Abstract
Diabetes mellitus is one of the most common causes of neuropathy. Although antioxidant and antidiabetic effects of the aqueous extract of purslane (Portulaca oleracea) (AEOP) have been demonstrated before by other researchers, we did not find any study that assessed the psychobiological effects of AEOP in diabetes induced animals. Thirty ovariectomized (OVX) female Wistar rats were randomly divided into 3 groups of control, Dia and Dia+AEOP. The latter group was orally treated by 300 mg/kg of AEOP for 35 days. Dia and Dia+AEOP groups were made diabetic by IP injection of 60 mg/kg of streptozotocin (STZ). The psychobiological effects of AEOP were assessed by Morris water maze (MWM), elevated plus maze (EPM), forced swimming test (FST) and tail pinch stressor (TPS). AEOP significantly decreased hyperglycemia (p<0.001). Diabetes significantly decreased their spatial cognitive performance at the training trial as well as the total distance traveled at the probe trial in MWM (p<0.05). All the diabetes related deficits at training trials were improved by AEOP treatment (p<0.05). AEOP treatment not only improved the motor deficit of Dia group in EPM, but also showed anxiolytic effects compared to both control and Dia groups (p<0.05). In the FST, no differences were observed between any groups (p>0.05). Diabetes significantly increased their non-functional masticatory activity in TPS (p≤0.001) while it was improved in Dia+AEOP group. We showed that AEOP has significant anxiolytic effects and it can improve spatial cognitive performance, locomotor deficit and stress in diabetic OVX rats.
Key Words: Portulaca oleracea, Diabetes, Psychobiological assays, Spatial memory, Locomotor activity
Introduction
Diabetes mellitus (DM) which is characterized by hyperglycemia is a common metabolic disorder that happens due to insulin deficiency and insulin resistance (1). It is associated with central nervous system (CNS) and peripheral nervous system (PNS) neurological complication (2). DM in Long-term causes a vast range of peripheral neuronal deficits including reduced motor nerve conduction velocity, axonal shrinkage, impaired nerve regeneration, and deficient axonal transport (3).
STZ-induced diabetes is a well-defined experimental model for DM because the nerve damages are similar to the nerve degeneration that occurs in human diabetic neuropathy (4). Different kinds of diabetic neuropathies are some of the major complications of DM. It has been suggested that diabetes is one of the primary risk factors in the creation of senile dementia of the Alzheimer's type (SDAT) (5). Although the complete mechanisms through which diabetes could mediate these effects are not fully understood, it seems that hyperglycemia, oxidative stress and subsequent free radicals generation, lead to increased cell membrane damage (lipid peroxidation) and initiates death signaling pathways )6, 7).
Portulaca oleracea L. (Portulacaceae) which is referred to as Purslane (Khorfeh in Persian) has been given the term ‘Global Panacea’ and is listed by the World Health Organization as one of the most used medicinal plants. Purslane is a rich source of antioxidants (vitamins A, C, and E, β-carotene, and glutathione) and omega-3 fatty acids (8). Purslane also contains various kinds of flavonoids (9). Other bioactives found in purslane are coumarins, alkaloids, saponins, polyphenols and anthocyanin (10). The aqueous extract of purslane hasn't shown any cytotoxicity or genotoxicity, and has been certified safe for daily consumption as a vegetable (11).
A wide range of biological effects related to purslane has been reported including being neuroprotective (12, 13), effective in cognition improvement (14), being anxiolytic (15, 16), analgesic (17), antidiabetic (18, 19) and having antioxidant effects (8-10).
Although there are many different aspects in brain's structure, neuroendocrine regulation and neurotransmitter systems of males and females (20, 21), most of the studies have been focused on the neurobehavioral changes which take place in male animals following diabetes. On the other hand, incidence of diabetes increases after menopause (22). Therefore, due to lack of enough knowledge concerning the effects of purslane on neurobehavioral changes and hyperglycemia that happen in female diabetic animals, the present study was designed and conducted. We assessed the effects of AEOP on ovariectomized (OVX) rats. OVX rats not only are well-defined models to assess post-menopausal events (23, 24), but also they don't get influenced by the effects of ovarian steroids which may alter the behavior of rats during the estrus cycle.
Experimental
Drugs
STZ was purchased from Enzo, Life sciences, Inc. (USA). All the other chemicals were of analytical grade and were obtained from commercial sources.
Preparation of Aqueous Extract
Purslane which was cultured in Khouzestan, Ramin Agriculture and Natural Resources University, was obtained as a gift. AEOP was prepared by the maceration method. Briefly 1500 ml of distilled water was added to 300 grams of dried purslane powder in a sealed glass container and it was set aside for about 72 h. The filtrated extract was concentrated in a rotary evaporator under reduced pressure at 55 °C and dried in a bath of warm water.
Animals
Thirty virgin female Wistar rats (weighing 190 - 200 g, 12 weeks of age) were obtained from the Faculty of Veterinary Medicine of Shahid Chamran University (Ahvaz, Iran). They were maintained under standard laboratory conditions with 12 h light/dark cycle and free access to standard laboratory rat food and tap water. The used protocol and animal ethics were approved by the Research Council of the Faculty of Veterinary Medicine of Shahid Chamran University of Ahvaz.
OVX rats were randomly divided into three groups (10 in each group): normal control (control), diabetic (Dia) and AEOP treated diabetic (Dia+AEOP) groups. Following confirmation of diabetes (Blood sugar>300 mg/dL), Dia+AEOP group were treated daily by oral gavage of 300 mg/kg of AEOP dissolved in 4 ml of saline (25) for 35 days. Both control and Dia groups were treated similarly with the same procedure and same volume of the solvent of the extract (saline).
Surgery
All the animals used in this study were OVX. Anesthesia was induced by ketamine-xylazine mixture (100 mg/kg-10 mg/kg, respectively) to remove both of their ovaries. The dorsal mid lumbar area was shaved and swabbed with surgical scrub, iodine and alcohol. A 1-2 cm dorsal midline incision was made halfway between the caudal edge of their ribcage and base of the tail at both right and left sides. Ovaries and oviducts were exteriorized, clamped and removed. With 10 days of recovery, the OVX rats were randomly introduced to the above mentioned groups.
Diabetes induction
Diabetes was induced by intraperitoneal (i.p.) injection of freshly prepared STZ (60 mg/kg, dissolved in 0.1 M citrate buffer, pH 4.5). The control rats received the same volume of the buffer. Five days after STZ injection, animals with blood glucose greater than 300 mg/dL were used for the study.
Glucose measurement
Glucose was measured by a glucometer from a drop of blood taken from their tail. Glucose was measured twice: once 5 days after injection of STZ to assess the induction of diabetes and again at the last stage of the study to assess effects of AEOP on diabetic hyperglycemia.
Morris water maze (MWM)
The spatial learning and memory ability of the rats were evaluated using MWM (26). Briefly, the system included a circular pool (height: 50 cm, diameter: 150 cm) which was filled with water (22 ± 1 °C) to a depth of 25 cm and a video tracking device captured the swimming pathway by a video-tracking Software (Borj Sanat Co, Tehran, Iran). A hidden square platform (10 × 10 cm) was placed in the center of the southwest quadrant, submerged 1 cm below the surface of the water. The maze was geographically divided into four quadrants; northeast (NE), northwest (NW), southeast (SE) and southwest (SW), and four starting positions including north (N), south (S), east (E) and west (W); which were equally spaced around the perimeter of the pool. In the training trials, each rat was trained in the maze for four consecutive days, which was repeated four times per day and the swim path of every rat was recorded for 90 seconds. Swim speed, the escape latency and the distance traveled to reach the hidden platform were recorded by the software. In the probe trial which took place 24 h after the last training trial, spatial memory of the rats was evaluated in the maze without the platform. At this point, the activity of the rats was recorded for 60 s to compute the total distance traveled, the percentage of distance traveled in the target quadrant and the percentage of time spent in the target quadrant as a measure of retention and memory performance of the rats.
Elevated plus maze (EPM)
The EPM was designed according to Pellow and File (27). The EPM is comprised of two open arms) 50 × 10 cm (and two closed arms (50 × 10 × 40 cm), which are connected by a common central square 10 × 10 cm. Rats were placed in the central square, facing an open arm and were allowed to explore the apparatus for 5 minutes. The maze was cleaned with ethanol (70%) after each test. A video tracking device captured every rat's pathway by a video-tracking Software (Borj Sanat Co, Tehran, Iran) and measured: a) total time spent in open arms, b) total time spent in closed arms, c) number of entries into open arms and d) number of entries into closed arms. Finally, the following parameters were calculated: 1) percentage of time spent in the open arms relative to the total time spent in the maze 2) percentage of entries into open arms relative to the total number of entries into any arm.
Forced swimming test (FST)
The FST was performed according to Porsolt et al. (28). A vertical plastic cylinder (50 cm in height, 20 cm in diameter) was filled with water (23-25 °C) to a depth of 30 cm. Each animal was placed in the cylinder for 6 min, to measure immobility-floating and swimming behaviors during the last 4 min. Immobility-floating was considered as the rat being passively in the water and only making slight movements to keep its head above the water line and swimming was considered as making active swimming motions, more than necessary to merely keep the head above the water (i.e., moving around in the cylinder).
Tail pinch stressor (TPS)
Rats were subjected to a mild tail pinch according to the method which is described by Go´mez et al. (29, 30(. Rats were placed in individual chambers (18 × 26 × 18 cm) which one side of them was made of wire-mesh and they were allowed to acclimatize for 15 min. A softwood biting block was put in each chamber. The rat's tail was put through a hole in the center of the wire-mesh wall and at the distance of 8 cm from its tip a plastic clothes clip was put on it for 5 min. The duration of non-functional masticatory activity (NFMA) displayed during tail pinch exposure was measured by an observer. Vigorous gnawing⁄biting of the wooden block and/or audible experimental bruxism (chattering or scraping of the teeth) were considered to be NFMA. Duration was defined as the total time in which NFMA was displayed during the observation period which was 5 min; although a prerequisite was that rats had to exhibit a minimum of 5 consecutive seconds of NFMA for it to be considered valid. The reason that 5 s was decided upon as an appropriate portion of time, was to prevent the scoring any intermittent masticatory activity which was not significant.
Data analysis
Data were analyzed by the software SPSS, version 16 (SPSS; Chicago, IL, USA). All values were expressed as mean ± SEM. Repeated measures and one way analysis of variance (ANOVA) followed by tukey test were used to assess treatment differences among the groups. P values of <0.05 were accepted as statistically significant.
Results
Fasting blood sugar (FBS)
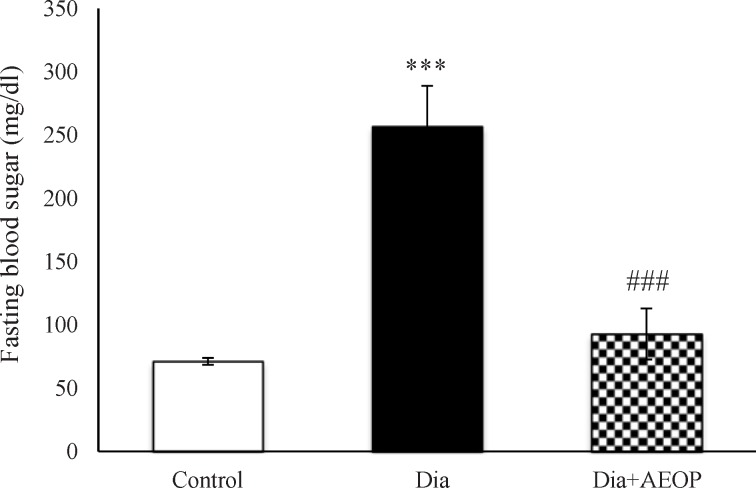
The results of measuring the FBS following 12 h of fasting are demonstrated in (Figure 1). Diabetes induction significantly increased FBS (p<0.001), while it was improved significantly in the Dia+AEOP group (p<0.001).
Figure 1.
Data on the STZ-induced diabetic rats both treated and not treated (Dia) with Purslane (Dia+AEOP, 300 mg/kg) and the control indicating FBS. Each bar represents mean ± SEM and is analyzed by ANOVA followed by Tukey test. *: indicates a significant difference vs. control group (***: p < 0.001); #: indicates a significant difference vs. Dia group (###: p < 0.001). In all the groups, n = 10.
MWM
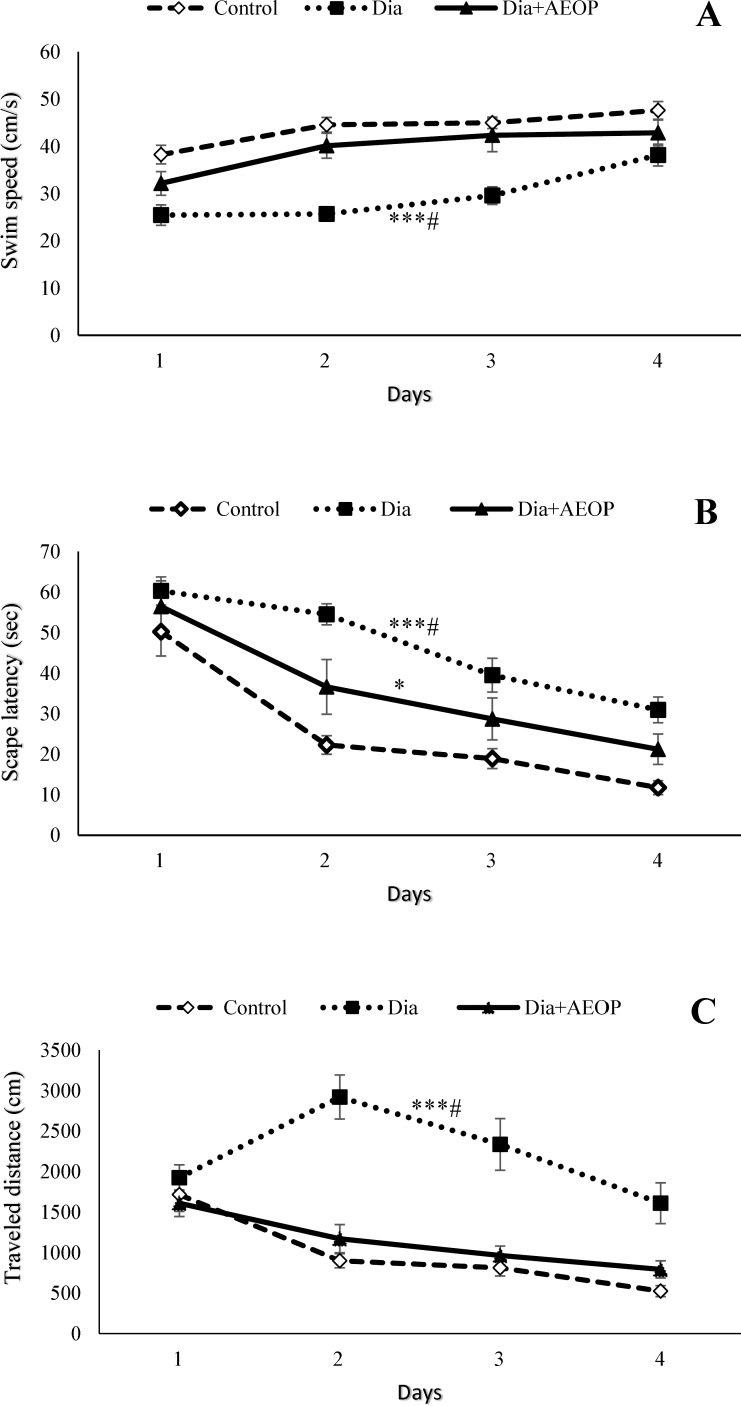
Diabetes significantly decreased swim speed while increased both the escape latency and the distance traveled to reach the hidden platform (p<0.001) at the training trials (Figure 2). All the three parameters were improved in the Dia+AEOP group compared to the Dia group (p<0.05). Although there were no significant differences in swim speed and the traveled distance between the control and Dia+AEOP groups, the escape latency to reach the hidden platform was longer in the Dia+AEOP (p<0.05).
Figure 2.
Data on the STZ-induced diabetic rats both treated and not treated (Dia) with Purslane (Dia+AEOP, 300 mg/kg) and the control demonstrating the swim speed (A), amount of escape latency to reach the hidden platform (B), and the distance traveled to reach it (C) in training trials of MWM. Each bar is represented as mean ± SEM and is analyzed by repeated measures. *: indicates the significant difference in the area below the curve vs. control group (*: p<0.05, ***: p<0.001). #: indicates the significant difference in the area under the curve vs. Dia+AEOP group (#: p<0.05). In all the groups, n = 10.
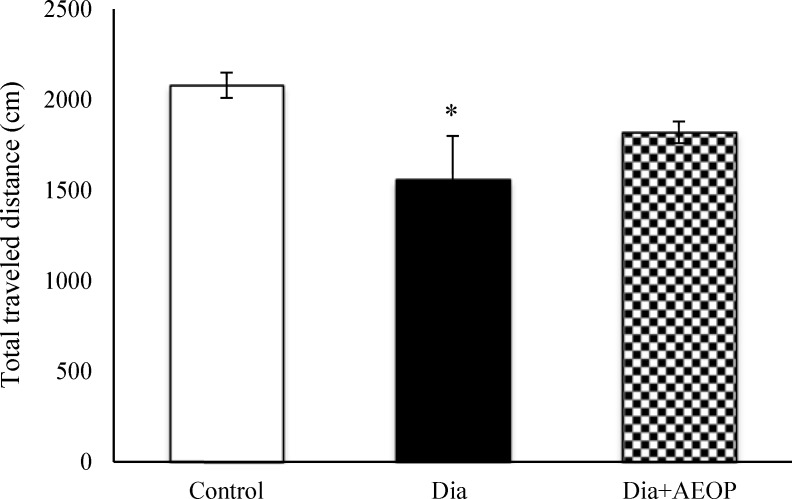
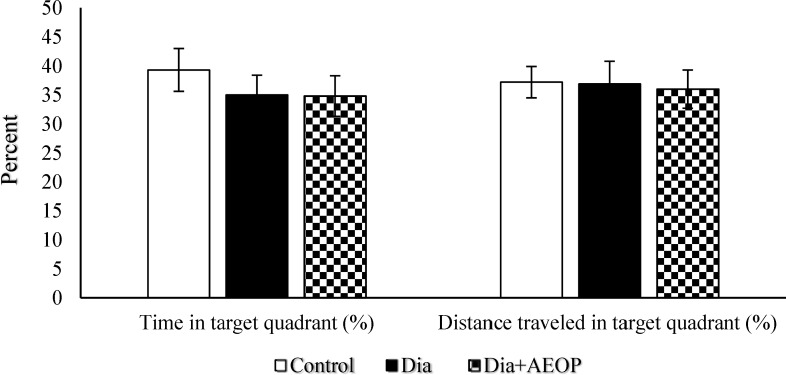
Data obtained from the probe trial is shown in Figures 3 and 4. Total distance traveled were significantly lower in Dia group compared to the control (P<0.05, Figure 3), but there were no significant differences in the percentages of target quadrant travel and target quadrant time (p>0.05, Figure 4).
Figure 3.
Data on the STZ-induced diabetic rats both treated and not treated (Dia) with Purslane (Dia+AEOP, 300 mg/kg) and the control indicating the total distance traveled in the probe trial of MWM. Each bar is represented as mean ± SEM and is analyzed by ANOVA followed by Tukey test. *: indicates the significant difference vs. control (*: p<0.05). In all the groups, n = 10
Figure 4.
Data on the STZ-induced diabetic rats both treated and not treated (Dia) with Purslane (Dia+AEOP, 300 mg/kg) and the control indicating the percentage of time spent in target quadrant and percentage of distance traveled in the target quadrant in the probe trial of MWM. Each bar is represented as mean ± SEM and is analyzed by ANOVA followed by Tukey test. No significant differences were detected. In all the groups, n = 10
EPM
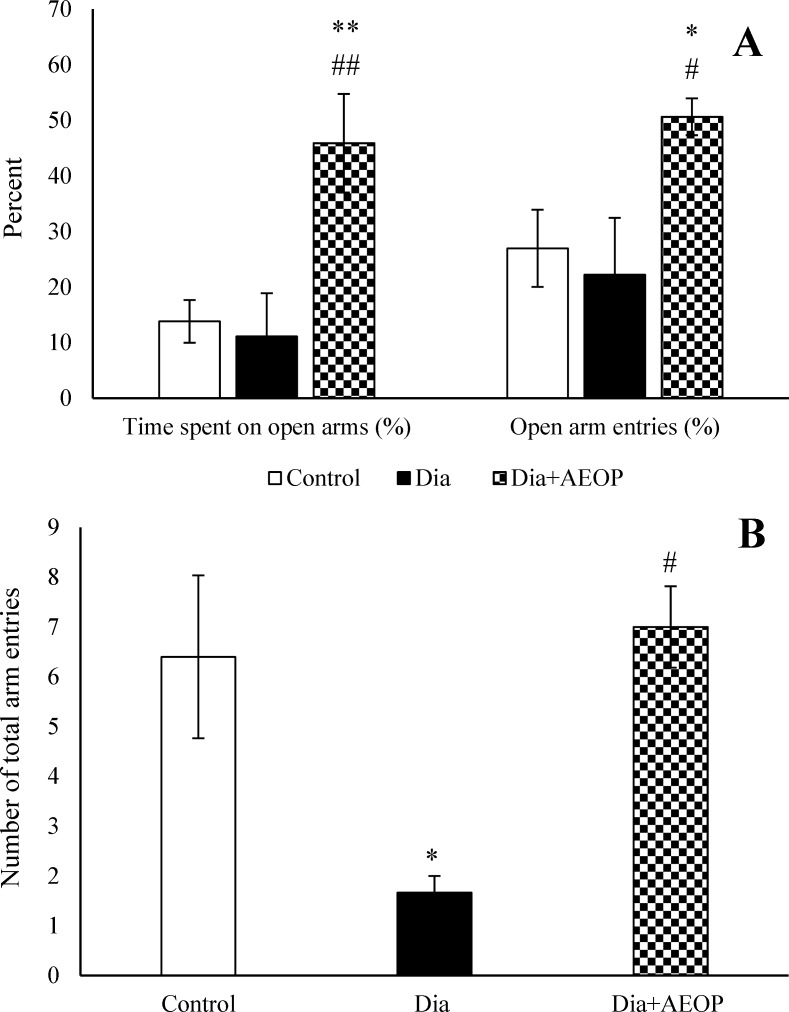
There were no significant differences in the percentages of open arm entries and open arm spent time between the control and Dia groups (P>0.05), but the number of total arm entries were significantly lower in Dia group (P<0.05). Treatment of diabetic rats with AEOP not only improved the number of total arm entries (p<0.05), but also increased the percentage of open arm entries (p<0.05) and the percentage of open arm spent time (p<0.01) compared to both the control and Dia groups (Figure 5).
Figure 5.
Data on the STZ-induced diabetic rats both treated and not treated (Dia) with Purslane (Dia+AEOP, 300 mg/kg) and the control, showing the percentage of time spent in the open arms, percentage of open arm entries (A) and number of total arm entries (B) in EPM. Each bar is represented as mean ± SEM and is analyzed by ANOVA followed by Tukey test. *: indicates a significant difference vs. Control group (*: p<0.05, **: p<0.01). #: indicates a significant difference vs. Dia group (#: p<0.05, ##: p<0.01). In all the groups, n = 10
FST
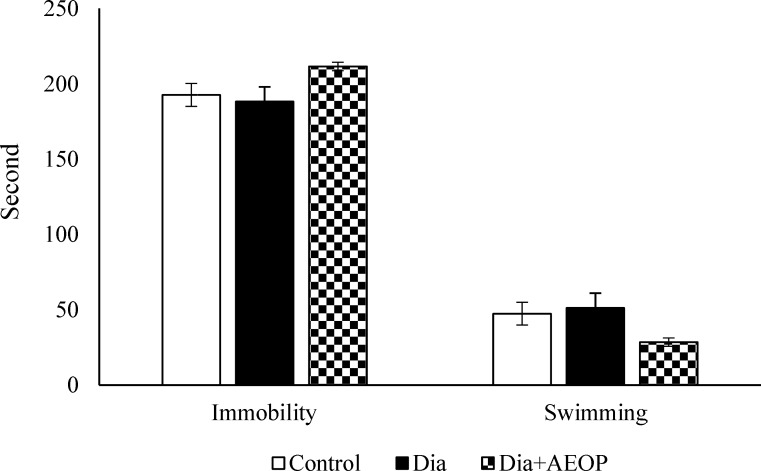
As shown in Figure 6, there were no significant differences between swimming/immobility times between any groups (P>0.05).
Figure 6.
Data on the STZ-induced diabetic rats both treated and not treated (Dia) with Purslane (Dia+AEOP, 300 mg/kg) and the control indicating immobility and swimming behavior assessed by FST. Each bar is represented as mean ± SEM and is analyzed by ANOVA followed by Tukey test. No significant differences were detected. In all the groups, n = 10
TPS
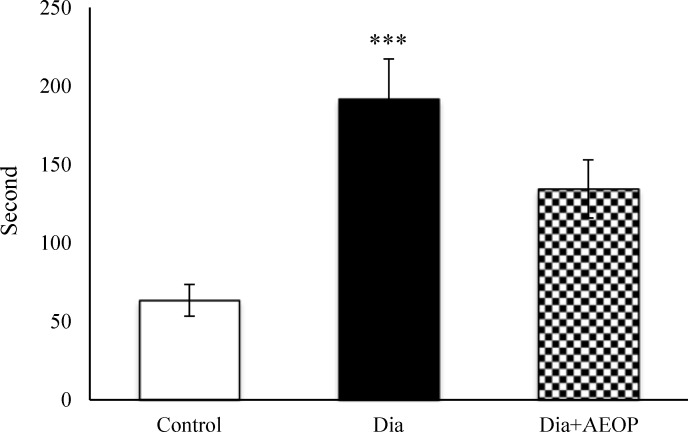
Total time of stress-induced NFMA was severely greater in the Dia group compared to the control (p≤0.001, Figure 7).
Figure 7.
Data one the STZ-induced diabetic rats both treated and not treated (Dia) with Purslane (Dia+AEOP, 300 mg/kg) and the control indicating NFMA in TPS. Each bar is represented as mean ± SEM and is analyzed by ANOVA followed by Tukey test. *: indicates the significant difference vs. control group (***: p≤0.001). In all the groups, n = 10
Discussion
The aim of the present study was to assess psychobiological effects of AEOP in diabetes induced OVX rats, as a model of postmenopausal women. AEOP not only ameliorated hyperglycemia, but also improved the diabetes related deficits in MWM, as well as motor deficit in EPM and NFMA in TPS. Furthermore, it showed significant anxiolytic effects.
Prolonged hyperglycemia, the most important cause of most diabetes complications, is thought to lead to cognitive impairments in diabetic patients (31, 32). Therefore, amelioration of diabetes related neurobehavioral impairment by AEOP may be partly related to its ability to attenuate hyperglycemia.
The MWM is a commonly used paradigm for experimenting on learning abilities and memory of rodents (33). Increased escape latency and distance traveled to reach the hidden platform in Dia group could be related to spatial learning impairment, while reduction of swim speed as well as the total distance traveled in this group are indications of motor impairment. This finding in the diabetic group is in accordance with the decrease in total arm entries in EPM. In general diabetes decreases locomotor activity (34). Since swim speed was significantly lower in diabetic animals, interpretation of learning and memory should be with caution. However, the distance traveled to reach the hidden platform may be more decisive in this context. All these deficits were improved by AEOP treatment. Deficits in spatial cognitive tasks have been reported in DM animal models, such as the STZ-diabetic rat in the MWM (35). The hippocampus and cerebral cortex of mammals which play a pivotal role in a diverse set of cognitive functions are very sensitive to the oxidative damage that occurs in STZ-diabetic animals (36). Enhancement of lipid peroxidation in these regions leads to a significant motor and memory deficit in behavioral functions of diabetic animals (37). In summary, chronic oral administration of AEOP did enhance the memory consolidation and the capability to recall stored information and spatial memory in diabetic OVX rats.
Many plant ingredients, including polyphenols, flavonoids and various plant extracts, have antioxidant effects (38). There are various antioxidants in purslane including anthocyanins, antioxidant vitamins (vit A, C and E), melatonin, flavonoids and etc. that have antioxidant properties (8-10, 39).
The EPM is widely used to assess anxiety-related behaviors in rodents (40). The percentages of open arm entries and time spent in the open arms which have been approved as a measure of anxiety (41), decreased significantly in Dia group. Both ovariectomy, via estrogen depletion (23, 24), and diabetes (42) increases anxiety-like behavior in rodents. AEOP not only reversed the diabetes related decrease of locomotor activity but also increased the percentage of time spent in the open arms and the percentage of open arm entries. The anxiety-like behavior of AEOP-treated group not only was lower than diabetic rats, but also was lower than the control group. Therefore the anxiolytic effect of AEOP was greater than we expected. This may be related to higher anxiety of OVX rats compared to intact ones (23, 24) or to high anxiolytic effects of AEOP. The later assumption is supported by the results of the previous studies which have indicated the anxiolytic effect of AEOP and ethanol extract of purslane on normal mice (15, 16). However, we did not find any report that indicates the anxiolytic effect of AEOP on diabetic or OVX models. Since both the naturally occurring and synthetic flavones have been shown to bind to GABAA benzodiazepine receptors with high affinity and to exert anxiolytic-like effects in rodents, they have been suggested as ligands for these binding sites (43). Furthermore flavonoids naturally are the ligands of benzodiazepine receptors and have anxiolytic effects (44). Thus, the anxiolytic effects of AEOP may be related to flavonoids.
FST is frequently used to assess antidepressant-like activities in rodents (45). In the present study, we utilized FST to evaluate the effects of AEOP on the depressive behavior of diabetic OVX rats. In some studies immobility was increased in STZ-induced diabetic rats (46, 47). However in our study there were no differences in the depressive behavior of normal and diabetic rats. This is in accordance with the study of Khanam and Pillai (48). AEOP did not show any significant effects on FST performance of diabetic OVX rats.
Tail pinch is used to stimulate stress-related behavioral activities in rats (29, 30). The use of a peripheral stressor which is unavoidable for the animal, like tail pinch, produces a series of behaviors including chewing and gnawing/biting (29, 30). The total time of stress-induced NFMA which was severely increased in the Dia group, was improved by AEOP-treatment. These responses attenuate a variety of stress-related changes in rats, such as increase in the activity of hypothalamo-pituitary-adrenal axis and catecholamine utilization in CNS (49). In this context, central dopamine is traditionally considered related to the etiopathogenesis of stereotyped behavior and bruxism (50). Rutledge et al. (51) showed that diabetes alter the sensitivity of dopaminergic system, which leads to modulation of nociception in STZ-diabetic rats. They reported that these changes are related to endogenous Met-enkephalinergic system. These changes may be related to the mechanism of increase of NFMA in diabetic rats. The stress reduction effect of AEOP may be correlated with its anxiolytic effect.
Purslane has many valuable compounds, it is rich in antioxidants (8-10), and also has neuroprotective (12, 13) and antidiabetic effects (17, 18). Thus, the positive effects of AEOP observed in this study may be attributed to these properties.
Conclusion
In the present study we showed anti-stress, antidiabetic and spatial memory improving effects of AEOP in diabetic OVX rats, as well as its anxiolytic effect on both diabetic OVX and normal OVX rats for the first time.
Acknowledgements
Authors offer their thanks to the research council of Shahid Chamran University of Ahvaz for its financial support. This paper is part of a research project approved by Shahid Chamran University Research Council.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care . 2014;37:81–90. [Google Scholar]
- 2.Manschot SM, Biessels GJ, Rutten GE, Kessels RP, Gispen WH, Kappelle LJ. Peripheral and central neurologic complications in type 2 diabetes mellitus: no association in individual patients. J. Neurol. Sci. . 2008;264:157–162. doi: 10.1016/j.jns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy JM, Zochodne DW. Impaired peripheral nerve regeneration in diabetes mellitus. J. Peripher. Nerv. Syst. . 2005;10:144–157. doi: 10.1111/j.1085-9489.2005.0010205.x. [DOI] [PubMed] [Google Scholar]
- 4.Jin HY, Liu WJ, Park JH, Baek HS, Park TS. Effect of dipeptidyl peptidase-IV (DPPIV) inhibitor (vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats. Arch. Med. Res. . 2009;40:536–544. doi: 10.1016/j.arcmed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson CM. Type 2 diabetes mellitus, dyslipidemia and Alzheimer’s disease. J. Alzheimers Dis. . 2010;20:711–722. doi: 10.3233/JAD-2010-100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang TJ, Price SA, Chilton L, Calcutt NA, Tomlinson DR, Verkhratsky A, Fernyhough P. Insulin prevents depolarization of the mitochondrial inner membrane in sensory neurons of type 1 diabetic rats in the presence of sustained hyperglycemia. Diabetes . 2003;52:2129–2136. doi: 10.2337/diabetes.52.8.2129. [DOI] [PubMed] [Google Scholar]
- 7.Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid. Redox. Signal . 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- 8.Kamal Uddin Md, Juraimi AS, Sabir Hossain Md, Nahar MA, Ali ME, Rahman MM. Purslane weed (Portulaca oleracea): a prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci. World J. . 2014 doi: 10.1155/2014/951019. Article ID 951019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang X, Li L, Tian J, Wu Y, Gao P, Li D, Zhang Q, Song S. A rapid extraction and analysis method for the simultaneous determination of 26 bioflavonoids in Portulaca oleracea L. Phytochem. Anal. . 2014;25:537–543. doi: 10.1002/pca.2524. [DOI] [PubMed] [Google Scholar]
- 10.Dkhil MA, Abdel Moniem AE, Al-Quraishy S, Saleh RA. Antioxidant effect of Purslane (Portulaca oleracea) and its mechanism of action. J. Med. Plants Res. . 2011;5:1589–1593. [Google Scholar]
- 11.Yen GC, Chen HY, Peng HH. Evaluation of the cytotoxicity, mutagenicity and antimutagenicity of emerging edible plants. Food Chem. Toxicol. . 2001;39:1045–1053. doi: 10.1016/s0278-6915(01)00053-9. [DOI] [PubMed] [Google Scholar]
- 12.Abdel Moneim AE, Dkhil MA, Al-Quraishy S. The potential role of Portulaca oleracea as a neuroprotective agent in rotenone-induced neurotoxicity and apoptosis in the brain of rats. Pestic. Biochem. Physiol. . 2013;105:203–212. [Google Scholar]
- 13.Abdel Moneim AE. The neuroprotective effects of purslane (Portulacaoleracea) on rotenone-induced biochemical changes and apoptosis in brain of rat. CNS Neurol. Disord. Drug Targets . 2013;12:830–841. doi: 10.2174/18715273113129990081. [DOI] [PubMed] [Google Scholar]
- 14.Hongxing Z, Nancai Y, Guofu H, Jianbo S, Yanxia W, Hanju H, Qian L, Wei M, Yandong Y, Hao H. Neuroprotective effects of purslane herb aqueous extracts against D-galactose induced neurotoxicity. Chem. Biol. Interact. . 2007;170:145–152. doi: 10.1016/j.cbi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Yoon BH, Ryu JH, Jung JW. Anxiolytic-like effects of Portulacaoleraceae L using the elevated plus-maze in mice. Orient. Pharm. Exp. Med. . 2009;9:135–141. [Google Scholar]
- 16.Miladi-Gorgi H, Vafaei AA, Rashidy-Pour A, Taherian AA, Jarrahi M, Emami-Abargoei M. Investigation of anxiolytic effects of aqueous extract of Portulacaoleracea in mice. Iran. J. Pharm. Res. . 2004;3:57–57. [Google Scholar]
- 17.Chan K, Islam MW, Kamil M, Radhakrishnan R, Zakaria MN, Habibullah M, Attas A. The analgesic and anti-inflammatory effects of Portulacaoleracea L subsp sativ (Haw) Celak. J. Ethnopharmacol. . 2000;73:445–451. doi: 10.1016/s0378-8741(00)00318-4. [DOI] [PubMed] [Google Scholar]
- 18.Gong F, Li F, Zhang L, Li J, Zhang Z, Wang G. Hypoglycemic effects of crude polysaccharide from purslane. Int. J. Mol. Sci. . 2009;10:880–888. doi: 10.3390/ijms10030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao D, Li Q, Fan Y. Hypoglycemic effects and mechanisms of Portulacaoleracea L in alloxan-induced diabetic rats. J. Med. Plants Res. . 2010;4:1996–2003. [Google Scholar]
- 20.Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog. Neurobiol. . 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- 21.Cahill L. Why sex matters for neuroscience. Nat. Rev. Neurosci. . 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 22.Godsland IF. Oestrogens and insulin secretion. Diabetologia . 2005;48:2213–2220. doi: 10.1007/s00125-005-1930-0. [DOI] [PubMed] [Google Scholar]
- 23.de Chaves G, Moretti M, Castro AA, Dagostin W, da Silva GG, Boeck CR, Quevedo J, Gavioli EC. Effects of long-term ovariectomy on anxiety and behavioral despair in rats. Physiol. Behav. . 2009;97:420–425. doi: 10.1016/j.physbeh.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Patki G, Allam FH, Atrooz F, Dao AT, Solanki N, Chugh G, Asghar M, Jafri F, Bohat R, Alkadhi KA, Salim S. Grape powder intake prevents ovariectomy-induced anxiety-like behavior, memory impairment and high blood pressure in female Wistar rats. PLoS One . 2013;8:74522. doi: 10.1371/journal.pone.0074522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AS, Lee YJ, Lee SM, Yoon JJ, Kim JS, Kang DG, Lee HS. An aqueous extract of Portulaca oleracea ameliorates diabetic nephropathy through suppression of renal fibrosis and inflammation in diabetic db/db mice. Am. J. Chin. Med. . 2012;40:495–510. doi: 10.1142/S0192415X12500383. [DOI] [PubMed] [Google Scholar]
- 26.Babanejad S, Naghdi N, Haeri Rohani SA. Microinjection of dihydrotestosterone as a 5α-reduced metabolite of testosterone into ca1 region of hippocampus could improve spatial learning in the adult male rats. Iran. J. Pharm. Res. . 2012;11:661–669. [PMC free article] [PubMed] [Google Scholar]
- 27.Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol. Biochem. Behav. . 1986;4:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 28.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 29.Go´mez FM, Giralt MT, Sainz B, Arru´ e A, Prieto M, Garcia-Vallejo P. A possible attenuation of stress-induced increases in striatal dopamine metabolism by the expression of nonfunctional masticatory activity in the rat. Eur. J. Oral. Sci. . 1999;107:461–467. doi: 10.1046/j.0909-8836.1999.eos107607.x. [DOI] [PubMed] [Google Scholar]
- 30.Go´mez FM, Ortega JE, Horrillo I, Meana JJ. Relationship between non-functional masticatory activity and central dopamine in stressed rats. J. Oral Rehabil. . 2010;37:827–833. doi: 10.1111/j.1365-2842.2010.02110.x. [DOI] [PubMed] [Google Scholar]
- 31.Kucukatay V, Ağar A, Gumuslu S, Yargiçoğlu P. Effect of sulfur dioxide on active and passive avoidance in experimental diabetes mellitus: relation to oxidant stress and antioxidant enzymes. Int. J. Neurosci. . 2007;117:1091–1107. doi: 10.1080/00207450600934531. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez EO, Beauquis J, Revsin Y, Banzan AM, Roig P, De Nicola AF, Saravia F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav. Brain Res. . 2009;198:224–230. doi: 10.1016/j.bbr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Assadian-narenji S, Naghdi N, Oryan S, Azadmanesh K. Intrahippocampal injection of 3α diol (a testosterone metabolite) and indomethacin (3α-HSD blocker), impair acquisition of spatial learning and memory in adult male rats. Iran. J. Pharm. Res. . 2013;12:457–469. [PMC free article] [PubMed] [Google Scholar]
- 34.Kou ZZ, Li CY, Hu JC, Yin JB, Zhang DL, Liao YH, Wu ZY, Ding T, Qu J, Li H, Li YQ. Alterations in the neural circuits from peripheral afferents to the spinal cord: possible implications for diabetic polyneuropathy in streptozotocin-induced type 1 diabetic rats. Front Neural Circuits . 2014;8:6. doi: 10.3389/fncir.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao CH, Liu HQ, Cao R, Ji AL, Zhang L, Wang F, Yang RH. Effects of dietary fish oil on learning function and apoptosis of hippocampal pyramidal neurons in streptozotocin-diabetic rats. Brain Res. . 2012;31:33–43. doi: 10.1016/j.brainres.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 36.Reagan LP, McEwen BS. Diabetes, but not stress, reduces neuronal nitric oxide synthase expression in rat hippocampus: implications for hippocampal synaptic plasticity. Neuroreport . 2002;13:1801–1804. doi: 10.1097/00001756-200210070-00022. [DOI] [PubMed] [Google Scholar]
- 37.Parihar MS, Chaudhary M, Shetty R, Hemnani T. Susceptibility of hippocampus and cerebral cortex to oxidative damage in streptozotocin treated mice: prevention by extracts of Withania somnifera and Aloe vera. J. Clin.Neurosci. . 2004;11:397–402. doi: 10.1016/j.jocn.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Reus MI, Gomez del Rio MA, Iglesias I, Elorza M, Slowing K, Benedi J. Standardized Hypericum perforatum reduces oxidative stress and increases gene expression of antioxidant enzymes on rotenone-exposed rats. Neuropharmacology . 2007;52:606–616. doi: 10.1016/j.neuropharm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Simopoulos AP, Tan DX, Manchester LC, Reiter RJ. Purslane: a plant source of omega-3 fatty acids and melatonin. J. Pineal. Res. . 2005;39:331–332. doi: 10.1111/j.1600-079X.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 40.Hassanzadeh F, Rabbani M, Khodarahmi GA, Moosavi M. Synthesis and evaluation of the anxiolytic activity of some phthalimide derivatives in mice model of anxiety. Iran. J. Pharm. Res. . 2012;11:109–115. [PMC free article] [PubMed] [Google Scholar]
- 41.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. . 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ates M, Dayi A, Kiray M, Sisman AR, Agilkaya S, Aksu I, Baykara B, Buyuk E, Cetinkaya C, Cingoz S, Uysal N. Anxiety- and depression-like behavior are correlated with leptin and leptin receptor expression in prefrontal cortex of streptozotocin-induced diabetic rats. Biotech. Histochem. . 2014;89:161–171. doi: 10.3109/10520295.2013.825319. [DOI] [PubMed] [Google Scholar]
- 43.Hanrahan JR, Chebib M, Johnston GA. Flavonoid modulation of GABA (A) receptors. Br. J. Pharmacol. . 2011;163:234–245. doi: 10.1111/j.1476-5381.2011.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girish C, Raj V, Arya J, Balakrishnan S. Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. Eur. J. Pharmacol. . 2013;710:49–58. doi: 10.1016/j.ejphar.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Yaghmaei P, Oryan S, Mohammadi K, Solati J. Role of serotonergic system on modulation of depressogenic-like effects of silymarin. Iran. J. Pharm. Res. . 2012;11:331–337. [PMC free article] [PubMed] [Google Scholar]
- 46.da Silva Haeser A, Sitta A, Barschak AG, Deon M, Barden AT, Schmitt GO, Landgraff S, Gomez R, Barros HM, Vargas CR. Oxidative stress parameters in diabetic rats submitted to forced swimming test: the clonazepam effect. Brain Res. . 2007;1154:137–143. doi: 10.1016/j.brainres.2007.03.088. [DOI] [PubMed] [Google Scholar]
- 47.Wayhs CA, Manfredini V, Sitta A, Deon M, Ribas G, Vanzin C, Biancini G, Ferri M, Nin M, Barros HM, Vargas CR. Protein and lipid oxidative damage in streptozotocin-induced diabetic rats submitted to forced swimming test: the insulin and clonazepam effect. Metab. Brain Dis. . 2010;25:297–304. doi: 10.1007/s11011-010-9211-0. [DOI] [PubMed] [Google Scholar]
- 48.Khanam R, Pillai KK. Effect of chromium picolinate on modified forced swimming test in diabetic rats: involvement of serotonergic pathways and potassium channels. Basic Clin. Pharmacol. Toxicol. . 2006;98:155–159. doi: 10.1111/j.1742-7843.2006.pto_288.x. [DOI] [PubMed] [Google Scholar]
- 49.Okada S, Hori N, Kimoto K, Onozuka M, Sato S, Sasaguri K. Effects of biting on elevation of blood pressure and other physiological responses to stress in rats: biting may reduce allostatic load. Brain. Res. . 2007;1185:189–194. doi: 10.1016/j.brainres.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 50.Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K. Bruxism physiology and pathology: an overview for clinicians. J. Oral Rehabil. . 2008;35:476–494. doi: 10.1111/j.1365-2842.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 51.Rutledge LP, Ngong JM, Kuperberg JM, Samaan SS, Soliman KF, Kolta MG. Dopaminergic system modulation of nociceptive response in long-term diabetic rats. Pharmacol. Biochem. Behav. . 2002;74:1–9. doi: 10.1016/s0091-3057(02)00949-8. [DOI] [PubMed] [Google Scholar]