Abstract
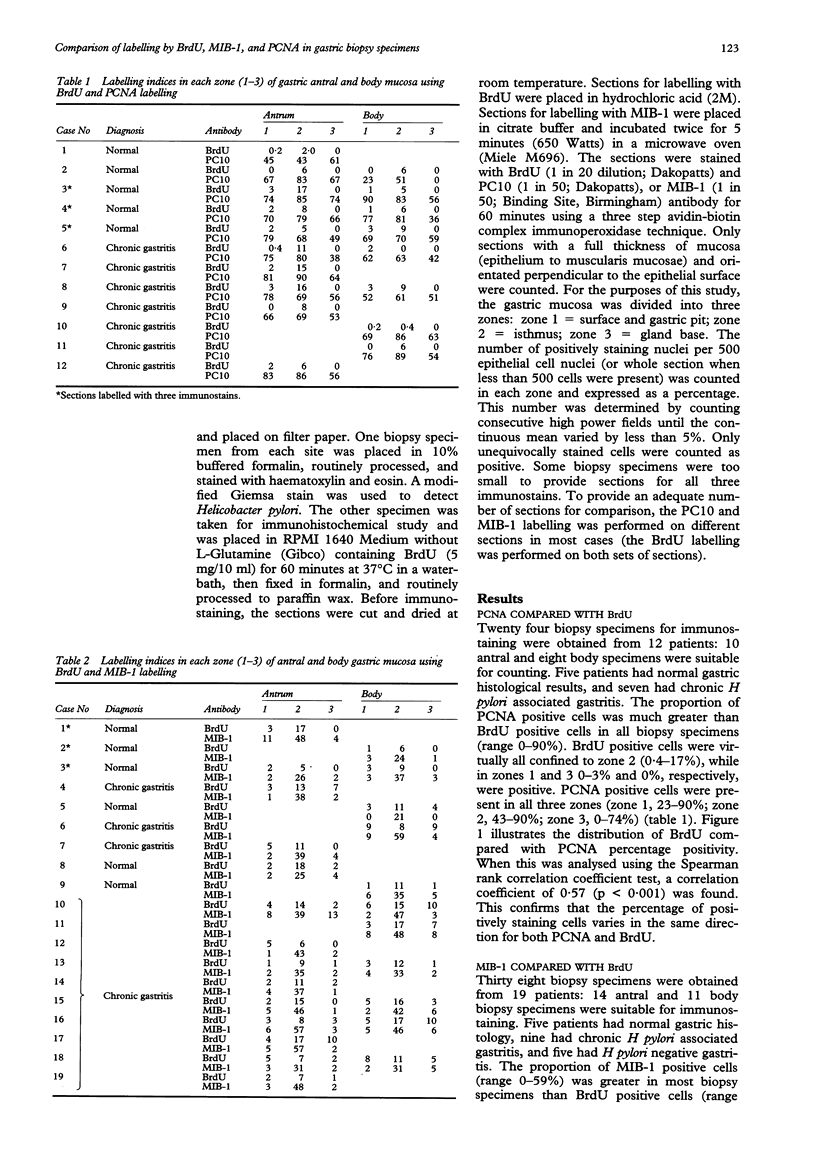
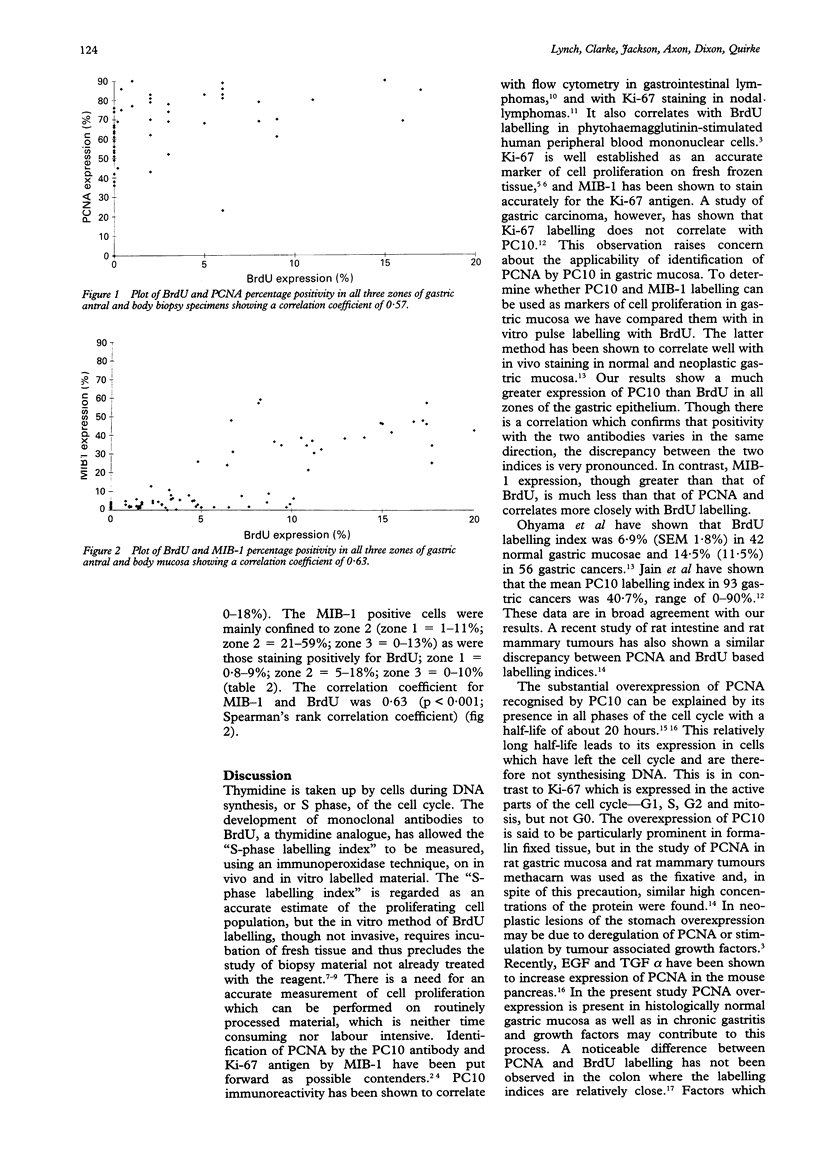
AIMS--To compare proliferating cell nuclear antigen (PCNA) and MIB-1 with bromodeoxyuridine (BrdU) pulse labelling, a specific marker of cell proliferation, in endoscopic gastric biopsy specimens. METHODS--Twenty four biopsy specimens were obtained from 12 patients: 10 antral and eight body specimens were suitable. Each specimen was routinely processed and stained with haematoxylin and eosin. A modified Giemsa stain was used to detect the presence of Helicobacter pylori. Sections of the specimens were labelled with BrdU, MIB-1, and PC10. Gastric mucosa specimens were divided into three zones. The numbers of positively staining nuclei for 500 epithelial cell nuclei were counted in each zone and expressed as a percentage. RESULTS--The proportion of PCNA positive cells (range 0-90%) was much greater in all specimens (10 antrum, eight body). BrdU positive cells were virtually all confined to zone 2 (0-17% cells in this zone were positive) (zone 1 = surface and gastric pit, zone 2 = isthmus, zone 3 = gland base), while PCNA positive cells were present in all three zones (1 = 23-90%, 2 = 43-90%, 3 = 0-74%). Spearman's rank coefficient correlation of 0.57 confirmed that the percentage of positively staining cells varied in the same direction for both PCNA and BrdU (p < 0.001). PCNA, however, was overexpressed in all zones of the gastric epithelium compared with BrdU. In 38 biopsy specimens from 19 patients, of which 14 antrum and 11 body were suitable, the proportion of MIB-1 positive cells (0-59%) was greater than BrdU in most. As with BrdU labelling, the MIB-1 positive cells were confined to zone 2 (zone 1 = 1-11%); zone 2 = 21-59%; zone 3 = 0-13%) and the coefficient correlation for MIB-1 and BrdU was 0.63 (p < 0.001). CONCLUSIONS--MIB-1 accurately reflects the S-phase fraction in gastric mucosa, determined by BrdU labelling in conventionally processed gastric biopsy material. Caution is needed in the interpretation of PCNA labelling detected by PC10, which should not be accepted uncritically as a marker of cell proliferation in paraffin wax embedded material.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Gerdes J. Ki-67 and other proliferation markers useful for immunohistological diagnostic and prognostic evaluations in human malignancies. Semin Cancer Biol. 1990 Jun;1(3):199–206. [PubMed] [Google Scholar]
- Hall P. A., Crocker J., Watts A., Stansfeld A. G. A comparison of nucleolar organizer region staining and Ki-67 immunostaining in non-Hodgkin's lymphoma. Histopathology. 1988 Apr;12(4):373–381. doi: 10.1111/j.1365-2559.1988.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Lemoine N. R. Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas. J Pathol. 1992 Feb;166(2):97–103. doi: 10.1002/path.1711660203. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Woods A. L. Immunohistochemical markers of cellular proliferation: achievements, problems and prospects. Cell Tissue Kinet. 1990 Nov;23(6):505–522. doi: 10.1111/j.1365-2184.1990.tb01343.x. [DOI] [PubMed] [Google Scholar]
- Jain S., Filipe M. I., Hall P. A., Waseem N., Lane D. P., Levison D. A. Prognostic value of proliferating cell nuclear antigen in gastric carcinoma. J Clin Pathol. 1991 Aug;44(8):655–659. doi: 10.1136/jcp.44.8.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Mathews M. B. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989 Aug 15;264(23):13856–13864. [PubMed] [Google Scholar]
- Morstyn G., Hsu S. M., Kinsella T., Gratzner H., Russo A., Mitchell J. B. Bromodeoxyuridine in tumors and chromosomes detected with a monoclonal antibody. J Clin Invest. 1983 Nov;72(5):1844–1850. doi: 10.1172/JCI111145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama S., Yonemura Y., Miyazaki I. Proliferative activity and malignancy in human gastric cancers. Significance of the proliferation rate and its clinical application. Cancer. 1992 Jan 15;69(2):314–321. doi: 10.1002/1097-0142(19920115)69:2<314::aid-cncr2820690207>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Roberts C. G., deFazio A., Tattersall M. H. A simple and rapid method for the identification of cycling cells in freshly excised tumours. Cytobios. 1985;43(174S):313–318. [PubMed] [Google Scholar]
- Wijsman J. H., Van Dierendonck J. H., Keijzer R., van de Velde C. J., Cornelisse C. J. Immunoreactivity of proliferating cell nuclear antigen compared with bromodeoxyuridine incorporation in normal and neoplastic rat tissue. J Pathol. 1992 Sep;168(1):75–83. doi: 10.1002/path.1711680113. [DOI] [PubMed] [Google Scholar]
- Woods A. L., Hall P. A., Shepherd N. A., Hanby A. M., Waseem N. H., Lane D. P., Levison D. A. The assessment of proliferating cell nuclear antigen (PCNA) immunostaining in primary gastrointestinal lymphomas and its relationship to histological grade, S+G2+M phase fraction (flow cytometric analysis) and prognosis. Histopathology. 1991 Jul;19(1):21–27. doi: 10.1111/j.1365-2559.1991.tb00890.x. [DOI] [PubMed] [Google Scholar]


