Abstract
This review discusses the status, antimicrobial mechanisms, application, and regulation of natural preservatives in livestock food systems. Conventional preservatives are synthetic chemical substances including nitrates/nitrites, sulfites, sodium benzoate, propyl gallate, and potassium sorbate. The use of artificial preservatives is being reconsidered because of concerns relating to headache, allergies, and cancer. As the demand for biopreservation in food systems has increased, new natural antimicrobial compounds of various origins are being developed, including plant-derived products (polyphenolics, essential oils, plant antimicrobial peptides (pAMPs)), animal-derived products (lysozymes, lactoperoxidase, lactoferrin, ovotransferrin, antimicrobial peptide (AMP), chitosan and others), and microbial metabolites (nisin, natamycin, pullulan, ε-polylysine, organic acid, and others). These natural preservatives act by inhibiting microbial cell walls/membranes, DNA/RNA replication and transcription, protein synthesis, and metabolism. Natural preservatives have been recognized for their safety; however, these substances can influence color, smell, and toxicity in large amounts while being effective as a food preservative. Therefore, to evaluate the safety and toxicity of natural preservatives, various trials including combinations of other substances or different food preservation systems, and capsulation have been performed. Natamycin and nisin are currently the only natural preservatives being regulated, and other natural preservatives will have to be legally regulated before their widespread use.
Keywords: natural preservative, antimicrobial, safety, food application
Introduction
The food industry has developed along with globalization, resulting in an increased risk of foodstuffs being contaminated with pathogens, chemical residues, and toxins. The proliferation of pathogenic and spoilage bacteria should be controlled to guarantee food safety. Conventional preservatives are a group of synthetic chemical substances including nitrates/nitrites, sulfites, sodium benzoate, propyl gallate, and potassium sorbate. The use of these conventional preservatives in food has known side effects (Sharma, 2015). Nitrites and nitrate have been linked to leukemia, colon, bladder, and stomach cancer. Sorbate and sorbic acid are rare; however, they are related to urticaria and contact dermatitis. Benozates have been suspected to relating to allergies, asthma, and skin rashes.
During recent decades, investigation on food preservation have focused on more natural and healthier food (Caminiti et al., 2011; Fangio and Fritz, 2014). Biopreservation has dealt with extending food shelf life and enhancing food safety using plants, animals, microorganisms, and their metabolites (Settanni and Corsetti, 2008). Particularly, meat and meat products are perishable materials, and are controlled by the Hazard Analysis Critical Control Point (HACCP) approach. The risk of contracting foodborne illnesses is reduced by various food preservation methods; thermal processing, drying, freezing, refrigeration, irradiation, modified atmosphere packaging, and the addition of antimicrobial agents, salts, or other chemical preservatives. Unfortunately, these techniques cannot be applied to all food products because of undesired effects (texture, color, etc.) depending on food type, such as ready-to-eat foods and fresh foods. Especially, preserving meat products is more complex, with higher pH and mild pasteurization temperatures required.
Natural preservative are the chemical agents derived from plants, animals, and microorganisms, and are usually related to the host defense system (Singh et al., 2010; Tiwari et al., 2009). As the demand for biopreservation in food systems has increased, new natural antimicrobial compounds of various origin are being developed, including animal-derived systems (lysozyme, lactoferrin, and magainins), plant-derived products (phytoalexins, herbs, and spices), and microbial metabolites (bacteriocins, hydrogen peroxide, and organic acids) (Lavermicocca et al., 2003). The requirements of natural preservatives are: safety, stability during food processing (pH, heat, pressure, etc.), and antimicrobial efficacy. The representative food pathogens are Escherichia coli, Salmonella spp., Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus, Yersinia enterocolytica, Clostridium perfringens, Clostridium botulinum, and Campylobacter jejuni. The pathogenic fungi often related to food-borne diseases are toxin-producing Aspergillus flavus and Aspergillus paraciticus (Prange et al., 2005).
This review summarizes the current knowledge about natural preservatives regarding their antimicrobial effects, antimicrobial mechanism, application, and regulation in food systems.
Natural Preservatives of Plant Origin
Plant preservatives are composed to polyphenols and phenolics, essential oils, and plant antimicrobial peptides (pAMPs). These substances have evolved to possess antibacterial and antioxidant effect (Dua et al., 2013). Phenolics and polyphenols have various antimicrobial structures: simple phenols (caffeic acid, catechol, eugenol, and epicatechin) and phenolic acids (caffeic acid and cinnamic acid), quinones (hypericin), flavones, flavonols, flavonoids (epigallocatechin-3-gallate, catechin, and chrysin), tannins (pentagalloylglucose, procyanidine B-2), coumarins (coumarin, warfarin, and 7-hydroxycourmarin), terpenoids (menthol, artemisin, and capsaicin), and alkaloids (berberine and harmane) (Table 1) (Cowan, 1999; Hintz et al., 2015). The pAMPs are represented by thionin, plant defensins, lipid transfer proteins (LTPs), myrosinase-binding proteins (MBPs), hevein- and knottin-like peptides, snakins, cyclotides, and peptides from hydrolysates (Hintz et al., 2015).
Table 1. Major classes of natural preservatives of plant origin.
| Class | Subclass | Examples | References |
|---|---|---|---|
| Phenolics | Simple phenols | Catechol | Hintz et al., 2015 |
| Epicatechin | Mason and Wasserman, 1987 | ||
| Phenolic acids | Cinnamic acid | Cowan, 1999 | |
| Hintz et al., 2015 | |||
| Quinones | Hypericin | Cowan, 1999 | |
| Hintz et al., 2015 | |||
| Flavonoids | Chrysin | Dua et al., 2013 | |
| Quercetin | Lee et al., 2011 | ||
| Flavones | Abyssinone | Cowan, 1999 | |
| Flavonols | Totarol | Cowan, 1999 | |
| Tannins | Ellagitannin | Dua et al., 2013 | |
| Lee et al., 2016 | |||
| Coumarins | Coumarin | Cowan, 1999 | |
| Warfarin | Hintz et al., 2015 | ||
| 7-Hydroxycoumarin | |||
| Terpenoids, essential oils | Capsaicin | Bajpai et al., 2008 | |
| Eugenol | Gutierrez et al., 2008 | ||
| Thymol | Helander et al., 1998 | ||
| Carvacrol | Tiwari et al., 1998 | ||
| Alkaloids | Berberine | Cowan, 1999 | |
| Harmane | Garba and Okeniyi, 2012 | ||
| Piperine | |||
| Lectins and polypeptides | Mannose-specific agglutinin | Cowan, 1999 | |
| Fabatin | Cowan, 1999 | ||
| Polyacetylenes | 8S-Heptadeca-2(Z),9(Z)-diene-4,6-diyne-1,8-diol | Cowan, 1999 | |
| Antimicrobial peptide (pAMP) | Potato defensin, hevein, thionines, | Hintz et al., 2015 | |
| snakins, lipid transfer protein etc. | Jessen et al., 2006 |
Status of plant preservatives
Plant polyphenol extracts have been used as natural meat preservatives, including extracts from oregano, cranberry, sage, rosemary, grape seed, and others. Polyphenols can act as reducing agents and metal ion chelators in the presence of various hydroxyl radicals.
Oregano and cranberry extracts were evaluated for antimicrobial activity against L. monocytogenes in laboratory media, beef, and fish (Lin et al., 2004). These phenolicbased plant extracts are widely used in food preparation and are classified as Generally Regarded as Safe (GRAS). The effects of neem oil on the meat pathogens Carnobacterium maltaromaticum, Brochothrix thermosphacta, E. coli, and Pseudomonas fluorescens, were investigated as a preservative for fresh retail meat (Del Serrone et al., 2015a, Del Serrone et al., 2015b). Citrus species extracts were investigated as antifungal agents against spoilage fungi including Mucor sp. and Rhizophus sp. (Mohanka and Priyanka, 2014). Ethanol extract of Citrus species showed a higher antifungal effect than water extract did, and the minimum inhibitory concentration of the extract ranged from 6.25 to 25 mg/mL. Inula britannica ethanol extract showed an antimicrobial effect against five B. cereus strains in low fat milk, and the antimicrobial effect depended on terpene and polyphenol compounds (Lee et al., 2012). Brassica juncea extract showed an antiviral effect against influenza virus A/H1N1 in nonfat milk (Lee et al., 2014). Chestnut inner shell extract containing gallic acid and quercetin was shown an antimicrobial effect against C. jejuni in chicken meat at 1 and 2 mg/mL (Lee et al., 2016). Eight different flavonoids [quercetin, kaempferol, apigenin, luteolin, 5,4-dihydroxy-7-methnozyflavone (genkwanin), narigenin, hesperetin and hesperidin] were tested for antimicrobial effects against B. cereus strains (P14 and KCCM 40935) (Lee et al., 2011). Among these flavonoids, only kaempferol and apigenin were inhibitory, and kaempferol showed the greatest antimicrobial effect at 100 μM.
Essential oil or terpenes are secondary metabolites that provide flavor and aroma. The addition of adding essential oils from marjoram and rosemary was investigated in beef patties (Mohamed and Mansour, 2012). These essential oils were beneficial for antioxidant activity and sensory evaluation.
Plant antimicrobial peptides (pAMPs) were discovered in 1942 as natural defense compounds against pathogens (Hintz et al., 2015). The pAMPs were named as thionins, plant defensins, lipid transfer proteins (LTPs), myrosinase-binding proteins (MBPs), hevein- and knottin-like peptides, snakins, cyclotides, and peptides from hydrolysates. These substances have been isolated from Triticum aestivum (wheat), Impatients balsamina, Hordeum vulgare (barley), Arabidopsis thaliana, Hevea brasiliensis, Solanum tuverosum (potato), Oldenlandia offinis, etc.
Antimicrobial mechanisms of plant preservatives
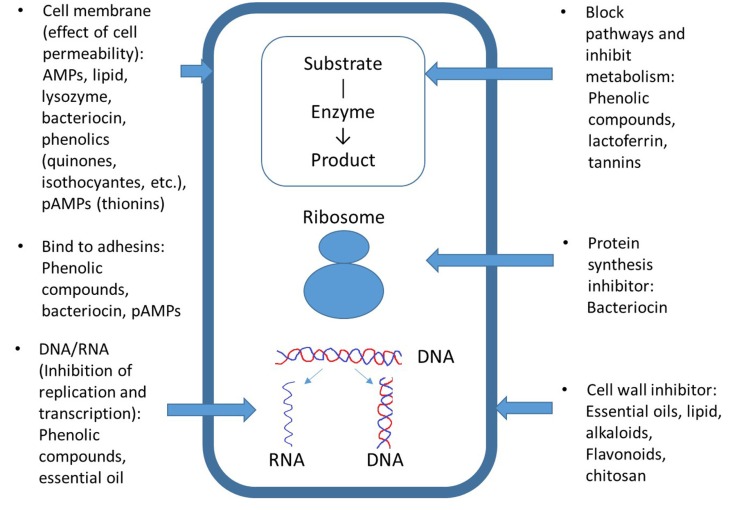
The antimicrobial mechanisms of phenol compounds depend on their concentration. Phenols affect enzyme activity related to energy production at low concentrations; however, they cause protein denaturation at high concentrations (Fig. 1). These abilities affect microbial cell permeability, thereby interfering with membrane function (material transport, nucleic acid synthesis, and enzyme activity) (Bajpai et al., 2008; Fung et al., 1977; Rico-Munoz et al., 1987). The high antibacterial activity of phenolic compounds can be due to alkyl substitution into the phenol nucleus, forming phenoxy radicals, which does not occur in more stable molecules such as the ethers myristicin or anethole (Dorman and Deans, 2000; Gutierrez et al., 2008). Catechol and pyrogallol possess phenolic toxicity to microorganisms through enzyme inhibition by oxidized compounds, possibly by reacting with sulfhydryl groups or through more nonspecific interactions with proteins (Mason and Wasserman, 1987). The antimicrobial targets of quinones may include surface-exposed adhesins, cell wall polypeptide, and membrane-bound enzymes (Cowan, 1999). The antimicrobial activities of isothiocynates derived from Brassicaceae vegetables, such as cauliflower, broccoli, mustard, and cabbage are related to 1) loss of cell membranes integrity, 2) inhibiting enzyme or regulatory activity by quorum sensing (in Helicobacter pylori, Pseudomonas aeruginosa, Chromobacterium violaceum, etc.), 3) inhibition of respiratory enzymes, 4) induction of heat-shock and oxidative stress, and 5) induction of a stringent response (Dufort et al., 2015). Carvacrol, (þ)-carvone, thymol, and trans-cinnamaldehyde decrease the intracellular ATP (adenosine triphosphate) content of E. coli O157:H7 cells (Helander et al., 1998).
Fig. 1. Antimicrobial mechanisms of natural preservatives. AMPs, antimicrobial peptides; pAMPs, plant antimicrobial peptides.
Essential oils have multiple cellular targets. Their hydrophobicity results in reactions with lipids on bacterial and fungal cell membranes, increasing membrane permeability and disturbing the original cell structure (Hintz et al., 2015; Pinto et al., 2009). In addition, antiviral effects are achieved by inhibiting viral protein synthesis at multiple stages of viral infection and replication (Wu et al., 2010).
The antimicrobial mechanism of most pAMPs involves cell membranes of targeted organisms and is driven by net positive charge, flexibility, and hydrophobicity to enable interaction with bacterial membranes (Jessen et al., 2006). Their antifungal mechanisms are involved in cell lysis, interference with fungal cell wall synthesis, permeabilization, binding to ergosterol/cholesterol in the membrane, depolymerization of the actin cytoskeleton, and targeting intracellular organelles such as mitochondria. Antiviral activity is also related to viral adsorption and entry processes.
Natural Preservatives of Animal Origin
There are numerous antimicrobial systems of animal origin related to host defense mechanisms. Preservatives of animal origin include lysozymes, lactoperoxidase, lactoferrin, ovotransferrin, antimicrobial peptide (AMP), chitosan, and others (Table 2).
Table 2. Natural preservatives of animal origin.
| Examples | Sources | Bacterial target | References |
|---|---|---|---|
| Chitosan | Crustaceans and arthropods | Antifungal and antimicrobial activity | Ben-Shalom et al., 2003; Je and Kim, 2006; Liu et al., 2006 |
| Defensin | Vertebrates and invertebrates | Bacteria, fungi, and virus | Ganz, 2003 |
| Dermaseptin S4 | Frog skin | Bacteria, fungi, and virus | Mor and Nicolas, 1994 |
| Lactoperxoidase | Milk | Gram-negative and -positive bacteria | Russell, 1991; de Wit and van Hooydonk, 1996 |
| Lactoferrin | Milk | Gram-negative and -positive bacteria, fungi, and parasites | Al-Nabulsi and Holley, 2005 |
| Lipids | Milk, animal | Gram-negative and -positive bacteria | Isaacs et al., 1990; Lampe et al., 1998; Wang and Johnson, 1997 |
| Lysozyme | Chicken egg whites | Gram-negative and -positive bacteria | Tiwari et al., 2009 |
| Magainin | African clawed frog | Gram-negative and -positive bacteria | Zasloff et al., 1988 |
| Ovotranferrin | Egg | S. aureus and E. coli | Ibrahim et al., 2000 |
| Pleurocidin | Skin of winter flounder | Bacteria, fungi, and virus | Cole et al., 1997 |
| PR-39 | Porcine | Gram-negative and -positive bacteria | Shi et al., 1996 |
Status of animal preservatives
Lysozyme is obtained from chicken egg whites, and is known as a bacteriolytic enzyme. Lysozyme has been used commercially under the name Inovapure, and can be used against a wide range of food spoilage organisms for extending the shelf life of various food products including raw and processed meats, cheese, and other dairy products (Tiwari et al., 2009).
The lactoperxoidase is a naturally active enzyme in milk with strong antimicrobial effects against both Gramnegative and -positive bacteria (de Wit and van Hooydonk, 1996; Russell, 1991) and fungi. Lactoperoxidase catalyzes the hydrogen peroxide (H2O2) oxidation of several acceptors; it has been utilized in industries such as dairy, oral care, cosmetics, cancer, and viral infection.
Lactoferrin and ovotranferrin are glycoproteins derived from bovine milk and hen egg respectively, that can bind iron, thereby restricting or preventing bacterial growth. Lactoferrin shows strong antimicrobial effects against various Gram-negative and -positive bacteria, fungi, and parasites in neutral pH and refrigeration temperature (Al-Nabulsi and Holley, 2005). Ovotranferrin peptide fragment OTAP-92 has strong bactericidal activity against both S. aureus and E. coli strains through membrane damage (Ibrahim et al., 2000). However, these transferrin peptides cannot be utilized in food systems because of their high cost.
AMPs are widely distributed in nature and are essential components of nonspecific host defense systems (Park et al., 1997; Tossi et al., 2000). The AMPs produced by animal cells include magainin (Zasloff et al., 1988), MSI-78 (Ge and Yan, 2002), PR-39 (Shi et al., 1996), pleurocidin (Cole et al., 1997), and dermaseptin S4 (Mor and Nicolas, 1994). AMPs are considered a promising solution for antibiotic resistance because of their non-specific molecular targets and fast membrane destruction. Pleurocidin is isolated from the winter flounder (Pleuronectes americanus) is active against Gram-negative and -positive bacteria (Cole et al., 2000). It is stable in heat and salt; however, it is unstable in supraphysiological concentrations to magnesium and calcium. An antimicrobial effect of pleurocidin was reported in foodborne organisms including Vibrio parahemolyticus, L. monocytogenes, E. coli O157: H7, Saccharomyces cerevisiae, and Penicillium expansum (Burrowes et al., 2004). Defensins are widely found in mammalian epithelial cells from chicken, turkey, and others (Brockus et al., 1998). Their antimicrobial spectrum included Gram-negative and -positive bacteria, fungi, and enveloped viruses (Ganz, 2003; Murdock et al., 2007).
Chitosan is a natural biopolymer obtained from the exoskeletons of crustaceans and arthropods, and has been used as an antifungal and antimicrobial agent (Ben-Shalom et al., 2003; Je and Kim, 2006; Liu et al., 2006). Chitooligosaccharides have a bacteriostatic effect on Gramnegative bacteria, E. coli, Vibrio cholera, Shigella dysenteriae, and Bacteriodes fragilis (Benhabiles et al., 2012). Chitosan (0.25, 0.5, and 1%) was studied as an antimicrobial ingredient in fresh pork sausage (Bostan and ’Isin Mahan, 2011).
Lipids of animal origin have antimicrobial activity against a wide range of microorganisms. Free fatty acids at mucosal surfaces have been shown to inactivate S. aureus (Bibel et al., 1989). Milk lipids are active against Gram-positive bacteria including S. aureus, C. botulinum, B. subtilis, B. cereus, and L. monocytogenes, and Gramnegative bacteria such as P. aeruginosa, E. coli, and Salmonella enteritidis (Isaacs et al., 1990; Lampe et al., 1998; Wang and Johnson, 1997).
Antimicrobial mechanisms of animal preservatives
AMPs, transferrins, and lipids can influence cell membranes and peptide synthesis (Fig. 1) (Brogden, 2005; Zasloff, 2002). AMPs can interact directly with the microbial cell membrane and result in the leaching out of vital cell components (Cole et al., 2000; Hancock, 1997). Lipids mainly inhibit bacterial cell walls or membranes, intracellular replication, or intracellular targets. Lysozymes inhibit bacterial cell membranes by hydrolyzing β-1,4-glycosidic linkages between N-acetylmuramic acid and N-acetylglucosamine in bacterial peptidoglycan.
Natural Preservatives from Microorganisms
The preservative of microbial origin include nisin, natamycin, pullulan, ε-polylysine, organic acid, and others (Singh et al., 2010) (Table 3).
Table 3. Natural preservatives from microorganisms.
| Examples | Sources | Bacterial target | References |
|---|---|---|---|
| Bacteriocins | |||
| Nisin, diplococcin, acidophilin, bulgaricin, helveticin, lactacin, pediocin, and plantarin | Lactococcus lactis, Lactobacillus acidophilus, Lactobacillus bulgaricus, etc. | Gram positive bacteria | Lee et al., 2013; Anastasiadou et al., 2008; Bhunia et al., 1988 |
| Natamycin | Streptomyces natalensis | Molds and yeasts | EFSA, 2009 |
| Reuterin | Lacotobacillus reuteri | Gram-negative and -positive bacteria, yeasts, and filamentous fungi | Axelsson et al., 1989 |
Status of microbial preservatives
Lactic acid bacteria produce antimicrobial compounds like organic acids, diacetyl, hydrogen peroxide, and proteinaceous bacteriocins (Lee et al., 2013). Bacteriocins are antimicrobial peptides or proteins produced by mainly lactic acid bacteria; these compounds are small and ribosomally synthesized. Most bacteriocins have potential as food preservatives because of their antimicrobial effect against food pathogens. The representative bacteriocins are nisin, diplococcin, acidophilin, bulgaricin, helveticin, lactacin, pediocin, and plantarin. Of these bacteriocins, nisin and pediocin have been used as commercial natural preservatives.
Nisin is a representative bacteriocin produced by various Lactococcus lactis strains, and has activity against food pathogens including Alicyclobacillus spp., L. monocytogenes, Bacillus spp., Micrococcus spp., Clostridium spp., Pediococcus spp., Desulfotomaculus spp., S. aureus, Enterococcus spp., Streptococcus haemolyticus, Lactobacillus spp., Sporolactobacillus spp., and Leuconostoc spp. Nisin is proteinaceous polypeptide that is most stable in acidic conditions. Nisin is soluble in aqueous conditions and is unstable in alkali solutions and heat. It has been used in various food products alone or in combination with other compounds. Nisin is the most widely used bacteriocin approved by the FDA as a food preservative. Dairy and meat products are applied with doses of 50-200 mg/kg. In the USA, nisin is used to inhibit outgrowth of C. botulinum spores and toxin formation in pasteurized processed cheese spread with fruits, vegetables or meats with a limited dose of about 250 ppm in finished products.
Pediocin is GRAS bacteriocin produced by strains of Pediococcus acidilactici (AcH, PA-1, JD, and 5) and P. pentosaceus (A, N5p, ST18, and PD1) (Anastasiadou et al., 2008). Most pediocins are stable in heat and a wide range of pH vlues. Pediocin AcH is effective against both spoilage and pathogenic organisms, including L. monocytogenes, Enterococcus faecalis, S. aureus, and Clostridium perfringens (Bhunia et al., 1988).
Natamycin is an antifungal substance produced by Streptomyces natalensis that is effective against almost all molds and yeasts; however, it has little or no effect on bacteria (EFSA, 2009). Natamycin has been used in dairy, meats, and other foods for antifungal effects, and its use as a surface preservative is regulated (E 235).
Reuterin (β-hydroxypropionaldehyde), an antimicrobial compound produced by Lacotobacillus reuteri, is a watersoluble nonproteinaceous metabolite of glycerol (Axelsson et al., 1989). Its broad antimicrobial spectrum includes Gram-negative and -positive bacteria, yeasts, and filamentous fungi (Nom and Rombouts, 1992).
Antimicrobial mechanisms of microbial preservatives
The antimicrobial mechanism of bacteriocin involves pore formation in the cytoplasmic membrane of target microorganisms (Fig. 1). This leads to cell death by loss of intracellular molecules and a collapse of the proton motive force (Driessen et al., 1995). Bacteriocin originating from Gram-positive bacteria is only effective for Grampositive bacteria, and is less effective on Gram-negative bacteria due to their selective membrane permeability (Lee et al., 2003). These disadvantages could be compensated for by using other preservatives and preservative methods.
Natamycin has an antimicrobial effect by binding to ergosterol, a cell membrane sterol, in fungal membranes (EFSA, 2009). The structure of natamycin contains a large lactone ring with a rigid lipophilc chain containing conjugated double bonds, and a flexible hydrophilic portion bearing several hydroxyl groups. The hydrophobic groups form a polar pore with ergosterol in the membrane, and this complex affects material passage (K+, H+, amino acids, and other metabolites) (Deacon, 1997).
Application of Natural Preservatives toward Livestock Food Systems
Raw meat, meat products, milk, and milk products are major sources of foodborne pathogens, and a variety of methods have been considered to reduce bacterial contaminants. These methods include (a) chemical decontamination with organic acids (Gill and Badoni, 2004; Goncalves et al., 2005; Nissen et al., 2001) and trisodium phosphate (Bashor et al., 2004; Okolocha and Ellerbroek, 2005); (b) physical processes such as irradiation (Badr, 2005; Kim et al., 2004), high pressure processing (Oliveira et al., 2015), steam (Kang et al., 2001a; Kang et al., 2001b; Logue et al., 2005; Stivarius et al., 2002), and UV; (c) natural antimicrobials such as bacteriocins (de Martinez et al., 2002; Gogus et al., 2004) and iron chelating compounds; and (d) combination treatment (Bashor et al., 2004; Koohmaraie et al., 2005).
Challenge studies using meat samples mainly reported efficacy against L. monocytogenes, B. cereus, C. jejuni, and S. aureus (Barman et al., 2014). The efficacy of natural preservatives was tested against commercial formulation (Microgard 100, Microgard 300, nisin, Altak 2002, Perlack 1902) (Lemay et al., 2002). These natural preservatives were investigated in an acidified chicken meat model (pH 5.0). E. coli ATCC 25922 and Brochothrix thermosphacta CRDAV452 were inhibited, however Lactobacillus alimentarius BJ33 (FloraCarn L-2) was not inhibited.
The use of fruit byproducts, including rinds of grapefruit, orange, and mandarin with or without γ-irradiation, was applied in raw ground beef (Abd El-khalek and Zahran, 2013). These substances demonstrated antioxidant and antimicrobial properties on microbial growth, lipid oxidation, and color change of raw ground beef meat. The antimicrobial effects on the survival of Salmonella typhimurium, E. coli and B. cereus were demonstrated.
A combination of plant extracts and MAPs was applied in meat products. Thymol and thymol-MAP were applied in sausage to inhibit Pseudomonas spp.; however, the performance is unacceptable respect to sensory acceptability (Mastromatteo et al., 2011). Bay essential oil with MAP (20% CO2 and 80% N2) was applied in ground chicken meat, and extend the shelf life without L. monocytogenes and E. coli (Irkin and Esmer, 2010). Oregano oil was added to fresh chicken breast meat under MAP (Chouliara et al., 2007). At 1%, oregano oil had a very strong taste in the sensory evaluation; however 0.1% oregano oil and MAP extended the shelf life by 5-6 d without strong taste.
Plant preservatives like clove oil showed a synergistic effect with lactic acid and vitamin C for antioxidant and antimicrobial effects (Naveena et al., 2006). Ntzimani et al. (2010) used a mixture of EDTA, lysozymes, rosemary, and oregano oil, and the shelf life of semi-cooked coated chicken fillets was extended under vacuum packaging at 4℃ to more than 2 wk.
Nisin was applied with lactoferrin in Turkish-style meatballs. Counts of total aerobic bacteria, coliform, E. coli, and other species were decreased by lactoferrin alone and by the mixture of lactoferrin and nisin (Colak et al., 2008). A mixture of lysozyme, nisin, and EDTA effectively inhibited L. monocytogenes and meat-borne spoilage bacteria in ostrich patties packaged in air and vacuum (Kim et al., 2002; Mastromatteo et al., 2010).
Regulation of Natural Preservatives in Livestock Foods
Preservatives permitted in livestock foods are sodium acetate, natamycin, pimamycin, nisin, nitrites (potassium nitrite and sodium nitrite), nitrates (potassium nitrate and sodium nitrate), sorbates (sorbic acid, sodium sorbate, potassium sorbate, and calcium sorbate), and sulphites (sulfur dioxide, sodium sulfite, sodium bisulfite, sodium metabisulfite, potassium metabisulfite, potassium sulfite, and potassium bisulfite) (Food and Drug Administration, 2016).
Natural food preservatives are regulated by maximum permitted levels for food safety and health (Table 4). The only natural preservatives regulated by legislation are natamycin and nisin. Natamycin (E235) is permitted for use in over 150 countries in the surface treatment of hard, semi-hard and semi soft cheeses and dried, cured sausages with a maximum permitted level of 6-40 mg/kg. Nisin (E234) is permitted for use in over 80 countries worldwide, including the United States and European Union, and has been in use as a food preservative for over 50 years (Adams, 2003; EFSA, 2006). The maximum permitted levels in meat, poultry, game products are 5.5-7 mg/kg.
Table 4. Representative natural preservatives and their maximum permitted level from codex.
| Preservative | Codex general standard for food additives | Maximum permitted levels (mg/kg) |
|---|---|---|
| Natamycin | Cheese analogues, processed cheese, ripened cheese, unripened cheese, whey protein cheese | 40 (USA, UK) |
| 20 (Germany) | ||
| Cured (including salted) and dried non-heat treated processed comminuted meat, poultry, and game products | 20 (USA, Germany) | |
| 6 (Germany) | ||
| Cured (including salted) and dried non-heat treated processed meat, poultry, and game products in whole pieces or cuts | 6 (USA) | |
| Surface of processed cheeses | 1 mg/dm2 (Korea) | |
| Nisin | Heat-treated processed meat, poultry, and game products in whole pieces or cuts | 5.5 (USA) |
| 6 (Japan) | ||
| Heat-treated processed comminuted meat, poultry, and game products | 5.5 (USA) | |
| 7 (Japan) | ||
| Edible casings (e.g., sausage casings) | 7 (USA) | |
| Processed cheeses | 250 (Korea) |
ESFA (2006, 2009); GSFA (1995); KFDA (2016).
Natural preservatives are considered safer than synthetic preservatives because of their existence in nature and long history of use. However, the use of natural preservatives in food is not powerful enough when considering added amounts in food system. Therefore, effective use levels of conventional and plant extracts/oils against microorganisms are less than 0.1% and 10-20%, respectively (Browne et al., 2012). Therefore, the regulation of these natural preservatives as food additives is necessary regarding their safety, toxicity, and effectiveness.
Conclusion
Chemical preservative have side effects related to the emergence of drug-resistant strains and chronic toxicity. Traditional methods of preservation including refrigeration, pasteurization, and low pH are not completely effective in controlling food pathogens. Therefore, the efficacy of combining natural preservatives with traditional methods has been tested. Combination with other substances or different food preservation systems, coatings, or microand nano-capsulation should be tested to assure safety and nontoxicity of natural preservatives. In addition, the use of natural preservatives must be regulated by law for safety, toxicity, and effectiveness.
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012-0006686).
References
- 1.Abd El-khalek H. H., Zahran D. A. Utilization of fruit by-product in ground meat preservation. Food Sci. Qual. Man. 2013;11:49–60. [Google Scholar]
- 2.Adams M. Nisin in multifactorial food preservation In: Roller S., editor. Natural antimicrobials for the minimal processing of foods. CRC Press LLC; Boca Raton, FL: 2003. pp. 11–33. [Google Scholar]
- 3.Al-Nabulsi A. A., Holley R. A. Effect of bovine lactoferrin against Carnobacterium viridans. Bioresource Technol. 2005;22:179–187. [Google Scholar]
- 4.Anastasiadou S., Papagianni M., Filiousis G., Ambrosiadis I., Koidis P. Pediocin SA-1, an antimicrobial peptide from Pediococcus acidilactici NRRL B5627: Production conditions, purification and characterization. Bioresources Technol. 2008;99:5384–5390. doi: 10.1016/j.biortech.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Axelsson L. T., Chung T. C., Dobrogosz W. J., Lindgren S. E. Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb. Ecol. Health D. 1989;2:131–136. doi: 10.3109/08910608909140210. [DOI] [Google Scholar]
- 6.Badr H. M. Elimination of Escherichia coli O157:H7 and Listeria monocytogenes from raw beef sausage by γ-irradiation. Mol. Nutr. Food Res. 2005;49:343–349. doi: 10.1002/mnfr.200400095. [DOI] [PubMed] [Google Scholar]
- 7.Bajpai V. K., Rahman A., Dung N. T., Huh M. K., Kang S. C. In vitro inhibition of food spoilage and foodbourne pathogenic bacteria by essential oil and leaf extracts of Magnolia liliflora Desr. J. Food Sci. 2008;73:M314–M320. doi: 10.1111/j.1750-3841.2008.00841.x. [DOI] [PubMed] [Google Scholar]
- 8.Barman S., Ghosh R., Mandal N. C. Use of bacteriocin producing Lactococcus lactis subsp. lactis LABW4 to prevent Listeria monocytogenes induced spoilage of meat. Food Nutr. Sci. 2014;5:2115–2123. doi: 10.4236/fns.2014.522224. [DOI] [Google Scholar]
- 9.Bashor M. P., Curtis P. A., Keener K. M., Sheldon B. W., Kathariou S., Osborne J. A. Effects of carcass washers on Campylobacter contamination in large broiler processing plants. Poultry Sci. 2004;83:1232–1239. doi: 10.1093/ps/83.7.1232. [DOI] [PubMed] [Google Scholar]
- 10.Benhabiles M. S., Salah R., Lounici H., Drouiche N., Goosen M. F. A., Mameri N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocolloid. 2012;29:48–56. doi: 10.1016/j.foodhyd.2012.02.013. [DOI] [Google Scholar]
- 11.Ben-Shalom N., Ardi R., Pinto R., Aki C., Fallik E. Controlling gray mould caused by Botytis cinerea in cucumber plants by means of chitosan. Crop Prot. 2003;22:285–290. doi: 10.1016/S0261-2194(02)00149-7. [DOI] [Google Scholar]
- 12.Bhunia A. K., Johnson M. C., Ray B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J. Appl. Microbiol. 1988;65:261–268. doi: 10.1111/j.1365-2672.1988.tb01893.x. [DOI] [PubMed] [Google Scholar]
- 13.Bibel D. J., Miller S. J., Brown B. E., Pandey B. B., Elias P. M., Shinefield H. M., Aly R. Antimicrobial activity of stratum corneum lipids from normal and essential fatty acid-deficient mice. J. Invest. Dermatol. 1989;92:632–638. doi: 10.1111/1523-1747.ep12712202. [DOI] [PubMed] [Google Scholar]
- 14.Bostan K., ’Isin Mahan F. Microbiological quality and shelf-life of sausage treated with chitosan. J. Fac. Vet. Med. Istanbul Univ. 2011;37:117–126. [Google Scholar]
- 15.Brogden K. A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 16.Browne B. A., Geis P., Rook T. Conventional vs. natural preservatives. HPPI. 2012;2012:69–73. [Google Scholar]
- 17.Brockus C. W., Jackwood M. W., Hamon B. G. Characterization of β-defensin prepropeptide mRNA from chicken and turkey bone marrow. Anim. Genet. 1998;29:283–289. doi: 10.1046/j.1365-2052.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 18.Burrowes O. J., Hadjicharalambous C., Diamond G., Lee T. C. Evaluation of antimicrobial spectrum and cytotoxic activity of pleurocidin for food application. J. Food Sci. 2004;69:66–71. [Google Scholar]
- 19.Caminiti I. M., Noci F., Muñoz A., Whyte P., Morgan D. J., Cronin D. A., Lyng J. G. Impact of selected combinations of non-thermal processing technologies on the quality of an apple and cranberry juice blend. Food Chem. 2011;124:1387–1892. doi: 10.1016/j.foodchem.2010.07.096. [DOI] [Google Scholar]
- 20.Chouliara E., Karatapanis A., Savvaidis I. N., Kontominas M. G. Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4℃. Food Microbiol. 2007;24:607–617. doi: 10.1016/j.fm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Colak H., Hampikyan H., Bingol E. B., Aksu H. The effect of nisin and bovine lactoferrin on the microbiological quality of Turkish-style meatball (Tekirdag k öfte) J. Food Safety. 2008;28:355–375. doi: 10.1111/j.1745-4565.2008.00105.x. [DOI] [Google Scholar]
- 22.Cole A. M., Darouiche R. O., Legarda D., Connell N., Diamond G. Characterization of a fish antimicrobial peptide: gene expression, subcellular localization and spectrum of activity. Antimicrob. Agents Chemother. 2000;44:2039–2045. doi: 10.1128/AAC.44.8.2039-2045.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole A. M., Weis P., Diamond G. Isolation and characterization of plurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 1997;272:12008–12013. doi: 10.1074/jbc.272.18.12008. [DOI] [PubMed] [Google Scholar]
- 24.Cowan M. M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Martinez Y. B., Ferrer K., Salas E. M. Combined effects of lactic acid and nisin solution in reducing levels of microbiological contamination in red meat carcasses. J. Food Prot. 2002;65:1780–1783. doi: 10.4315/0362-028x-65.11.1780. [DOI] [PubMed] [Google Scholar]
- 26.de Wit J. N., van Hooydonk A. C. M. Structure, functions and applications of lactoperoxidase in natural antimicrobial systems. Neth. Milk Dairy J. 1996;50:227–244. [Google Scholar]
- 27.Deacon J. W., editor. Modern Mycology. 3rd Ed. Blackwell Science; Oxford: 1997. pp. 289–290.Prevention and control of fungal growth [Google Scholar]
- 28.Del Serrone P., Toniolo C., Nicoletti M. Neem (Azadirachta indica A. Juss) oil to tackle enteropathogenic Escherichia coli. BioMed Res. Int. 2015a doi: 10.1155/2015/343610. Article ID 343610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Serrone P., Toniolo C., Nicoletti M. Neem (Azadirachta indica A. Juss) oil: A natural preservative to control meat spoilage. Foods. 2015b;4:3–14. doi: 10.3390/foods4010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorman H. J. D., Deans S. G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 31.Driessen A. J. M., van den Hoov H. W., Kuiper W., van der Kamp M., Sahl H. G., Konings R. N. H., Konings W. N. Mechanistic studies of lantibiotic-induced permeabilization of phospholipids vesicles. Biochem. 1995;34:1606–1614. doi: 10.1021/bi00005a017. [DOI] [PubMed] [Google Scholar]
- 32.Dua A., Garg G., Mahajan R. Polyphenols, flavonoids and antimicrobial properties of methanolic extract of fennel (Foeniculum vulgare Miller) Euro. J. Exp. Bio. 2013;3:203–208. [Google Scholar]
- 33.Dufort V., Stahl M., Baysse C. The antibacterial properties of isothiocyantes. Microbiol. 2015;161:229–243. doi: 10.1099/mic.0.082362-0. [DOI] [PubMed] [Google Scholar]
- 34.EFSA. Opinion of the scientific panel on food additives, flavorings, processing aids and material in contact with food on the safety in use of nisin as a food additive in an additional category of liquid eggs and on the safety of nisin produced using a modified production process as a food additive. EFSA J. 2006;314:1–8. [Google Scholar]
- 35.EFSA. Scientific opinion on the use of natamycin (E 235) as a food additive EFSA panel on food additives and nutrient sources added to food (ANS) EFSA J. 2009;1412:1–25. [Google Scholar]
- 36.Fangio M. F., Fritz R. Potential use of a bacteriocin-like substance in meat and vegetable food biopreservation. Int. Food Res. J. 2014;21:677–683. [Google Scholar]
- 37.Food and Drug Administration. [Accessed April 8, 2016];Food additive status list. 2016 Available from: http://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm091048.htm .
- 38.Fung D. Y. C., Taylor S., Kahan J. Effects of butylated hydroxyanisole (BHA) and butylated hydroxitoluene (BHT) on growth and aflatoxin production of Aspergillus flavus. J. Food Safety. 1977;1:39–51. doi: 10.1111/j.1745-4565.1977.tb00258.x. [DOI] [Google Scholar]
- 39.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 40.Garba S., Okeniyi S. O. Antimicrobial activities of total alkaloids extracted from some Nigerian medicinal plants. J. Microbiol. Antimicrob. 2012;4:60–63. [Google Scholar]
- 41.Ge Y., Yan H. Extraction of natural vitamin E from wheat germ by supercritical carbon dioxide. J. Agric. Food Chem. 2002;50:685–689. doi: 10.1021/jf010615v. [DOI] [PubMed] [Google Scholar]
- 42.Gill C. O., Badoni M. Effects of peroxyacetic acid, acidified sodium chlorite or lactic acid solutions on the microflora of chilled beef carcasses. Int. J. Food Microbiol. 2004;91:43–50. doi: 10.1016/S0168-1605(03)00329-5. [DOI] [PubMed] [Google Scholar]
- 43.Gogus U., Bozoglu F., Yurdugul S. The effects of nisin, oil-wax coating and yogurt on the quality of refrigerated chicken meat. Food Control. 2004;15:537–542. doi: 10.1016/j.foodcont.2003.08.007. [DOI] [Google Scholar]
- 44.Goncalves A. C., Almeida R. C. C., Alves M. A. O., Almeida P. F. Quantitative investigation on the effects of chemical treatments in reducing Listeria monocytogenes populations on chicken breast meat. Food Control. 2005;16:617–622. doi: 10.1016/j.foodcont.2004.06.026. [DOI] [Google Scholar]
- 45.GSFA. General Standard for Food Additives. Codex STAN 192. 1995.
- 46.Gutierrez J., Rodriguez G., Barry-Ryan C., Bourke P. Efficacy of plant essential oils against foodborne pathogens and spoilage bacteria associated with ready-to-eat vegetables: Antimicrobial and sensory screenin. J. Food Prot. 2008;71:1846–1854. doi: 10.4315/0362-028x-71.9.1846. [DOI] [PubMed] [Google Scholar]
- 47.Hancock R. E. W. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 48.Helander I. M., Alakomi H. L., Latva-Kala K., Mattila-Sandholm T., Pol I., Smid E. J., Gorris L. G. M., von Wright A. Characterisation of the action of selected essential oil components on gram-negative bacteria. J. Agric. Food Chem. 1998;46:3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- 49.Hintz T., Matthews K. K., Di R. The use of plant antimicrobial compounds for food preservation. Biomed Res. Int. 2015 doi: 10.1155/2015/246264. Article ID 246264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibrahim H. R., Sugimoto Y., Aoki T. Ovotranferrin antimicrobial peptide (OTAP-92) kills bacteria through a membrane damage mechanism. Biochim. Biophys. Acta. 2000;1523:196–205. doi: 10.1016/S0304-4165(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 51.Irkin R., Esmer O. K. Control of Listeria monocytogenes in ground chicken breast meat under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of bay essential oil at 4℃. Food Sci. Technol. Res. 2010;16:285–290. doi: 10.3136/fstr.16.285. [DOI] [Google Scholar]
- 52.Isaacs C. E., Kashyap S., Heird W. C., Thormar H. Antiviral and antibacterial lipids in milk and infant formula feeds. Arch. Dis. Child. 1990;65:861–864. doi: 10.1136/adc.65.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Je J. Y., Kim S. K. Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. J. Agric. Food Chem. 2006;54:6629–6633. doi: 10.1021/jf061310p. [DOI] [PubMed] [Google Scholar]
- 54.Jessen H., Hamill P., Hancock R. E. W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:479–491. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang D. H., Koohmaraie M., Dorsa W. J., Siragusa G. R. Development of a multiple-step process for the microbial decontamination of beef trim. J. Food Prot. 2001a;64:63–71. doi: 10.4315/0362-028x-64.1.63. [DOI] [PubMed] [Google Scholar]
- 56.Kang D. H., Koohmaraie M., Siragusa G. R. Application of multiple antimicrobial interventions for microbial decontamination of commercial beef trim. J. Food Prot. 2001b;64:168–171. doi: 10.4315/0362-028x-64.2.168. [DOI] [PubMed] [Google Scholar]
- 57.Kim B. H., Jang A. R., Lee S. O., Min J. S., Lee M. H. Combined effect of electron-beam (beta) irradiation and organic acids on shelf life of pork loins during cold storage. J. Food Prot. 2004;67:168–171. doi: 10.4315/0362-028x-67.1.168. [DOI] [PubMed] [Google Scholar]
- 58.Kim Y. M., Paik H. D., Lee D. S. Shelf-life characteristics of fresh oysters and ground beef as affected by bacteriocin-coated plastic package film. J. Sci. Food Agric. 2002;82:998–1002. doi: 10.1002/jsfa.1125. [DOI] [Google Scholar]
- 59.Koohmaraie M., Arthur T. M., Bosilevac J. M., Guerini M., Shackelford S. D., Wheeler T. L. Post-harvest interventions to reduce/eliminate pathogens in beef. Meat Sci. 2005;71:79–91. doi: 10.1016/j.meatsci.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 60.KFDA. [Accessed Feb. 20, 2016];Korea Food Additives Code. 2015 Available from: http://www.foodnara.go.kr:9010/20121228_051106 .
- 61.Lampe M. F., Ballweber L. M., Isaacs C. E., Patton D. L., Stamm W. E. Killing of Chlamydia trachomatis by novel antimicrobial lipids adapted from compounds in human breast milk. Antimicrob. Agents Chemother. 1998;42:1239–1244. doi: 10.1128/aac.42.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lavermicocca P., Valerio F., Visconti A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microbiol. 2003;69:634–640. doi: 10.1128/AEM.69.1.634-640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee D. U., Heinz V., Knorr D. Effects of combination treatments of nisin and high-intensity ultrasound with high pressure on the microbial inactivation in liquid whole egg. Innov. Food Sci. Emerg. Technol. 2003;4:387–393. doi: 10.1016/S1466-8564(03)00039-0. [DOI] [Google Scholar]
- 64.Lee J. H., Lee Y. J., Ahn S. H., Lee N. K., Paik H. D. Antimicrobial properties of whole milk with Inula britannica extract against Bacillus cereus strains during storage. Milchwissenschaft. 2012;67:315–317. [Google Scholar]
- 65.Lee K. A., Moon S. H., Kim K. T., Nah S. Y., Paik H. D. Antimicrobial effect of kaempferol on psychrotrophic Bacillus cereus strains outbreakable in dairy products. Korean J. Food Sci. An. 2011;31:311–315. doi: 10.5851/kosfa.2011.31.2.311. [DOI] [Google Scholar]
- 66.Lee N. K., Han E. J., Han K. J., Paik H. D. Antimicrobial effect of bacteriocin KU24 produced by Lactococcus lactis KU24 against methicillin-resistant Staphylococcus aureus. J. Food Sci. 2013;78:M465–M469. doi: 10.1111/1750-3841.12053. [DOI] [PubMed] [Google Scholar]
- 67.Lee N. K., Jung B. S., Na D. S., Yu H. H., Kim J. S., Paik H. D. The impact of antimicrobial effect of chestnut inner shell extracts against Campylobacter jejuni in chicken meat. LWT-Food Sci. Technol. 2016;65:746–750. doi: 10.1016/j.lwt.2015.09.004. [DOI] [Google Scholar]
- 68.Lee N. K., Lee J. H., Lim S. M., Lee K. A., Kim Y. B., Chang P. S., Paik H. D. Antiviral activity of subcritical water extract of Brassica juncea against influenza virus A/H1N1 in nonfat milk. J. Dairy Sci. 2014;97:5383–5386. doi: 10.3168/jds.2014-8016. [DOI] [PubMed] [Google Scholar]
- 69.Lemay M. J., Choquette J., Delaquis P. J., Gariépy C., Rodrigue N., Saucier L. Antimicrobial effect of natural preservatives in a cooked and acidified chicken meat model. Int. J. Food Microbiol. 2002;78:217–226. doi: 10.1016/S0168-1605(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 70.Lin Y. T., Labbe R. G., Shetty K. Inhibition of Listeria monocytogenes in fish and meat systems by use of oregano and cranberry phytochemical synergies. Appl. Environ. Microbiol. 2004;70:5672–5678. doi: 10.1128/AEM.70.9.5672-5678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu N., Chen X. G., Park H. J., Liu C. G., Liu C. S., Meng X. H., Yu L. J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006;64:60–65. doi: 10.1016/j.carbpol.2005.10.028. [DOI] [Google Scholar]
- 72.Logue C. M., Sheridan J. J., Harrington D. Studies of steam decontamination of beef inoculated with Escherichia coli O157:H7 and its effect on subsequent storage. J. Appl. Microbiol. 2005;98:741–751. doi: 10.1111/j.1365-2672.2004.02511.x. [DOI] [PubMed] [Google Scholar]
- 73.Mason T. L., Wasserman B. P. Inactivation of red beet beta-glucan synthase by native and oxidized phenolic compounds. Phytochem. 1987;26:2197–2202. doi: 10.1016/S0031-9422(00)84683-X. [DOI] [Google Scholar]
- 74.Mastromatteo M., Incoronato A. L., Conte A., Del Nobile M. A. Shelf life of reduced pork back-fat content sausages as affected by antimicrobial compounds and modified atmosphere packaging. Int. J. Food Microbiol. 2011;150:1–7.. doi: 10.1016/j.ijfoodmicro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Mastromatteo M., Lucera A., Sinigaglia M., Corbo M. R. Synergic antimicrobial activity of lysozyme, nisin, and EDTA against Listeria monocytogenes in ostrich meat patties. J. Food Sci. 2010;75:M422–M429. doi: 10.1111/j.1750-3841.2010.01732.x. [DOI] [PubMed] [Google Scholar]
- 76.Mohamed M. H., Mansour H. A. Incorporating essential oils of marjoram and rosemary in the formulation of beef patties manufactured with mechanically deboned poultry meat to improve the lipid stability and sensory attributes. LWT-Food Sci. Technol. 2012;45:79–87. doi: 10.1016/j.lwt.2011.07.031. [DOI] [Google Scholar]
- 77.Mohanka R., Priyanka. Plant extract as natural food preservative against spoilage fungi from processed food. Int. J. Curr. Microbiol. App. Sci. 2014;3:91–98. [Google Scholar]
- 78.Mor A., Nicolas P. Isolation and structure of novel defensive peptides from frog skin. Eur. J. Biochem. 1994;219:145–154. doi: 10.1111/j.1432-1033.1994.tb19924.x. [DOI] [PubMed] [Google Scholar]
- 79.Murdock C. A., Cleveland J., Matthews K. R., Chikindas M. L. The synergistic effect of nisin and lactoferrin on the inhibition of Listeria monocytogenes and Escherichia coli O157:H7. Lett. Appl. Microbiol. 2007;44:255–261. doi: 10.1111/j.1472-765X.2006.02076.x. [DOI] [PubMed] [Google Scholar]
- 80.Naveena B. M., Muthukumar M., Sen A. R., Babji Y., Murthy T. R. K. Improvement of shelf life of buffalo meat using lactic acid, clove oil and vitamin C during retail display. Meat Sci. 2006;74:409–415. doi: 10.1016/j.meatsci.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 81.Nissen H., Maugesten T., Lea P. Survival and growth of Escherichia coli O157:H7, Yersinia enterocolitica and Salmonella enteritidis on decontaminated and untreated meat. Meat Sci. 2001;57:291–298. doi: 10.1016/S0309-1740(00)00104-2. [DOI] [PubMed] [Google Scholar]
- 82.Nom M. J. R., Rombouts F. M. Fermentative preservation of plant foods. Appl. Bacterial Symp. Suppl. 1992;73:1365–1478. [Google Scholar]
- 83.Ntzimani A. G., Giatrakou V. I., Savvaidis I. N. Combined natural antimicrobials treatments (EDTA, lysozyme, rosemary and oregano oil) on semi cooked chicken meat stored in vacuum packages at 4℃: Microbiological and sensory evaluation. Innov. Food Sci. Emerg. Technol. 2010;11:187–196. doi: 10.1016/j.ifset.2009.09.004. [DOI] [Google Scholar]
- 84.Okolocha E. C., Ellerbroek L. The influence of acid and alkaline treatments on pathogens and the shelf life of poultry meat. Food Control. 2005;16:217–225. doi: 10.1016/j.foodcont.2004.01.015. [DOI] [Google Scholar]
- 85.Oliveira T. L. C., Junior B. R. C., Ramos A. L. S., Ramos E. M., Piccoli R. H., Cristianini M. Phenolic carvacrol as a natural additive to improve the preservative effects of high pressure processing of low-sodium sliced vacuumpacked turkey breast ham. LWT-Food Sci. Technol. 2015;64:1297–1308. doi: 10.1016/j.lwt.2015.06.011. [DOI] [Google Scholar]
- 86.Park C. B., Lee J. H., Park I. Y., Kim M. S., Kim S. C. Novel antimicrobial peptide from loach, Misgurnus anguillicaudatus. FEMS Lett. 1997;411:173–178. doi: 10.1016/s0014-5793(97)00684-4. [DOI] [PubMed] [Google Scholar]
- 87.Pinto E., Vale-Silva L., Cavaleiro C., Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009;58:1454–1462. doi: 10.1099/jmm.0.010538-0. [DOI] [PubMed] [Google Scholar]
- 88.Prange A., Birzele B., Hormes J., Modrow H. Investigation of different human pathogenic and food contaminating bacteria and mould grown on selenite/selenate and tellurite/tellurate by X-ray absorption spectroscopy. Food Control. 2005;16:713–728. [Google Scholar]
- 89.Rico-Munoz E., Eriotou E., Davidson P. M. Effect of selected phenolic compounds on the membrane-bound adenosine triphosphate of Staphylococcus aureus. Food Microbiol. 1987;4:239–249. doi: 10.1016/0740-0020(87)90006-2. [DOI] [Google Scholar]
- 90.Russell A. D. Mechanisms of bacterial resistance to nonantibiotics: Food additives and food and pharmaceutical preservatives. J. Appl. Bacteriol. 1991;71:191–201. doi: 10.1111/j.1365-2672.1991.tb04447.x. [DOI] [PubMed] [Google Scholar]
- 91.Settanni L., Corsetti A. Application of bacteriocins in vegetable food biopreservation. Int. J. Food Microbiol. 2008;121:123–138. doi: 10.1016/j.ijfoodmicro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Sharma S. Food preservatives and their harmful effects. Int. J. Sci. Res. Pub. 2015;5:1–2. [Google Scholar]
- 93.Shi J., Ross C. R., Chengappa M. M., Style M. J., McVey D. S., Blecha F. Antibacterial activity of a synthetic peptide (PR-26) derived from PR-39, a proline-argininerich neutrophil antimicrobial peptide. Antimicrob. Agents Chemother. 1996;40:115–121. doi: 10.1128/aac.40.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singh A., Sharma P. K., Garg G. Natural products as preservatives. Int. J. Pharm. Bio Sci. 2010;1:101–612. [Google Scholar]
- 95.Stivarius M. R., Pohlman F. W., McElyea K. S., Waldroup A. L. Effects of hot water and lactic acid treatment of beef trimmings prior to grinding on microbial, instrumental color and sensory properties of ground beef during display. Meat Sci. 2002;60:327–334. doi: 10.1016/S0309-1740(01)00127-9. [DOI] [PubMed] [Google Scholar]
- 96.Tiwari B. K., Valdramidis V. P., O’Donnell C. P., Muthukumarappan K., Bourke P., Cullen P. J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009;57:5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- 97.Tossi A., Sandri L., Giangaspero A. Amphipathic, - helical antimicrobial peptides. Biopolymers. 2000;55:4–30. doi: 10.1002/1097-0282(2000)55:1<4::AID-BIP30>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 98.Wang L. L., Johnson E. A. Control of Listeria monocytogenes by monoglycerides in foods. J. Food Prot. 1997;60:131–138. doi: 10.4315/0362-028X-60.2.131. [DOI] [PubMed] [Google Scholar]
- 99.Wu S., Patel K. B., Booth L. J., Metcalf J. P., Lin H. K., Wu W. Protective essential oil attenuates influenza virus infection: an in vitro study in MDCK cells. BMC Complement. Altern. Med. 2010;10 doi: 10.1186/1472-6882-10-69. article 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zasloff M., Martin B., Chen H. C. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc. Natl. Acad. Sci. USA. 1988;85:910–913. doi: 10.1073/pnas.85.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]