Abstract
Metastatic Ewing sarcoma has a very poor prognosis and therefore new investigations into the biologic drivers of metastatic progression are key to finding new therapeutic approaches. The tumor microenvironment is highly dynamic, leading to exposure of different regions of a growing solid tumor to changes in oxygen and nutrient availability. Tumor cells must adapt to such stress in order to survive and propagate. In the current study, we investigate how Ewing sarcoma cells respond to the stress of growth factor deprivation and hypoxia. Our findings reveal that serum deprivation leads to a reversible change in Ewing cell cytoskeletal phenotypes. Using an array of migration and invasion techniques, including gelatin matrix degradation invadopodia assays, we show that exposure of Ewing sarcoma cells to serum deprivation and hypoxia triggers enhanced migration, invadopodia formation, matrix degradation and invasion. Further, these functional changes are accompanied by and dependent on activation of Src kinase. Activation of Src, and the associated invasive cell phenotype, were blocked by exposing hypoxia and serum-deprived cells to the Src inhibitor dasatinib. These results indicate that Ewing sarcoma cells demonstrate significant plasticity in response to rapidly changing micro-environmental stresses that can result from rapid tumor growth and from necrosis-causing therapies. In response to these stresses, Ewing cells transition to a more migratory and invasive state and our data show that Src is an important mediator of this stress response. Our data support exploration of clinically available Src inhibitors as adjuvant agents for metastasis prevention in Ewing sarcoma.
Introduction
Ewing sarcoma is a tumor of the bone or soft tissue that is driven by EWS-ETS fusion oncoproteins, most commonly EWS-FLI1. The incidence of Ewing sarcoma peaks in adolescents and young adults and most patients in this age group present with localized bone tumors and no overt evidence of metastatic disease [1], [2]. Treatment for localized Ewing sarcoma has been intensified over the past two decades and upfront, compressed cycles of alternating vincristine/doxorubicin/cyclophosphamide and ifosfamide/etoposide is the current standard of cared and has led to a significant improvement in survival in this patient population [3]. However, for patients who present with overt metastatic disease or who relapse following initial therapy, survival estimates remain dismal. Common sites of Ewing sarcoma metastasis are the lungs, bones and bone marrow and metatastic spread can be detected at any time, including many years after initial presentation [4], [5]. Despite attempts to identify biomarkers of aggressive disease, it is still not clear why some patients never develop metastasis and others go on to relapse at distant sites despite experiencing initial clinical remissions [6]. Therefore, an improved understanding of the underlying biologic processes that contribute to Ewing sarcoma metastasis is needed if we are to advance therapies to prevent and treat progressive disease in this high-risk population [2].
The development and progression of solid tumors is dependent on both tumor cell autonomous factors, such as the presence of oncogenic mutations, and on the contributions of the tumor microenvironment. The tumor microenvironment includes the collection of secreted factors and cells that support and surround the tumor cells [7]. In addition, while a number of secreted factors can locally alter cell signaling [8], a more overarching influence is the impact of hypoxia or nutrient deprivation on tumor cell behavior. These micro-environmental stresses occur when tumors outreach their blood supply or experience a rapid loss in blood flow due to surgery, radiation or rapid tumor shrinkage secondary to chemotherapy-induced tumor necrosis. Previous reports have noted that conditions of hypoxia alter the transcriptional signature of EWS-FLI1 [9], highlighting the potential impact of local stresses on Ewing sarcoma cell behavior.
Prior work in our lab demonstrated that Ewing cells have the ability to alter the expression of a key cell surface receptor, CXCR4, in a rapid, reversible manner in response to microenvironmental stress, including hypoxia and growth factor deprivation [10]. The plasticity in expression of this G-protein coupled receptor altered the ability of cells to migrate toward the chemokine ligand CXCL12, also known as SDF-1. Given the key observation that stress can rapidly and dynamically alter the CXCR4 axis in Ewing sarcoma to promote chemotactic migration and invasion, we postulated that micro-environmental stress might also have other more global effects on the tumor cells that could contribute to a migratory and/or invasive phenotype. Cell migration and invasion are essential components of the metastatic cascade and, therefore, elucidation of the mechanisms by which these processes are induced in Ewing sarcoma cells could provide novel therapeutic opportunities to specifically prevent tumor metastasis. In this study, we therefore sought to further explore the impact of micro-environmental stress on Ewing sarcoma cells and to investigate the biologic mechanisms that contribute to cell plasticity and emergence of migratory/invasive phenotypes.
Materials and methods
Cell lines and culture
The Ewing sarcoma cell lines A673 (ATCC, Bethesda MD, USA) and CHLA-25 (Children's Oncology Group, COGcell.org) were cultured in RPMI-1640 media (Gibco, Grand Island, NY, USA) and CHLA-10 (COGcell.org) in IMDM media (Gibco). Cell lines were supplemented with 10% (A673, CHLA-25) or 20% (CHLA10) FBS (Atlas Biologicals, Inc., Fort Collins, CO, USA) and 6 mM L-glutamine (Life Technologies, Grand Island, NY, USA). CHLA-10 cells were additionally supplemented with 1× insulin-transferrin-selenium (Life Technologies, Grand Island, NY, USA). HeLa (ATCC) and PANC1 (a kind gift from the laboratory of Dr. Diane Simeone, Univeristy of Michigan, Ann Arbor, MI) cells were also utilized as a control cell lines in some experiments and were cultured in RPMI-1640 or DMEM, respectively, and supplemented with 10% FBS and 6 mM L-glutamine. Standard cell cultures were maintained in ambient conditions at 37°C. For hypoxia studies, cells were incubated in consistent 1% O2 in an xVivo system (Biospherix, Parish, NY) at 37°C and 5% CO2. Serum deprivation consisted of 0% FBS containing media. All cell lines are routinely tested for mycoplasma and confirmed to be negative. Cell lines in the lab have also undergone STR profiling to ensure authenticity.
Reagents
Primary and conjugated antibodies used in these studies include phalloidin (Alexa Fluor 488 and 350, Molecular Probes, Eugene, OR), cortactin (4F11 Alexa Fluor 555 conjugate; Millipore, Temecula, CA), p-Y416 Src (Life Technologies, Frederick, MD), and GAPDH (Cell Signaling, Beverly, MA). Secondary antibodies for western blot analysis included IRDye 800CW goat anti-rabbit and goat anti-mouse (LiCor, Lincoln, NE). TGF-beta was purchased from R&D Systems (Minneapolis, MN) and was used at a concentration of 10 ng/mL. Vehicle control for the TGF-beta treatment was 4 mM HCl in sterile water.
Migration and invasion assays
Real-time cell analysis (RTCA) of cell migration and invasion was monitored using a CIM-plate 16 and xCELLigence DP System (Acea Bioscience, Inc.). Cells were serum starved overnight in 0% FBS containing media. For migration studies, 1×105 cells/well were placed in the upper chamber of a CIM-16 plate and then the plate was equilibrated for 30 minutes at room temperature. For migration assays done with a combination of stresses, cells were serum-starved and placed in either normoxic or hypoxic conditions overnight before evaluation of migration. For invasion studies, 1×105 cells/well were plated in the upper chamber of wells that had been previously coated with 5% (v/v) Growth Factor Reduced Matrigel Matrix (diluted 1:20 in serum-free base media). Matrigel-coated plates were allowed to equilibrate for 4 hours in an incubator at 37°C before the addition of cells. Cells were allowed to invade for 30 hours.
Transwell assays were also performed to assess migration and invasion. Briefly, after incubating cells (in conditions denoted in individual experiments) for 24 hours for migration and 48 hours for invasion, non-migrating/invading cells were gently removed from the upper surface and inserts were stained (Crystal Violet Stain; 0.5% crystal violet, 20% methanol), washed and cells that had migrated or invaded were imaged by light microscopy.
Invadopodia assay
FITC-gelatin invadopodia/matrix degradation assays were performed as previously described [11]. Briefly, chamber well glass slides (Lab-Tek II, Thermo Fisher Scientific, Rochester, NY) were coated with FITC-labeled gelatin (Oregon Green gelatin from pig skin, 488 conjugate, Molecular Probes, Eugene, OR). For invadopodia assays, cells were treated in conditions as dictated and described in the individual experiments and then placed on the FITC-gelatin coated chamber slides for 12–24 hours. Cells were then fixed using 2.75% paraformaldehyde for 30 minutes, stained via immunofluorescence (see below) and imaged using standard confocal microscopy (see details below) following slide mounting with ProLong Gold Anti-Fade reagent (Molecular Probes, Eugene, OR).
Immunofluorescence
Following fixation, cells were washed in 1× PBS, permeabilized using 0.5% triton × 100, and blocked with 5% goat serum at room temperature for 30 minutes. Cells were again washed in 1× PBS prior to incubating with primary antibodies in 1% goat serum overnight at 4°C. Staining for cortactin and phalloidin was performed for visualizing invadopodia (see reagents above).
Confocal microscopy
A Nikon A-1 Spectral Confocal microscope (Microscopy and Imaging Analysis Laboratory, University of Michigan, Ann Arbor, MI) was used to obtain all confocal microscopy images using a 60× water lens.
Western blot analysis
Western blot analysis was performed using the Biorad Mini-PROTEAN Tetra system (Hercules, CA). Following transfer, nitrocellulose membranes were blocked in 50/50 1×TBS/Odyssey Blocking Buffer (LiCor, Lincoln, NE) for 1 hour. Membranes were washed once in 1× TBST and then incubated (rocking) overnight at 4°C in primary antibody. Membranes were then washed three times in 1×TBST for 5 minutes each wash and then placed exposed to a 1:10,000 dilution of secondary antibody (see reagents above) in 5% milk/TBST for 1 hour at room temperature. The membrane was then washed in 1×TBST two times and lastly in one 1×TBS wash for 5 minutes prior to scanning the membrane using the LiCor Imaging system (LiCor, Lincoln, NE).
Cell proliferation
Standard MTS assays were used to measure cell proliferation. 5000 cells were seeded into 96 wells in at least triplicate per condition and allowed to incubate for 48 hours. Following this, 20 μl of MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) in the presence of phenazine methosulfate (PMS) was added to each well and allowed to incubate for 3 hours at 37°C in the dark. Plates were then read on a plate reader at a wavelength of 490 nm.
Quantitative real-time PCR
Total RNA was extracted from cells at 70% to 80% confluency using Quick-RNA MicroPrep (Zymo Research, Irvine, CA) and cDNA was generated using iScript (Bio-Rad Laboratories, Inc., Hercules, CA). Quantitative real-time PCR (qRT-PCR) was performed using universal SYBR-Green Supermix (BioRad) for designed primers (Supplementary Table S1). Analysis was performed in triplicate using the Light-Cycler 480 System (Roche Applied Science, Indianapolis, IN) and average Cp values were normalized relative to the housekeeping (HK) gene HPRT1.
Results
Serum deprivation and hypoxia result in enhanced cell migration and invasion
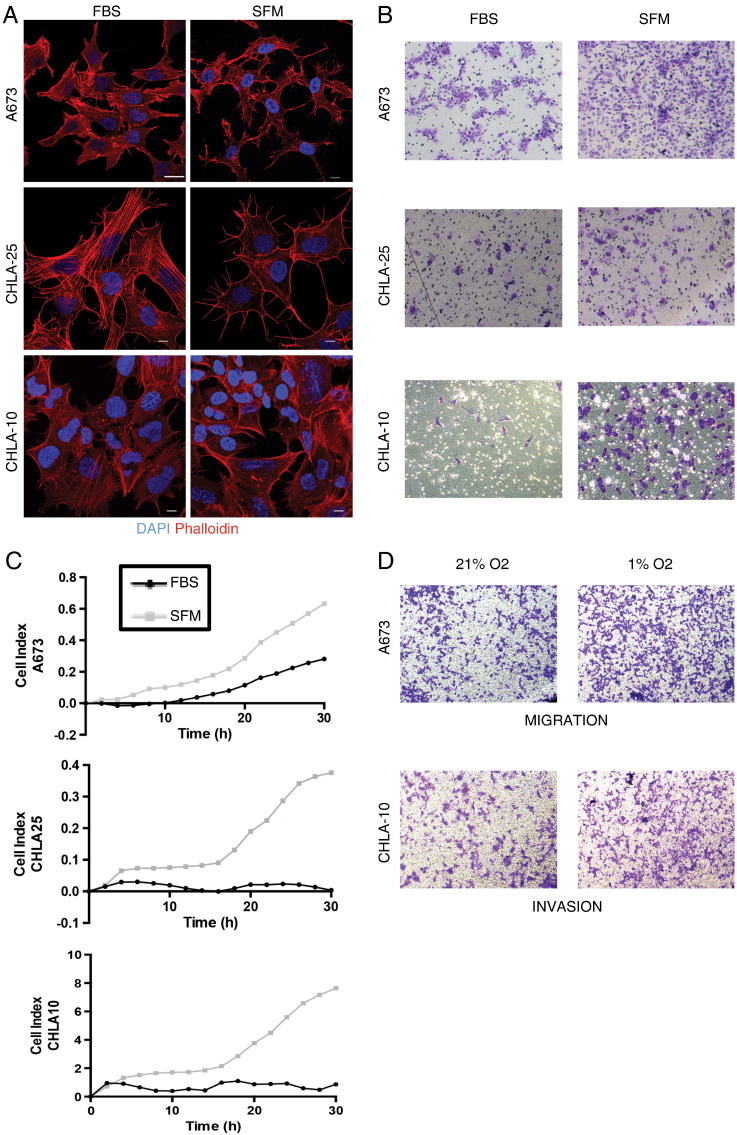
To determine the impact of stress on Ewing cell behavior, we first examined cells under conditions of serum deprivation (0% FBS containing media, SFM). Figure 1A shows that significant changes in the actin cytoskeleton were induced following serum withdrawal. Immunofluorescence labeling of Ewing cells with conjugated phalloidin revealed alterations in F-actin filament distribution in all three cell lines following 24 hours in SFM. With this change in the cell actin cytoskeleton noted, we next assessed whether this morphologic change was accompanied by any functional changes in cell behavior. When cells were subjected to stress (SFM, no gradient), all three cell lines demonstrated enhanced migration through transwell inserts compared to non-stressed controls (Figure 1B). Further, these stressed cells also showed an enhanced capability to invade through Matrigel (Figure 1C).
Figure 1.
Cell stress induces changes in the cell actin cytoskeleton and enhances cell migration and invasion. (A) Fixed cells previously treated with either 10% FBS (FBS, left column) or 0% FBS (SFM, right column) were stained with DAPI (blue) and phalloidin (red) to examine changes in the cell cytoskeletal structure upon stress. White scale bars represent 10 microns. (B) Cells (FBS versus SFM) were subjected to transwells to asses migration or (C) to Matrigel-coated xCELLignece chambers to assess invasion. The variation in the y-axis values between graphs is due to the inherent differences in the ability of the cells lines to migrate at baseline or under stress conditions. (D) Transwell assays for migration and invasion were also performed under a second type of stress (hypoxia = 1% O2). Cells were fixed and stained with crystal violet for visualization. Assays were performed at least in triplicate.
As a tumor grows and expands, it eventually outreaches its blood supply, leading to concurrent loss of nutrients and oxygen [7]. Prior reports showed that exposure to hypoxia can induce Ewing sarcoma cells to invade and form soft agar colonies more effectively [9]. Our studies corroborated these prior invasion results and also demonstrated that, like serum deprivation, hypoxic stress also induces both migration and invasion (Figure 1D). Thus, Ewing sarcoma cells demonstrate remarkable functional plasticity in response to micro-environmental stress and stressed cells transition to a more migratory and invasive cell state.
Changes in cell phenotype are not due to induction of classic EMT mediators
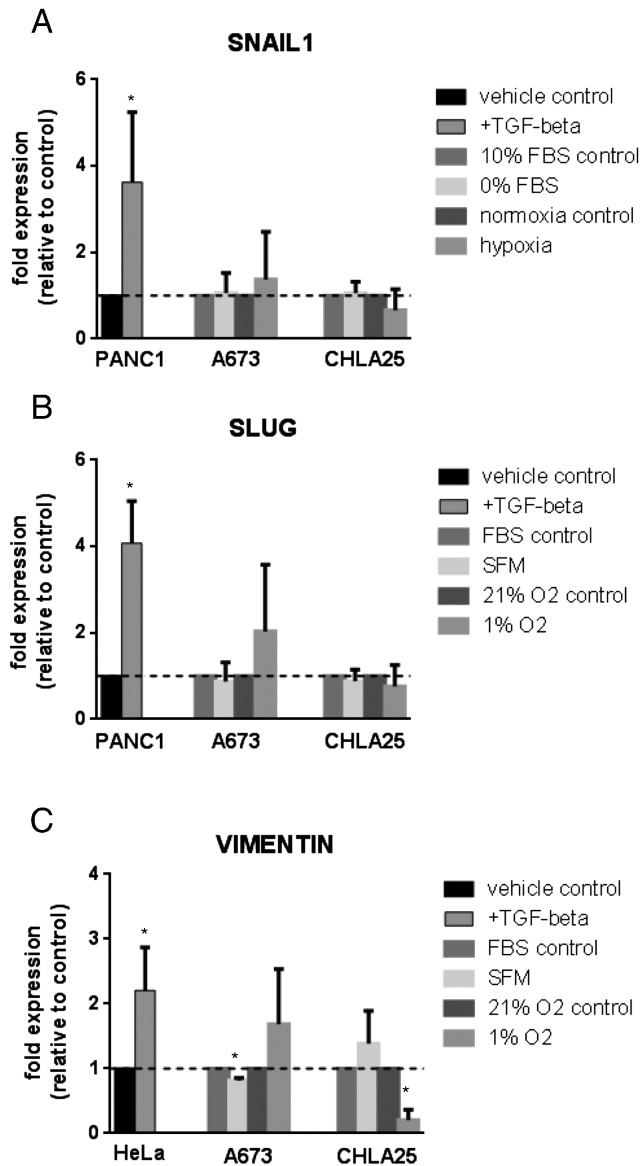
In carcinomas, cells classically change their cell cytoskeleton and migratory capabilities after undergoing epithelial-to-mesenchymal transition (EMT) [12]. Sarcomas, however, generally lack e-cadherin expression, do not undergo this transition and can even display baseline expression of some “classic” EMT markers [13], [14], [15]. Although a classic EMT would not be expected to occur in Ewing sarcoma cells, the morphologic and functional changes that we observed under stress were reminiscent of an EMT-like process. We therefore examined whether stressed Ewing sarcoma cells demonstrated evidence of up-regulation of transcription factors that regulate EMT, such as SNAIL (SNAI1, Figure 2A), SLUG (SNAI2, Figure 2B) or the intermediate filament protein encoding gene vimentin (VIM, Figure 2C). None of these markers showed a statistically significant increase in either SFM (Figure 2A) or hypoxic (Figure 2B) conditions confirming that stress-dependent changes in Ewing sarcoma cells are regulated independently of an EMT-like process. PANC1 or HeLa cells (established models of EMT induction) [16], [17], [18], [19], [20] were included as positive controls for EMT and showed the expected increase in EMT genes in response to TGF-beta.
Figure 2.
Changes in noted Ewing sarcoma cell migratory phenotype are not due to engagement of a classic EMT signature program. qRT-PCR was performed to analyze stressed (SFM, serum free media or hypoxia, 1% O2) versus unstressed (FBS, 10% fetal bovine serum or normoxia, 21% O2) A673 and CHLA-25 cells for up-regulation of the classic EMT genes SNAIL1 (A), SLUG (B) and Vimentin (C). Cells were treated with FBS versus SFM or with 21% O2 versus 1% O2 for 24 hours and fold change in expression relative to control is reported for the genes listed. PANC1 (A and B) or HeLa (C) cells treated with 10 mg/mL TGF-beta for 48 hours were included as positive controls for induction of each gene examined. Original Cp values were normalized relative to the housekeeping (HK) gene HPRT1. Asterisks (*) denote a p-value of <0.05.
Acute stress does not significantly alter cell proliferation
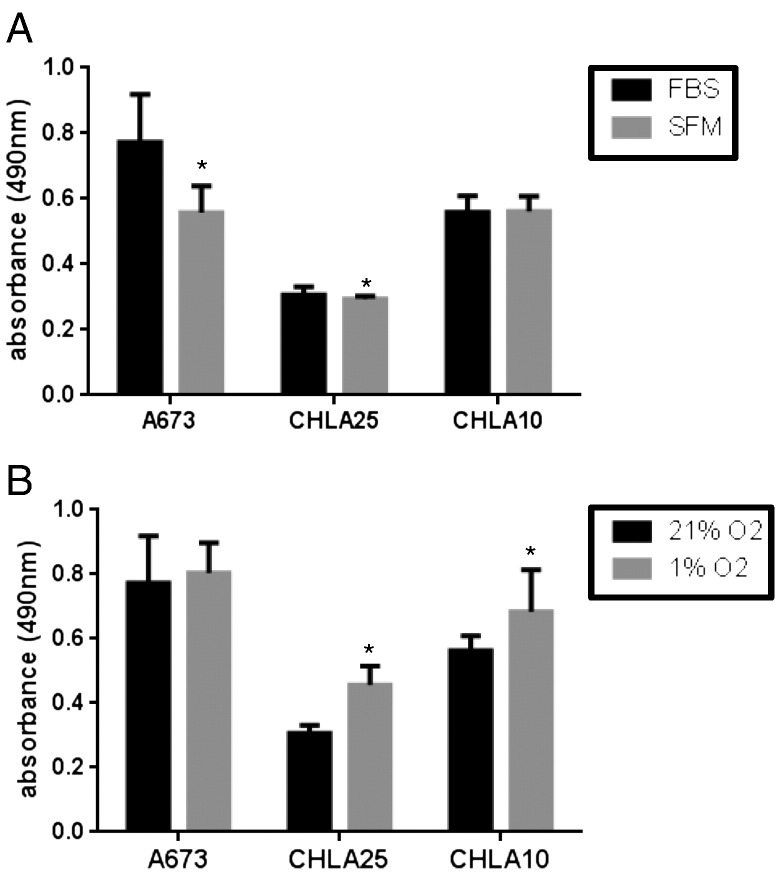
Transition of carcinoma cells to a more migratory state is often accompanied by cell cycle arrest and reduced cell proliferation [21]. We therefore performed MTS assays to examine whether Ewing sarcoma cells showed differential rates of proliferation under stressed and unstressed conditions. Despite their clear switch to a more migratory and invasive state, Ewing sarcoma cells continued to proliferate at nearly equivalent rates for up to 48 hours (Figure 3). Furthermore, these data confirm that the enhanced number of migrated and invaded cells that was observed under both stresses (Figure 1) was not due to enhanced cell proliferation.
Figure 3.
Enhanced cell proliferation is not responsible for the increase in the number of migratory/invasive cells. Standard MTS assays were used to measure cell proliferation under (A) FBS versus SFM and (B) 21% O2 versus 1% O2 conditions for a time-span of 48 hours. Data is reported as average absorbance read at 490 nm. Asterisks (*) denote a p-value of <0.05.
Stress drives invadopodia formation and matrix degradation
Invadopodia are actin-rich membrane protrusions that serve as lead-points for cell invasion due to their role as sites of matrix metalloproteinase concentration and secretion. As reviewed elsewhere [22], the induction and turnover of invadopodia structures in highly complex and dynamic and involves the coordinated activities of numerous positive and negative regulators. However, key molecules regulating the formation and function of invadopodia include Src, cortactin, fascin, Tks5, p53, and caldesmon [22]. Invadopodia structures have been identified both in vitro and in vivo and their contribution to matrix degradation is critical to the process of cell invasion, both in vitro and in vivo [22], [23], [24]. Therefore, examining the regulation of these structures can provide key insights into why some tumors cells may be are primed to metastasize.
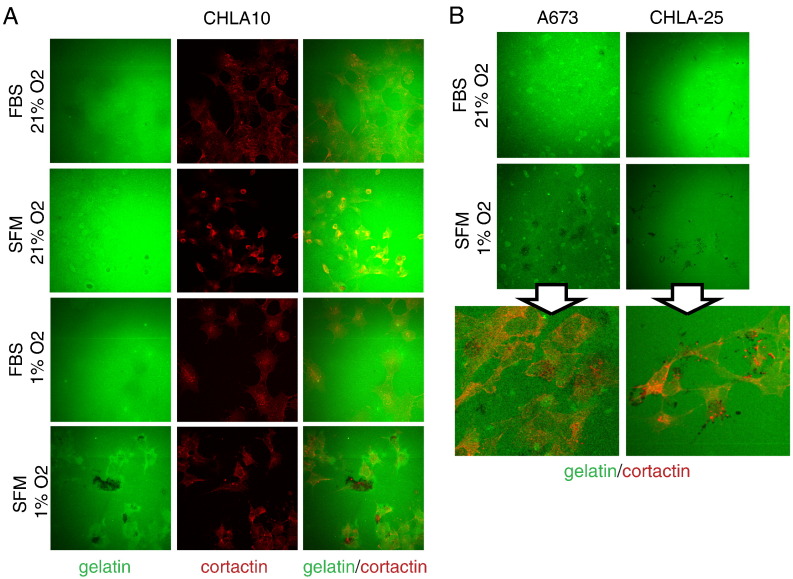
Given that both serum deprivation and hypoxia stimulated Ewing cells to more effectively migrate and invade, we examined the impact of stress on cell invadopodia formation and matrix degradation. In order to examine this, we subjected cells to culture conditions with and without stress and then applied the cells to FITC gelatin matrix degradation assays as previously described [11]. In these assays, the black (non-fluorescent) areas amongst the FITC (green)-labeled gelatin matrix are points of degradation. As shown in Figure 4A (top), in ambient conditions, CHLA-10 cells showed very little matrix degradation. In contrast, when exposed to the single stress of either serum deprivation or hypoxia CHLA-10 cells started to demonstrate small amounts of matrix degradation compared to ambient/non-stressed controls (Figure 4A, middle). Moreover, exposure to concurrent stresses led to a striking difference in matrix degradation (Figure 4A bottom). Cortactin is an actin binding protein that localizes to invadopodia, is critical for their function and is therefore used as a marker of these actin-based invasive cell structures [25]. Immunofluorescent labeling of CHLA-10 cells under ambient and stressed conditions with a conjugated cortactin antibody confirmed the presence of punctate cortactin-labeled structures co-localizing with the area of matrix degradation (Figure 4A, center and right).
Figure 4.
Application of dual stress drives Ewing sarcoma matrix degradation capabilities. Representative images of FITC gelatin matrix degradation assays in (A) CHLA10 cells when exposed to no stress (top panels), single stresses (middle panels) or combined serum deprivation (SFM) and hypoxia (1% O2, bottom panels) for 24 hours. Corresponding cell staining for cortactin (red) is included as a marker for punctate invadopodia structures. Black “holes” in the green gelatin represent areas of degradation. (B) Representative images of gelatin degradation in A673 and CHLA25 cells in unstressed (FBS, 21% O2) versus dual stress (SFM and 1% O2) conditions are shown in the top two panel rows. Bottom panels are magnified images of the dual stress condition with the cortactin overlay included to demonstrate the overlap of punctate structures with areas of matrix degradation. Assays were performed at least in triplicate.
This effect of combined hypoxia and serum deprivation leading to enhanced matrix degradation and invadopodia formation was also noted in two additional cell lines, A673 and CHLA-25 (Figure 4B). Thus, exposure of Ewing sarcoma cells to micro-environmental stress results in induction of invadopodia and enhanced matrix degradation, critical initial steps in the process of cell invasion.
Stress mediated Src activation and invadopodia formation can be blocked by dasatinib
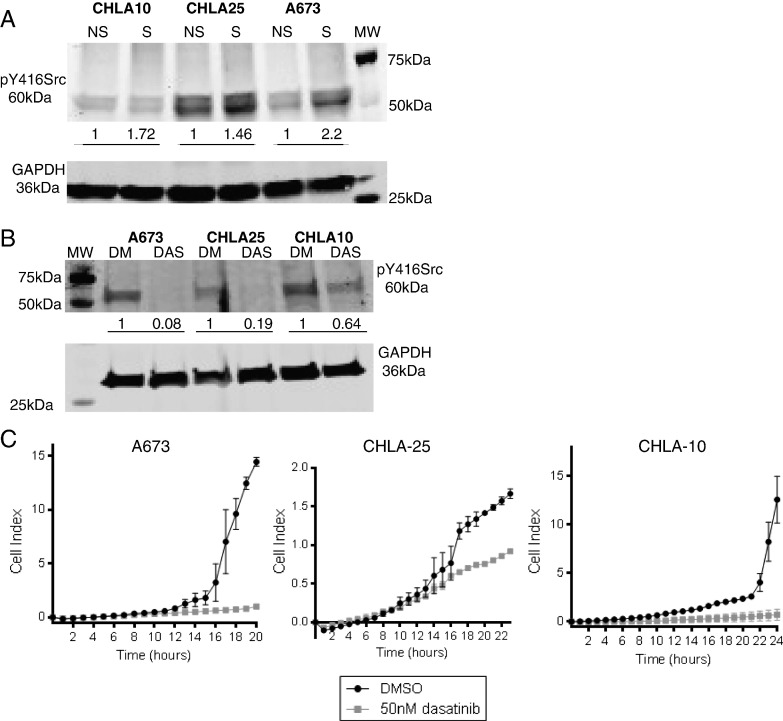
Src kinase is activated by auto-phosphorylation at tyrosine 416 and increased phosphorylation at this site has been described in a number of cancers, including sarcomas [26], [27]. Src is a key protein regulating invadopodia formation and function [22], [28], [29]. This led us to investigate whether Src was activated in stressed tumor cells. Indeed, we found that Src phosphorylation at tyrosine 416 increased in Ewing sarcoma cells following exposure to the dual stress of hypoxia and SFM (Figure 5A), raising the possibility that inhibition of Src might result in inhibition of stress-mediated invadopodia formation and matrix degradation. Dasatinib is a clinically available oral tyrosine kinase inhibitor used in the treatment of chronic myelogenous leukemia given its ability to target Abl in this Bcr-Abl fusion protein driven malignancy [30]. However, dasatinib is a dual tyrosine kinase inhibitor, and is also a potent ATP-competitive inhibitor of Src tyrosine kinase [31]. While other in vitro Src inhibitors such as PP2 and SU6656 are commercially available, we chose to test dasatinib as the Src inhibitor for our studies given its immediate clinical applicability. The dose of dasatinib used in these studies was based on previously reported data demonstrating Src inhibition at nanomolar, sub-cytotoxic concentrations [32] and this effect was confirmed in our cells. As shown, exposure of stressed cells to 50 nM dasatinib successfully blocked stress induced Src kinase phosphorylation at tyrosine 416 (Figure 5B).
Figure 5.
Stress-induced Src phosphorylation and cell migration can be blunted by dasatinib.
(A) Western blot for pY416Src and GAPDH loading control in CHLA10, CHLA25 and A673 cells upon application of no stress (NS) or stress (S, hypoxia and serum deprivation) conditions. (B) Western blot for pY416Src and GAPDH loading control in stressed cells treated with either DMSO (DM) or 50 nM dasatinib (DAS). Numbers listed under the blots in (A-B) correspond to normalized values of band pixel intensity per cell line examined. (C) Cells under stress conditions were treated with DMSO or 50 nM dasatinib and subjected to xCELLigence migration assays. Images are representative of experiments performed at least in triplicate. The variation in the y-axis is due to the inherent differences in the ability of the different cells lines to migrate at baseline or under stress conditions.
Next, we questioned whether this dasatinib-mediated inhibition of Src activation could block stress-enhanced cell migration in Ewing sarcoma. Dasatinib and DMSO vehicle treated cells were exposed to dual stress and subjected to real-time monitoring of cell migration. Exposure to dasatinib resulted in a marked inhibition of cell migration over time (Figure 5C).
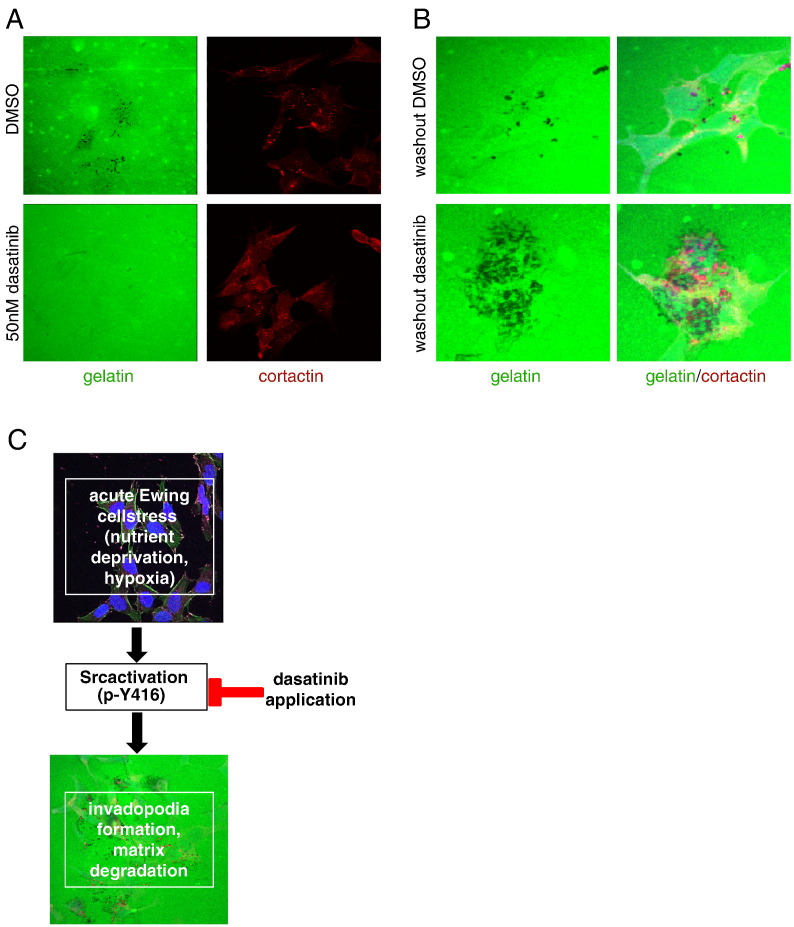
Finally, we assessed whether dasatinib could also inhibit stress-enhanced matrix degradation and observed that inhibition of Src kinase activation could indeed effectively block matrix degradation (Figure 6A). Significantly, however, following wash-out of dasatinib, stressed CHLA10 cells rapidly recovered and even augmented their ability to induce matrix degradation (Figure 6B). Altogether, these data highlight the key role of Src kinase in stress-mediated migration and invasion in Ewing sarcoma, as summarized in Figure 6C.
Figure 6.
Dasatinib blocks stress-induced matrix degradation in Ewing sarcoma cells. (A) CHLA10 cells were treated with dual stress (serum deprivation and hypoxia), applied to FITC matrix degradation assays and treated with either DMSO or 50 nM dasatinib. (B) Cells were treated as in (A) but after 12 hours, media containing DMSO or dasatinib was removed, cells were washed with 1× PBS and then placed back in serum free media. (C) Schematic of the stress/Src relationship in Ewing sarcoma.
Discussion
Enhanced migratory and invasive capabilities are essential for cancer cells to acquire the ability to metastasize. While carcinomas (epithelial derived cancers) can acquire these phenotypes via signaling regulating epithelial-to-mesenchymal transitions [12], sarcomas typically do not follow this pattern, as they are already phenotypically “mesenchymal”. However, not all sarcoma cells are primed to metastasize. The triggers fueling the transition of sarcoma cells to a more aggressive state are still not fully understood. In this study, we have demonstrated a means of priming Ewing sarcoma cells to be more motile and invasive by application of micro-environmental stress in the form of hypoxia and/or serum deprivation. Clinically, these are very relevant stresses, as with upfront therapy (chemotherapy, radiation, or surgery), tumors are acutely exposed to changes in both oxygen and nutrient availability via direct or indirect changes in tumor vasculature resulting from these therapies. Ewing sarcoma tumors are highly vascular and are therefore susceptible to such insults [33], [34].
The concept of cellular plasticity in the context of tumor heterogeneity is critical to finding better ways to prevent or treat sarcoma metastasis [35], [36], [37]. Subsets of cells within a bulk tumor respond differently to insults such as drugs, cytokines secreted by tumor stroma and stress, thereby making tumor heterogeneity a mechanism of therapy resistance. The tumor micro-environment can, in fact, manipulate sub-populations of tumor cells to behave in either more aggressive or dormant fashions [35]. Many prior studies of Ewing sarcoma have largely focused on inter-tumoral rather than intra-tumoral variances in cell behavior [6], [38], [39]. However, more recently, evidence has surfaced revealing the truly heterogeneous composition of Ewing sarcoma tumor cells within individual tumors and cell lines [40], [41]. We have demonstrated that acute, stress-induced Src activation is one means by which subsets of Ewing sarcoma cells can rapidly switch to a more aggressive state. Src and invadopodia have been shown to be key regulators of metastasis in other cancer types, such as melanoma [42], and our findings support that this signaling axis could also play a seminal role in inducing subsets of Ewing sarcoma cells to be primed to metastasize. This is a very clinically relevant issue, as while it is suspected that the majority of Ewing patients with localized tumors at diagnosis actually do have micro-metastases, most of these patients are cured with upfront, compressed cycles of chemotherapy. However, for patients that do progress to macro-metastatic disease or present with metastatic disease at diagnosis, the clinical challenge is preventing the vicious cycle of further spread of already established metastases, as worsening metastatic disease is still the number one cause of patient mortality.
Recently, the results of a Phase II clinical trial were published and reported the impact of single agent dasatinib on advanced sarcoma growth (including some cases of Ewing sarcoma) [43]. While slightly higher doses of dasatinib have been reported to cause cell death/apoptosis in sarcomas (such as osteosarcoma [44]), this trial did not find clinical benefit in the use of single agent dasatinib to control tumor growth in the majority of sarcomas examined. In the discussion of these results, the authors importantly point out that the preclinical data surrounding dasatinib was possibly more compelling for prevention of tumor invasion and metastasis as opposed to its ability to have cytotoxic effects on the tumor bulk. Our current results support this hypothesis. Given the plasticity of Ewing sarcoma cells under conditions of acute stress, we feel that further studies are warranted to examine the role of Src inhibitors, such as dasatinib, as a means by which to prevent acute stress-induced/Src-dependent invasion. It is possible that adding agents such as dasatinib during key portions of therapy could help to prevent stress-mediated plasticity in Ewing sarcoma and prevent cells from being primed to metastasize. However, given our data demonstrating a rapid rebound in matrix degradation capabilities following wash-out of dasatinib, such studies would need to be mindful of the short half-life of dasatinib and to ensure appropriate dosing intervals were applied to avoid significant Src reactivation during therapy in patient tumors.
The following is the supplementary data related to this article.
Full sequences of primers designed for use in this study
Acknowledgements
This work was supported by the following funding sources: NIH T32 training grant (5T32HL007622), NCI F30 (F30CA183276), NIH/NCI SARC SPORE 1 U01-CA114757, 5 T32-CA009676, and The University of Michigan's Cancer Center Support Grant (P30 CA046592) by the use of the following Cancer Center Core: microscopy. We would like to thank Linda Barthel (University of Michigan Microscopy and Image Analysis Laboratory) for her assistance with the confocal microscopy studies.
References
- 1.Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11(2):184–192. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 2.Lawlor ER, Sorensen PH. Twenty Years on: What Do We Really Know about Ewing Sarcoma and What Is the Path Forward? Crit Rev Oncog. 2015;20(3–4):155–171. doi: 10.1615/critrevoncog.2015013553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore DD, Haydon RC. Ewing's sarcoma of bone. Cancer Treat Res. 2014;162:93–115. doi: 10.1007/978-3-319-07323-1_5. [DOI] [PubMed] [Google Scholar]
- 4.Biswas B, Rastogi S, Khan SA, Mohanti BK, Sharma DN, Sharma MC, Mridha AR, Bakhshi S. Outcomes and prognostic factors for Ewing-family tumors of the extremities. J Bone Joint Surg Am. 2014;96(10):841–849. doi: 10.2106/JBJS.M.00411. [DOI] [PubMed] [Google Scholar]
- 5.Biswas B, Shukla NK, Deo SV, Agarwala S, Sharma DN, Vishnubhatla S, Bakhshi S. Evaluation of outcome and prognostic factors in extraosseous Ewing sarcoma. Pediatr Blood Cancer. 2014;61(11):1925–1931. doi: 10.1002/pbc.25095. [DOI] [PubMed] [Google Scholar]
- 6.Shukla N, Schiffman J, Reed D, Davis IJ, Womer RB, Lessnick SL, Lawlor ER, C. O. G. E. S. B. Committee Biomarkers in Ewing Sarcoma: The Promise and Challenge of Personalized Medicine. A Report from the Children's Oncology Group. Front Oncol. 2013;3:1–13. doi: 10.3389/fonc.2013.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ungefroren H, Sebens S, Seidl D, Lehnert H, Hass R. Interaction of tumor cells with the microenvironment. Cell Commun Signal. 2011;9:1–8. doi: 10.1186/1478-811X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aryee DN, Niedan S, Kauer M, Schwentner R, Bennani-Baiti IM, Ban J, Muehlbacher K, Kreppel M, Walker RL, Meltzer P. Hypoxia modulates EWS-FLI1 transcriptional signature and enhances the malignant properties of Ewing's sarcoma cells in vitro. Cancer Res. 2010;70(10):4015–4023. doi: 10.1158/0008-5472.CAN-09-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krook MA, Nicholls LA, Scannell CA, Chugh R, Thomas DG, Lawlor ER. Stress-induced CXCR4 promotes migration and invasion of ewing sarcoma. Mol Cancer Res. 2014;12(6):953–964. doi: 10.1158/1541-7786.MCR-13-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin KH, Hayes KE, Walk EL, Ammer AG, Markwell SM, Weed SA. Quantitative measurement of invadopodia-mediated extracellular matrix proteolysis in single and multicellular contexts. J Vis Exp. 2012;66:e4119. doi: 10.3791/4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen M, Lehto VP, Badley RA, Virtanen I. Expression of intermediate filaments in soft-tissue sarcomas. Int J Cancer. 1982;30(5):541–546. doi: 10.1002/ijc.2910300502. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Du X, Wang G, Sun Y, Chen K, Zhu X, Lazar AJ, Hunt KK, Pollock RE, Zhang W. Mesenchymal to epithelial transition in sarcomas. Eur J Cancer. 2014;50(3):593–601. doi: 10.1016/j.ejca.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Eddy JA, Pan Y, Hategan A, Tabus I, Wang Y, Cogdell D, Price ND, Pollock RE, Lazar AJ. Integrated proteomics and genomics analysis reveals a novel mesenchymal to epithelial reverting transition in leiomyosarcoma through regulation of slug. Mol Cell Proteomics. 2010;9(11):2405–2413. doi: 10.1074/mcp.M110.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandl M, Seidler B, Haller F, Adamski J, Schmid RM, Saur D, Schneider G. IKK(alpha) controls canonical TGF(ss)-SMAD signaling to regulate genes expressing SNAIL and SLUG during EMT in panc1 cells. J Cell Sci. 2010;123(Pt 24):4231–4239. doi: 10.1242/jcs.071100. [DOI] [PubMed] [Google Scholar]
- 17.Ellenrieder V, Hendler SF, Boeck W, Seufferlein T, Menke A, Ruhland C, Adler G, Gress TM. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001;61(10):4222–4228. [PubMed] [Google Scholar]
- 18.Horiguchi K, Shirakihara T, Nakano A, Imamura T, Miyazono K, Saitoh M. Role of Ras signaling in the induction of snail by transforming growth factor-beta. J Biol Chem. 2009;284(1):245–253. doi: 10.1074/jbc.M804777200. [DOI] [PubMed] [Google Scholar]
- 19.Kallergi G, Papadaki MA, Politaki E, Mavroudis D, Georgoulias V, Agelaki S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13(3):R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):314–323. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18(10):1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12(7):413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blouw B, Patel M, Iizuka S, Abdullah C, You WK, Huang X, Li JL, Diaz B, Stallcup WB, Courtneidge SA. The invadopodia scaffold protein Tks5 is required for the growth of human breast cancer cells in vitro and in vivo. PLoS One. 2015;10(3):e0121003. doi: 10.1371/journal.pone.0121003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy DA, Diaz B, Bromann PA, Tsai JH, Kawakami Y, Maurer J, Stewart RA, Izpisua-Belmonte JC, Courtneidge SA. A Src-Tks5 pathway is required for neural crest cell migration during embryonic development. PLoS One. 2011;6(7):e22499. doi: 10.1371/journal.pone.0022499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67(9):4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 26.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19(49):5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 27.Michels S, Trautmann M, Sievers E, Kindler D, Huss S, Renner M, Friedrichs N, Kirfel J, Steiner S, Endl E. SRC signaling is crucial in the growth of synovial sarcoma cells. Cancer Res. 2013;73(8):2518–2528. doi: 10.1158/0008-5472.CAN-12-3023. [DOI] [PubMed] [Google Scholar]
- 28.Destaing O, Sanjay A, Itzstein C, Horne WC, Toomre D, De Camilli P, Baron R. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol Biol Cell. 2008;19(1):394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schachtner H, Calaminus SD, Thomas SG, Machesky LM. Podosomes in adhesion, migration, mechanosensing and matrix remodeling. Cytoskeleton (Hoboken) 2013;70(10):572–589. doi: 10.1002/cm.21119. [DOI] [PubMed] [Google Scholar]
- 30.Breccia M, Salaroli A, Molica M, Alimena G. Systematic review of dasatinib in chronic myeloid leukemia. Onco Targets Ther. 2013;6:257–265. doi: 10.2147/OTT.S35360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47(27):6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 32.Buettner R, Mesa T, Vultur A, Lee F, Jove R. Inhibition of Src family kinases with dasatinib blocks migration and invasion of human melanoma cells. Mol Cancer Res. 2008;6(11):1766–1774. doi: 10.1158/1541-7786.MCR-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Castellano JM, Atallah Yordi N, Reyes C, Healey JH. Histopathologic and Radiologic Assessment of Chemotherapeutic Response in Ewing's Sarcoma: A Review. Sarcoma. 2012;2012:1–8. doi: 10.1155/2012/357424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brabletz T, Lyden D, Steeg PS, Werb Z. Roadblocks to translational advances on metastasis research. Nat Med. 2013;19(9):1104–1109. doi: 10.1038/nm.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell. 2014;54(5):716–727. doi: 10.1016/j.molcel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crompton BD, Stewart C, Taylor-Weiner A, Alexe G, Kurek KC, Calicchio ML, Kiezun A, Carter SL, Shukla SA, Mehta SS. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. 2014;4(11):1326–1341. doi: 10.1158/2159-8290.CD-13-1037. [DOI] [PubMed] [Google Scholar]
- 39.van Doorninck JA, Ji L, Schaub B, Shimada H, Wing MR, Krailo MD, Lessnick SL, Marina N, Triche TJ, Sposto R. Current treatment protocols have eliminated the prognostic advantage of type 1 fusions in Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2010;28(12):1989–1994. doi: 10.1200/JCO.2009.24.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson JL, Titz B, Akiyama R, Komisopoulou E, Park A, Tap WD, Graeber TG, Denny CT. Phosphoproteomic profiling reveals IL6-mediated paracrine signaling within the Ewing sarcoma family of tumors. Mol Cancer Res. 2014;12(12):1740–1754. doi: 10.1158/1541-7786.MCR-14-0159. [DOI] [PubMed] [Google Scholar]
- 41.Suva ML, Riggi N, Stehle JC, Baumer K, Tercier S, Joseph JM, Suva D, Clement V, Provero P, Cironi L. Identification of cancer stem cells in Ewing's sarcoma. Cancer Res. 2009;69(5):1776–1781. doi: 10.1158/0008-5472.CAN-08-2242. [DOI] [PubMed] [Google Scholar]
- 42.Hanna SC, Krishnan B, Bailey ST, Moschos SJ, Kuan PF, Shimamura T, Osborne LD, Siegel MB, Duncan LM, O'Brien ET., III HIF1alpha and HIF2alpha independently activate SRC to promote melanoma metastases. J Clin Invest. 2013;123(5):2078–2093. doi: 10.1172/JCI66715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuetze SM, Wathen JK, Lucas DR, Choy E, Samuels BL, Staddon AP, Ganjoo KN, von Mehren M, Chow WA, Loeb DM. SARC009: Phase 2 study of dasatinib in patients with previously treated, high-grade, advanced sarcoma. Cancer. 2015 doi: 10.1002/cncr.29858. [DOI] [PubMed] [Google Scholar]
- 44.Shor AC, Keschman EA, Lee FY, Muro-Cacho C, Letson GD, Trent JC, Pledger WJ, Jove R. Dasatinib inhibits migration and invasion in diverse human sarcoma cell lines and induces apoptosis in bone sarcoma cells dependent on SRC kinase for survival. Cancer Res. 2007;67(6):2800–2808. doi: 10.1158/0008-5472.CAN-06-3469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full sequences of primers designed for use in this study