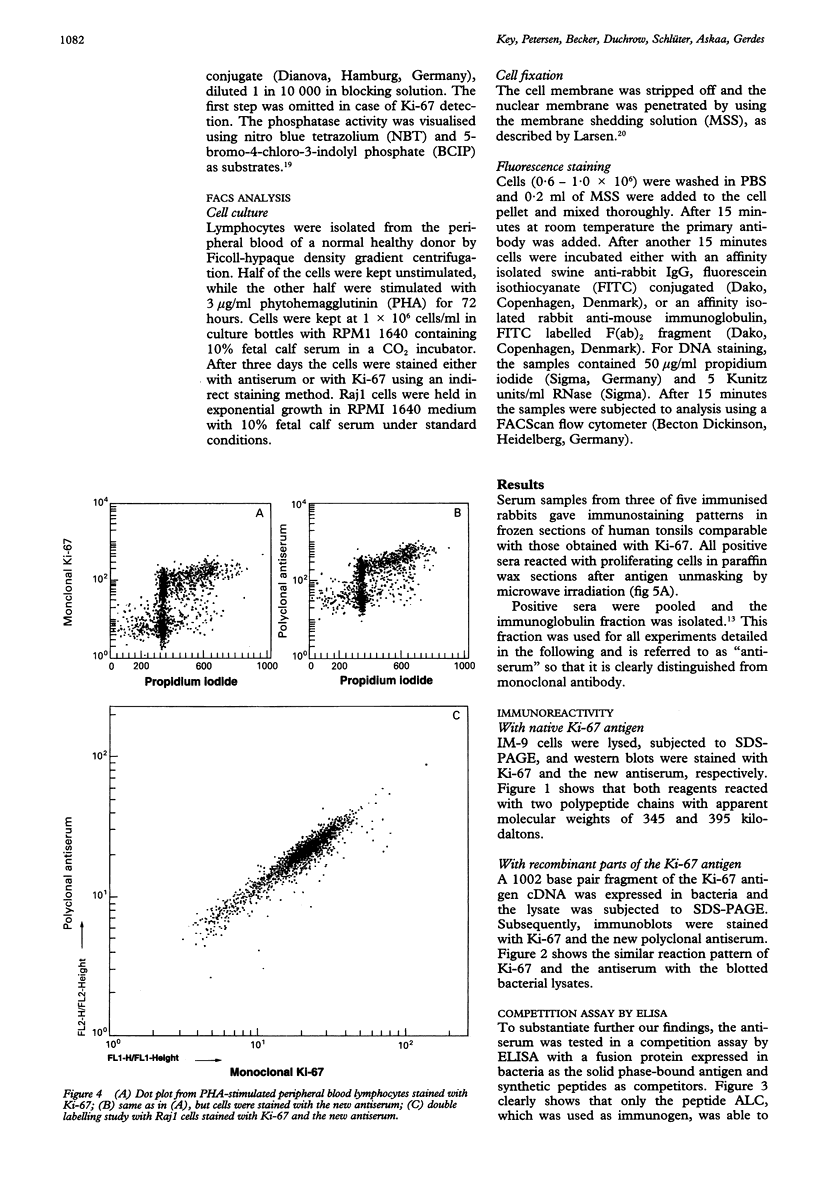
AIMS--To prepare a rabbit antiserum equivalent to MIB 1 to permit the simultaneous assessment of cell proliferation and other markers of interest using double labelling studies. METHODS--Rabbits were immunised with a synthetic peptide deduced from the cDNA sequence coding for the Ki-67 antigen. Serum samples were tested for immunoreactivity using different immunobiochemical methods. RESULTS--A polyclonal antiserum was derived which detects the native as well as recombinant parts of the Ki-67 antigen in different test systems. Furthermore, the antiserum stains the Ki-67 antigen in routinely processed, paraffin wax embedded material. CONCLUSIONS--After antigen unmasking by microwave treatment the antiserum described here represents a powerful tool for the determination of growth fractions even in archival material. It is especially suitable for double staining experiments in combination with monoclonal antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Becker M. H., Key G., Cattoretti G. Immunohistological detection of tumour growth fraction (Ki-67 antigen) in formalin-fixed and routinely processed tissues. J Pathol. 1992 Sep;168(1):85–86. doi: 10.1002/path.1711680114. [DOI] [PubMed] [Google Scholar]
- Gerdes J. Ki-67 and other proliferation markers useful for immunohistological diagnostic and prognostic evaluations in human malignancies. Semin Cancer Biol. 1990 Jun;1(3):199–206. [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Stein H., Pileri S., Rivano M. T., Gobbi M., Ralfkiaer E., Nielsen K. M., Pallesen G., Bartels H., Palestro G. Prognostic relevance of tumour-cell growth fraction in malignant non-Hodgkin's lymphomas. Lancet. 1987 Aug 22;2(8556):448–449. doi: 10.1016/s0140-6736(87)90977-9. [DOI] [PubMed] [Google Scholar]
- Grogan T. M., Lippman S. M., Spier C. M., Slymen D. J., Rybski J. A., Rangel C. S., Richter L. C., Miller T. P. Independent prognostic significance of a nuclear proliferation antigen in diffuse large cell lymphomas as determined by the monoclonal antibody Ki-67. Blood. 1988 Apr;71(4):1157–1160. [PubMed] [Google Scholar]
- Hall P. A., Richards M. A., Gregory W. M., d'Ardenne A. J., Lister T. A., Stansfeld A. G. The prognostic value of Ki67 immunostaining in non-Hodgkin's lymphoma. J Pathol. 1988 Mar;154(3):223–235. doi: 10.1002/path.1711540305. [DOI] [PubMed] [Google Scholar]
- Holte H., de Lange Davies C., Beiske K., Stokke T., Marton P. F., Smeland E. B., Høie J., Kvaløy S. Ki67 and 4F2 antigen expression as well as DNA synthesis predict survival at relapse/tumour progression in low-grade B-cell lymphoma. Int J Cancer. 1989 Dec 15;44(6):975–980. doi: 10.1002/ijc.2910440605. [DOI] [PubMed] [Google Scholar]
- Key G., Becker M. H., Baron B., Duchrow M., Schlüter C., Flad H. D., Gerdes J. New Ki-67-equivalent murine monoclonal antibodies (MIB 1-3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest. 1993 Jun;68(6):629–636. [PubMed] [Google Scholar]
- Key G., Meggetto F., Becker M. H., al Saati T., Schlüter C., Duchrow M., Delsol G., Gerdes J. Immunobiochemical characterization of the antigen detected by monoclonal antibody IND.64. Evidence that IND.64 reacts with the cell proliferation associated nuclear antigen previously defined by Ki-67. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62(4):259–262. doi: 10.1007/BF02899690. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen J. K. Washless double staining of a nuclear antigen (Ki-67 or bromodeoxyuridine) and DNA in unfixed nuclei. Methods Cell Biol. 1990;33:227–234. doi: 10.1016/s0091-679x(08)60528-2. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungekar M. F., Gatter K. C., Dunnill M. S., Mason D. Y. Ki-67 immunostaining and survival in operable lung cancer. Histopathology. 1991 Dec;19(6):545–550. doi: 10.1111/j.1365-2559.1991.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Wintzer H. O., Zipfel I., Schulte-Mönting J., Hellerich U., von Kleist S. Ki-67 immunostaining in human breast tumors and its relationship to prognosis. Cancer. 1991 Jan 15;67(2):421–428. doi: 10.1002/1097-0142(19910115)67:2<421::aid-cncr2820670217>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]





