Abstract
Despite dedicated efforts to identify interventions to delay aging, most promising interventions yielding dramatic life-span extension in animal models of aging are often ineffective when translated to clinical trials. This may be due to differences in primary outcomes between species and difficulties in determining the optimal clinical trial paradigms for translation. Measures of physical function, including brief standardized testing batteries, are currently being proposed as biomarkers of aging in humans, are predictive of adverse health events, disability, and mortality, and are commonly used as functional outcomes for clinical trials. Motor outcomes are now being incorporated into preclinical testing, a positive step toward enhancing our ability to translate aging interventions to clinical trials. To further these efforts, we begin a discussion of physical function and disability assessment across species, with special emphasis on mice, rats, monkeys, and man. By understanding how physical function is assessed in humans, we can tailor measurements in animals to better model those outcomes to establish effective, standardized translational functional assessments with aging.
Keywords: Translational, Animal models, Physical function, Behavior
Advancing age is coupled with declines in physiological functioning that have classically been defined under a framework of a disablement process consisting of four distinct, yet related phenomena: active pathology, impairment, functional limitation, and ultimately disability (1,2) (Figure 1). The progression from impairment to limitation and disability is of considerable importance considering the “Silver Tsunami” of baby-boomers who threaten to strain healthcare costs and other facets of community life due to age-related disability (3,4). It has been estimated that dependent functional status is present in fewer than 20% of older adults, but responsible for almost half (ie, 46%) of healthcare costs for older persons (5). The absolute and relative projected increases of the number of older adults, and the high costs for the management of disabling conditions make the design and development of effective interventions against disability essential (3).
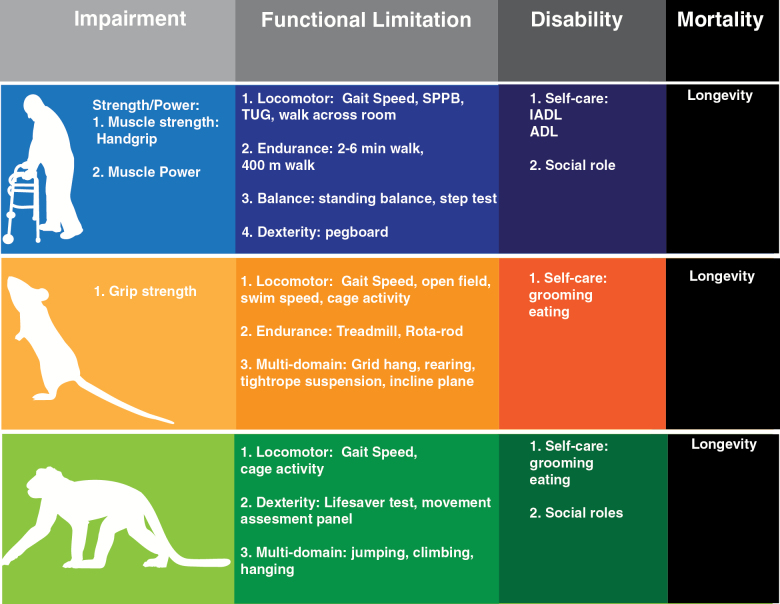
Figure 1.
Translational outcomes in the disablement process across species. Functional measures observed in humans at each stage of the disability spectrum can be reverse translated to outcomes in rodents and nonhuman primates (adapted from references 23,41). Impairment: a loss or abnormality of an anatomical, physiological, mental, or emotional nature. Functional limitation: manifestations at the level of the organism as a whole. Disability: social rather than organismic functioning; the inability or limitation in performing socially defined roles and expected tasks within a specific sociocultural and physical environment. Definitions originally derived from the disability framework established by Nagi (1).
Interventions targeting aging are frequently screened in animal models, including mammalian species such as rodents and nonhuman primates, often with extension of longevity as the primary outcome. However, the goal of geriatric medicine and gerontology has long been to focus on the compression of morbidity, that is, delaying the age of onset of chronic diseases and disability (6). More recently, this idea has spread to basic biology of aging community, and is referred to as healthspan, or the period of functional life in which “autonomy, control, independence, productivity and well-being” are maintained (7). Measures of healthspan are now considered important endpoints in the preclinical testing of interventions, and discussions are currently underway across the field to determine how independence, control, or well-being can be quantitatively defined in housed laboratory animals. One of the most obvious outcomes is the assessment of physical function or performance, as it is noninvasive, easy to assess, and relatively conserved across many species. Therefore, it is not surprising that many current studies have added objectively measured physical function outcomes (8–11). However, to date, there has been limited discussion or consensus on what constitutes healthy physical function status in animals that may actually translate to clinically meaningful assessments of functional impairments, limitations, or disability in humans (7). This is likely because specific investigators studying aging typically use a single model, and presently there is limited information about how functional assessments relate among these models or how they relate to physical function in humans.
Accordingly, below we present an initial discussion of what constitutes meaningful physical function clinically in humans and in laboratory animal models with special attention given to mice, rats, and nonhuman primates. We wish to emphasize that the assessment of physical function in humans is also particularly relevant in the context of detecting the so-called frailty syndrome (ie, the geriatric syndrome characterized by a decrease in physiological reserves and increased vulnerability to stressors) (12). In fact, several operational definitions of frailty rely (at least in part) on the evaluation of one or more physical performance tests (13,14). However, a thorough discussion of frailty in humans and model organisms is beyond the scope of the present review (see Mohler et al. 15), therefore, we will instead attempt to discuss the translation of physical function measures across species.
Humans
Geriatric medicine has long identified functional disability as a primary outcome for its activities. The assessment of physical function in older persons indeed represents a cornerstone of the geriatric evaluation (16). For example, the assessment of deficits in activities of daily living (ADLs) is today routinely performed in both clinical and research settings for estimating the capacity of the older person to interact with the surrounding environment and maintain his/her independence (17). Standardized medical models have been developed in humans to characterize the physical domain of the individual and the possible transitions across the spectrum of function (17–20). Multiple instruments are available for measuring each of the different phases of the conceptual framework proposed by Nagi (1), starting from the active pathology or underlying mechanisms, through physical impairment (ie, loss or abnormality of an anatomical, physiological, mental, or emotional nature) and limitation (ie, when the functional issue involves the organism as a whole), and ending with the disability condition (ie, the incapacity to interact with the surrounding environment and/or to function socially) (Figure 1). Such instruments range from muscle strength/power-related measures, objective physical performance tests of mobility and locomotion, to disability questionnaires and scales. In addition, novel technologies are today increasingly supporting the quantification of habitual activity and function in “real-world” settings using sensor-based monitoring approaches (21,22).
Disability Measures
Disability is defined in terms of restrictions in the ability to perform functional activities, including limitation in performance of socially defined roles or tasks (2,23). Common disability self-reports or proxy reports, such as index of ADLs (17), Instrumental ADLs (24), and the Pepper Assessment Tool for Disability (20), characterize the degree of difficulties the person faces in performing typical activities related to home or work life, including “typical” ADLs and Instrumental ADLs such as ambulatory ability, meal preparation, dressing, and managing medications and money. Such measures assess ability–disability and present environment- and subject-specific limitations in the heterogeneous contextualization of the condition of interest. In other words, a person may be more or less disabled according to the life environment in which his/her capacities are estimated. On the other hand, specific characteristics of the tested individual may significantly affect the assessment results, potentially under- or overestimating certain domains related to physical function. For example, functional independence is differently associated to quality of life in men and women, suggesting the importance of social role in the determination of disability (25). Thus, despite the efficacy of simple disability assessments, several limitations of these measurements exist, including difficulties associated with scoring the subject’s ability restrictions, interviewers adhering to standards and operational rules, and results biased by third factors (23). It is also noteworthy that disability scales/questionnaires tend to be less sensitive to the subject’s health status modifications. For these reasons, there is a growing tendency to assess objective measures of physical performance both in research as well as in clinical settings, especially for healthier older adults, ie, a population for which disability scales may present a “ceiling effect” (23,26).
Physical Performance Measures
Locomotion or mobility can be broadly defined as the ability to move from one place to another, and the ease with which this movement is accomplished (27). The most commonly used, standardized physical performance tests designed for older adults are aimed at assessing mobility and lower body function, as these domains are tightly correlated to the early phases of the disabling cascade. For example, these assessments usually encompass a wide range of ambulatory functional activities related to walking (either at a brisk or usual pace) and lower extremities’ muscle strength (eg, rising from a chair). Most of these tests are inexpensive, time effective, and easy to assess and interpret. However, more complex physical performance assessments also exist that: (i) incorporate specific measures of muscle strength, muscle power, and dexterity (eg, the National Institute of Health [NIH] Toolbox) (27); and (ii) may require special equipment (and in some cases ad hoc expertise) to be administered and interpreted (eg, gait analysis based on computerized systems) (28,29). Importantly, lower values of physical performance are strong predictors of future negative health-related outcomes (30–32).
Measures of physical performance in humans and corollary measures in other mammalian models of aging are illustrated in Figure 1. The most commonly used measures of locomotor- or mobility-related functions include the Short Physical Performance Battery (SPPB) (18,26), the gait speed test (typically conducted on distances ranging from 3 to 30 feet) (33,34), chair-rise ability (35), and the Timed-Up-and-Go test (36,37).
The SPPB is a brief testing battery that incorporates measures of standing balance, usual gait speed, and chair-rise ability. The results of these three timed tasks composing the SPPB are then used to generate a summary performance score according to predetermined and validated cut-points. The global score (ranging from 0 [worst performance] to 12 [best performance]) is a predictor (among other negative outcomes) of mobility disability (18) and incident ADL disability (26). Similarly, gait speed tests, the Timed-Up-and-Go test, and the simple chair stand test (36) are other examples of physical performance measures that are independently predictive of adverse health outcomes, including hospitalizations, institutionalization, disability, and quality of life decline (34,38–40). Recently, a large combined cohort analysis demonstrated that usual gait speed is a strong predictor of life expectancy in both men and women (34). Notably, this study also provided nomograms from which the life expectancy of the older individual (with no disabling condition) can be estimated from an individual’s gait speed, gender, and age (34).
Muscle Performance Measures
Measures of muscle strength and power decline with advancing age (41). These declines are associated with the cascade of developing disability, and precede more clinically evident conditions of mobility or functional limitation (41). Age-related decreases in maximal voluntary muscle strength and power (eg, handgrip, isometric knee extension, knee flexion torque, and lower extremity muscle power) are strongly related to poor mobility (31,42). The assessment of muscle power appears to be a more influential predictor of functional performance in older adults compared to muscle strength (41,43). Nevertheless, the greater complexity associated with measuring muscle power has led to a wider adoption of muscle strength parameters, in particular the handgrip strength. This simple marker is widely and increasingly used in research as well as in clinics because it is: (i) time-effective, easy-to assess, and does not require special training (27); (ii) relatively inexpensive (only the handheld dynamometer is required); (iii) adequately standardized and repeatable; and (iv) corroborated by a relevant body of evidence indicating it as a useful biomarker of aging (44,45) and predictor of negative outcomes (32,46).
Overall, recent systematic reviews have consistently demonstrated a strong predictive value of physical performance measures (in particular, gait speed and handgrip strength) for negative health-related outcomes, especially incident disability and mortality (47–50). Nevertheless, specific issues in this field still need to be clarified. For example, a better standardization and more consistent use of presently validated tools are necessary. Similarly, more widespread use of many current instruments will require understanding of population and/or ethnicity-specific thresholds of risk because of different risk profiles, body size, and other factors among groups (51). It also will be important to better establish specific aspects of physical performance measures used today (eg, gait-step patterns, modifications of center-of-pressure).
In recent years, novel technologies and mobile-sensing devices (including activity recognition and wearable monitoring systems) are increasingly being developed in order to facilitate/promote/improve the assessment of physical function in older persons (22,52). For example, accelerometer- and pedometer-based assessments demonstrate a reduction in volitional activity with advancing age (53,54). However, as many monitoring methods are still in development and a variety of data collection and analysis methods exist, there is little consensus regarding protocols and reported outcomes (55,56). In addition to monitoring daily activity, mobile-sensing devices can be used to determine physical functions relevant to mobility and fall risk, like gait parameters and postural transitions (57,58). As these technologies are developed and incorporated into future clinical investigation and practice, their usefulness in assessing physical function in humans will continue to expand. The advent of “big data” approaches for synthesizing large amounts of information invariably coming from the use such technologies likely will require ongoing adaptations of research methodologies.
Additional Considerations
Physical function assessments are implemented in clinical research and practice to screen, estimate risk profile, and increasingly, as major endpoints in clinical trials. Physical function can first be used to screen potential candidates for clinical trials. For example, working groups are developing operational algorithms to assess the baseline dysfunction of subjects to be included in clinical trials targeting sarcopenia (59,60) and mobility limitation (61). Functional measures are also incorporated as key biomarkers in comprehensive geriatric assessments (62), assisting with the identification of those elders presenting an increased vulnerability to stressors and exposed to increased risk of future negative health outcomes (32,34,46,63). Physical function assessment is then increasingly used as outcome to assess the efficacy of specific interventions (60). Collaborative groups, like the Functional Outcomes Working Group, are today working to standardize physical function measures and facilitate a wider adoption of them in large cohort studies and clinical trials of older adults (61).
Standardization and ease of use are of critical importance in the measure of physical performance. Through such necessary standardization, analyses from multiple clinical and research settings can be pooled and a “common currency” developed, supporting the development of innovative and cost-effective investigations (19). For example, an NIH-funded multicenter project aimed at designing a standardized battery for clinical research has recently been completed; such initiatives have provided a common metric of functionality including motor function (27). The recently unveiled NIH Toolbox battery for assessing motor function represents an accessible, valid, reliable, and versatile tool that is relatively brief, inexpensive, and easy to administer (19).
Physical performance measures also come with limitations that are important to consider. Results might be affected by body size (eg, taller individuals may take longer steps and reach higher gait speed on short tracks). This implies that proposed cut-points may require modification before being applied to different ethnic or other groups. These measures also can be influenced by a ceiling effect, reducing their discriminative capacity among individuals with a high performance status. For this reason, modifications of the traditional tests to make them more challenging, particularly healthy older adults, have been proposed (39).
Animal Models of Aging
Relying exclusively on humans as subjects in aging research is complicated by numerous issues, including ethical issues, long life span, and environmental and genetic influences (64). The use of animal models may circumvent many of these issues. When evaluating the significance of observations in animal models for human health it is important to evaluate the validity and reliability of the model in question. Reliability refers to the consistency and stability with which the variables of interest are observed, whereas validity refers to the extent to which a model is well-founded and corresponds accurately to the real world (65). Here, we briefly review physical function in the most common animal models currently in use. Rodent models are described in greatest detail, as they are the most commonly used approach in the preclinical literature, and primate models are emphasized because of their promise for recapitulating human function and behavior.
Nonmammalian Models
Round worms
Caenorhabditis elegans is the most widely used worm model of aging. Much of our understanding of the role of oxidative stress in aging (66), the influence of genes on life span, sarcopenia, and loss of physical function is based on initial discoveries using C. elegans (67). Important to the present review, mobility, as measured by viewing the rate of forward movement under a dissecting microscope, declines with age and has been used as a marker of healthspan (68). Thus, C. elegans represents a unique model system for the study of genetic and biochemical events that contribute to the relation between aging and physical function (69).
Fruit flies
The fruit fly Drosophila melanogaster is one of the principal model organisms used to study the biology of aging. Flies are well suited for aging studies because they have a short developmental phase to adulthood, a short life span, and are inexpensive to house. Most of the fly genome has been sequenced, sophisticated methods are available to manipulate it, and most fly genes have mammalian homologues. Historically, fly aging studies have focused on the regulation of life span, however, the fly is emerging as a powerful model of age-related functional decline. Declines in physical activity, as measured by the Drosophila Activity Monitoring System (70), and decreases in physical functions, including impairments in walking, preening, and flying, parallel reductions in physical activity and function in humans (71,72). Flies also exhibit decreased climbing ability, reductions in exploratory activity, and shortened flight times and distance with age (72).
Fish
Fish are small, inexpensive, and convenient to study in the laboratory, and have emerged as useful models of aging. This is particularly the case for the zebrafish (Danio rerio), as much is known about its genetics and physiology. Studies on physical function, such as endurance or sprint swimming against an adjustable water flow, as well as frequency and amplitude of tail beats (akin to stride length in humans), indicate performance declines with age (73). Additionally, the killifish, Nothobranchius spp. undergo deterioration of diurnal patterns of activity and sleep, and declines in overall activity with age (74).
Birds
Birds are a valuable model of aging because they live about three times longer than the average mammal of their size, appear to age relatively slowly, and display an impressive resistance to the degenerative processes associated with aging. Their activity levels and physical performance, as measured by flying activity, decline very little with age, consistent with the need to fly to survive. Thus, it could be argued that the healthspan of this model organism is nearly identical to its life span. Accordingly, the study of these species may provide novel insights into approaches to maintain physical function into later life (75,76).
Mammalian Models
Though lower organisms present many obvious advantages (77), mammalian models are more easily translatable to humans because of physiological similarities (eg, bioenergetics, central nervous system function) that support a diversity of behaviors that can be tested under laboratory conditions. The following section details physical function measures in mammalian models and discusses roadblocks and future developments needed to improve translation to humans.
Canines
Canines are easy to handle and train, and due to their role as pets, they are well characterized for clinical and biochemical status, structural changes (via medical imaging), and pathology associated with aging (78). Cognitive decline with aging has been studied for a number of years in dogs (79), and more recently sarcopenia (80). Interestingly, although dogs appear to represent a promising animal model of cognitive decline and dementia (81), there has been relatively little characterization of physical function or performance in healthy aging canines. The few tests involve range of motion assessment and general mobility in models of joint degeneration (82). Clearly, dogs represent a model with great potential in need of future development.
Rodents
Rodents are the most widely used experimental animals in aging research, with rats and mice being most common because their animal husbandry is well established, they reproduce rapidly, have relatively short developmental periods (~2–3 months), and genetic standardization of some species is possible (64). Importantly, laboratory rodents have a comparatively short life span, with a mean life span of common strains between ~2 and 3 years (83), as compared with the much longer life expectancy of humans (84) (Figure 2). Of relevance to the current review, many phenotypes of aging are preserved across species, and there is a substantial literature on laboratory-based assessments in rodents, including measures of physical function (11,85–88).
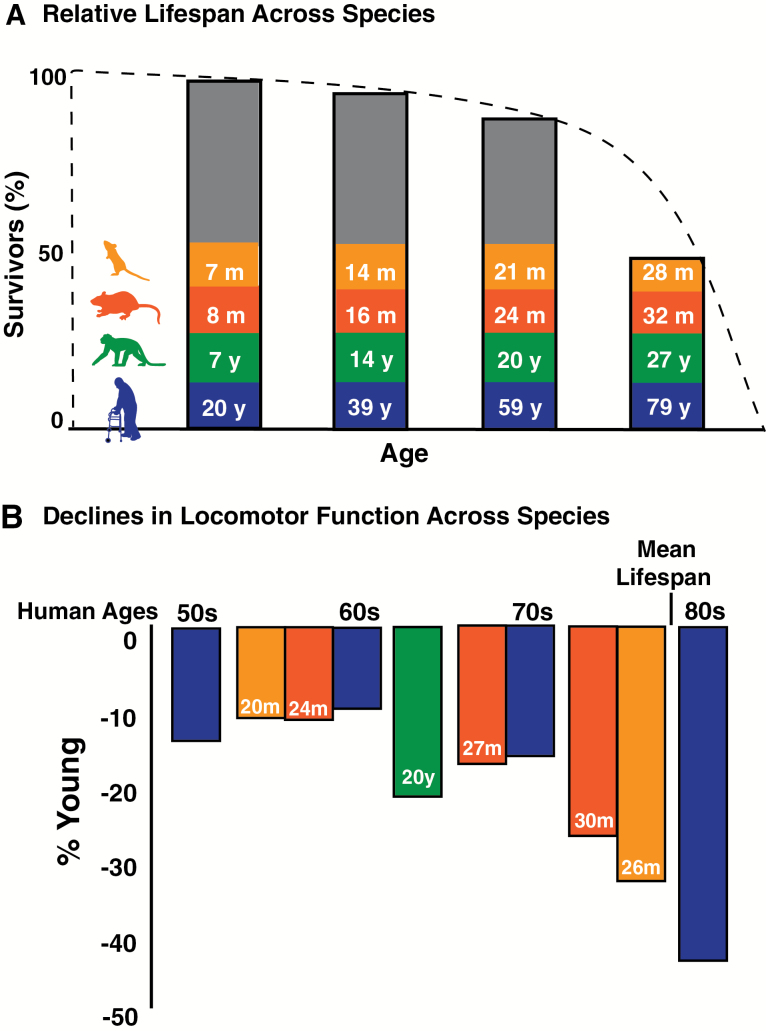
Figure 2.
Relative life spans and age-related declines in locomotor function across species. Analogous ages (A) in man (blue), old world monkeys (green), F1 344xBN rats (red), and C57Bl/6 mice (orange) are calculated based on percentages of mean life span for each species (10,83,84). The dotted line indicates the percent survival. The vertical bars indicate the relative ages, calculated as percent life span for each species. Declines in locomotor function (B) for each species are plotted at their respective relative ages determined by percentage of life span. Percent declines from young humans (20–29 y) were calculated from representative studies with normative data for gait speed (see Justice et al. 8 for detail). Percent declines in locomotor function were determined from scored tests of open field distance, walking track scurry speed, and rearing ability in mice (8), swim speed in rats (11), and gait speed in old world monkeys (10).
Physical Function Measures in Mice
Laboratory mice are the most common rodents used in aging research, and the majority of studies assessing physical function in mice have predominantly used the inbred strain C57BL/6 (8,9,86,89,90). Investigators are beginning to introduce motoric assessments in genetically heterogeneous mice, such as those produced from a four-way cross (91), which may represent an important translational advantage when investigating efficacy of late-life intervention. Regardless of strain, tests can be performed in mice that map to similar functional subdomains assayed in humans (Figure 1). However, little work has been performed to standardize noninvasive tests of physical function and performance across preclinical trials.
Recent attempts have been made to develop and assess the validity of a battery of tests to characterize motor function in mice (8,9,90). Justice and coworkers (8) identified and developed a battery of tests commonly used and validated in aging research with special attention given to measures aligned with the subdomains that comprise the Motor Function Domain of the NIH Toolbox: strength, locomotion, balance, and endurance (27). In addition, Graber and coworkers (9) developed a Neuromuscular Healthspan Scoring System incorporating functional assessment as part of an integrated battery to assess terminal treatment effects on sarcopenia. Instruments are also being developed to assess frailty in mice (92), including a physical function-based battery to detect clinically relevant criteria related to human frailty, including strength, gait speed, daily activity, and endurance (90).
In mice, locomotor capacity or mobility can be measured using approaches mimicking the gait speed and Timed-Up-and-Go tests designed for humans. Total distance traveled and maximum scurry speeds can be derived from forced exploration paradigms and use automated data collection video tracking software (8,92,93). Another gait speed proxy is the time required for a mouse to traverse a narrow straight path to escape into a dark box (8). Mobility in humans includes measures of chair-rise ability, which in mice could be translated to rearing activity assessed during forced exploration or in a rearing cylinder with counts tracked manually by a trained investigator (8,88).
Strength is most commonly assessed by forepaw grip strength using a force transducer with a trapeze, ring, or grid attachment (86), and must be conducted by well-trained personnel. As opposed to maximal voluntary grip strength measured in humans, grip strength in mice is derived from the force required to break a reflexive forepaw grasp. Assessment batteries may also include hybrid measures combining domains such as strength and muscular endurance. Examples are represented by an index of overall strength such as the inverted-cling grip test (9,90), and the tightrope or wire-suspension test, which also adds an element of motor coordination in the latency of the grasp on the wire or tightrope with the hindlimbs (8,86).
Another common apparatus used to assess motor function in mice is the accelerating rota-rod. The rota-rod is a suspended grooved rod affixed to an internally housed motor. As the rota-rod accelerates, the mice must increase their pace to keep from falling to the platform below. Depending on the protocol, this device may be used to characterize balance, motor coordination, “walking” speed, motor learning, and endurance (see below) (8,86,90,93). Most protocols recommend introductory or practice trials for 1–3 days. This is followed by an experimental day in which mice are placed on the rod as it accelerates from 4 to 40rpm over 5 minutes (usually × 3 trials), with the duration and corresponding maximal speed achieved recorded (8,9), though other protocols have been proposed (86,94). The improvement in rota-rod ability across trials may also be used as an index of motor learning. Importantly, once the rota-rod activity is learned, this ability is relatively stable (ie, may not adequately detect functional change with intervention). However, for balance, validation work comparing declines in mice to those observed in humans suggest that the declines observed in mice do not clearly follow the trajectory of declines seen in humans (8). Additional work remains to determine which functional subdomain is actually reflected by accelerating rota-rod performance (8).
Endurance in mice is measured using either treadmill or rota-rod devices locked at fixed speeds, or through hybrid measures described previously. Mice are motivated to run to exhaustion by shock grid or to mitigate a fall. Endurance capacity on either a standard or rota-rod treadmill is derived from the total time and distance run until exhaustion (8,95). The protocols used to determine endurance versus motor coordination with a rota-rod differ considerably. To test endurance, the device is locked at fixed, usually submaximal rotation speeds, and the run duration is assessed. Running for endurance tests can attain durations upward of 45 minutes or more compared with much shorter (typically 5-minute) durations used to assess coordination.
Habitual activity can be assessed by home-cage activity monitoring and voluntary wheel running. Home-cage activity can be monitored by surgically implanted telemetry devices or video software that track number of cage crossings (96,97), and have the major advantage minimizing human intervention which reduces stress caused by handling. Voluntary wheel running is an important index of daily physical activity (90) and running activity exhibits dramatic age-related declines (98). However, wheel habituation durations may introduce training confounds in assessments of late-life dietary or pharmacological interventions.
Physical Function Measures in Rats
Rats are also widely used in assessment of physical function and performance and, like the mouse, a variety of tests can be administered to measure both physical and cognitive function with age (88,99). The functional subdomains and specific tests in rats are very similar to those used in mice, and parallel age-related declines are observed in the two species (Figure 2). For example, strength can be assessed by forepaw grip strength devices, as well as by hybrid measures such as the inclined plane test, which measure overall muscular strength and endurance. Locomotor activity is measured by open field activity and rearing counts (88), but may also be reliably assessed as swim speed in rats (11). Endurance is easily assessed using similar procedures to those described above for mice though the majority of studies employ treadmill testing (100). Likewise, habitual activity and voluntary wheel running are as effectively assessed in rats as in mice, with similar declines observed across age in both species (8,88). Finally, functional scores derived from swim speed (similar to gait speed) and inclined plane (a strength measurement) in rats at midlife are predictive of survival (11), which supports observations of measures of physical function in middle-age as independent predictors of survival in humans (Rantanen, 2012 #37697). This relation has yet to be demonstrated in mice.
Additional Considerations
Rodent studies generally do not employ experimental designs in which baseline characteristics are used to balance treatment group assignment or as covariates when assessing outcomes. This practice is due to the high similarity of individual animals within a strain. However, heterogeneity of physical function outcomes and physiological characteristics increase with age, and is purposefully produced in some aging studies through strain crosses. Thus, baseline characterization may be more important than previously considered. Similar to assessment in humans, physical function outcomes in rodents can be used to screen animals prior to treatment (90), to determine risk of future adverse health events (11), and as an outcome in intervention testing (8,93,99).
As emphasized earlier, many markers of physical function exist in rodents that mimic those in humans. The majority of these are measures of mobility or locomotor activity, which also decline with aging in humans and are predictive of disability or mortality risk. Measures of mobility are the most consistent functional outcomes that demonstrate reproducible declines across multiple species and should therefore be prioritized if economy of time and effort is of the essence. However, because disability is characterized by more than one functional subdomain, if time permits, a more comprehensive set of noninvasive functional assessments should be performed, including measures of strength and endurance.
Further work needs to be conducted to standardize testing protocols in rodents, similar to efforts made in humans to develop the NIH Toolbox and SPPB. Such an effort could minimize discrepancies between laboratories and facilities testing life-span and healthspan outcomes in rodents and may improve translation from interventions in rodents to clinical trials. Standardization efforts should carefully control for potential strain and sex differences and demonstrate a keen understanding of motivational/learning components of various procedures. There should be a concerted effort to construct, perhaps through NIH funding, a standardized physical performance battery for both the rat and the mouse model that could be used in longitudinal and cross-sectional settings.
Key to these endeavors is the continued availability of a variety of aging rodent species. It is important to remember that mice are not “little rats” nor are rats “big mice.” The choice of whether to use mice or rats as an experimental model depends on the research question being asked, but a full discussion is beyond the scope of this review. However, the use of multiple species provides opportunity for convergence and divergence in scientific discovery. There are both consistent and discordant findings using mice and rats as models to the study of functional aging. Where convergence occurs in these models, scientific advances are most likely to emerge. Likewise, divergent findings from different models provide evidence of alternative mechanisms not recognizable in one model alone. Thus, replicating findings and understanding the differences across animal models in the context of aging engenders effective hypothesis testing.
Nonhuman primates
Though laboratory rodents are useful models, especially given their relatively short life span, there are aspects of aging that are not adequately modeled in mice and rats. For example, declines in physical function in humans are accelerated by comorbidities (101,102). Therefore, an animal model that exhibits age-related comorbidities as observed in humans may be useful. Old world monkeys exhibit multiple age-related morbidities that contribute to functional impairment and are characteristic of aging human beings, including obesity and fat redistribution (103), sarcopenia (104), type II diabetes (105), coronary heart disease (106,107), cerebrovascular disease (108), and osteoarthritis (109). However, until recently, there were no validated measures of physical functioning in nonhuman primates that decline with age similar to humans.
Physical Function Measures in Nonhuman Primates
Because of their availability, moderate size, and ability to adapt to laboratory conditions, macaques (including Macaca mulatta [rhesus], M. fascicularis [cynomolgus], and M. radiata [bonnet]) and African green (Chlorocebus aethiops) monkeys have been used to develop functional models of aging despite a relatively long life span (Figure 2). In particular, macaques have been extensively used to assess age-related declines in fine motor skills (110,111). Thus, primates offer unique translational insights into the age-related declines in dexterity observed in humans. Further, both cage-monitored locomotor activity and accelerometry have been used to study longitudinal declines in physical activity with age (and calorie restriction) in rhesus macaques (112,113). However, evaluation of gross motoric function predictive of negative health outcomes in humans has been limited in nonhuman primates, and no measures of strength, balance, or endurance have been established.
Recent efforts have been made to develop a nonhuman primate model of age-related physical decline that is similar to mobility measures used in humans (10). The model specifically targets measures that are inexpensive, sensitive to age, and allow monitoring of animals in social groups in a variety of settings that permit species-typical locomotion. Because these are relatively large animals (3–15kg depending on species, sex, and level of obesity) that require relatively large enclosures to achieve species-typical locomotion, automated video tracking is difficult to achieve. As a result, these measures of physical function are collected primarily by observation of natural activity in home cages. This approach has proved sensitive for capturing changes in locomotor activity between middle-aged (6–13 years) and old (18–25 years) monkeys that cannot be obtained by removing the monkey from its home pen and monitoring activity with an automated video tracking system (114), likely because the latter approach constrains activities of interest (10). Trained observers record the frequency and duration of all instances of locomotion, climbing, leaping and jumping, and hanging (eg, from chain-link ceilings and walls, or from furniture, with all limbs off the ground) for each monkey in four 15-min observations. In addition, usual walking speed is measured by identifying landmarks with known distances between them on various structures in the home pen. During the observation period, the monkeys are timed as they pass from landmark to landmark. Importantly, only self-motivated locomotor bouts are timed, as opposed to moving as a result of being chased or racing toward a desired food or toy, making this measure more similar to habitual walking speed as measured in humans (18,27,34). In this model, walking speed was the most sensitive to age, the simplest to use, and least expensive measure, and the least affected by differences in housing, whereas behaviors such as climbing and hanging may best reflect strength (10, 115).
Additional Considerations
Using reductions in walking speed, climbing, leaping, jumping, and hanging, correlations between age-related declines in these measures of physical function and age-related morbidities including sarcopenia and degeneration of the shoulder joint (116) have been reported, indicating that these monkeys may serve as a model of sarcopenia and osteoarthritis, and consequent functional limitations. Additionally, given the similarly expansive brains and age-related degeneration that are exclusive to primates (117), nonhuman primates may make a promising model for study of central nervous system contributions to declines in physical function. Because nonhuman primate motor function assessment in the context of aging research is relatively new, there are many future applications to be considered, including development of standardized screening and assessment tools, similar to those utilized in human and rodent models.
Conclusions and Future Directions
Declining physical function is a hallmark of the aging process, a major predictor of and contributor to overt physical disability and other morbidities, and a key indicator of diminishing quality of life. Functional assessments provide a unique phenotypic biomarker that can be easily, inexpensively, and noninvasively assessed with aging, as well as in response to later life interventions. Given the robustness of the decline in physical function with aging and challenges associated with validly measuring this decline across age and species, we propose a broad, consistent approach across species in the assessment of physical function (Figure 3).
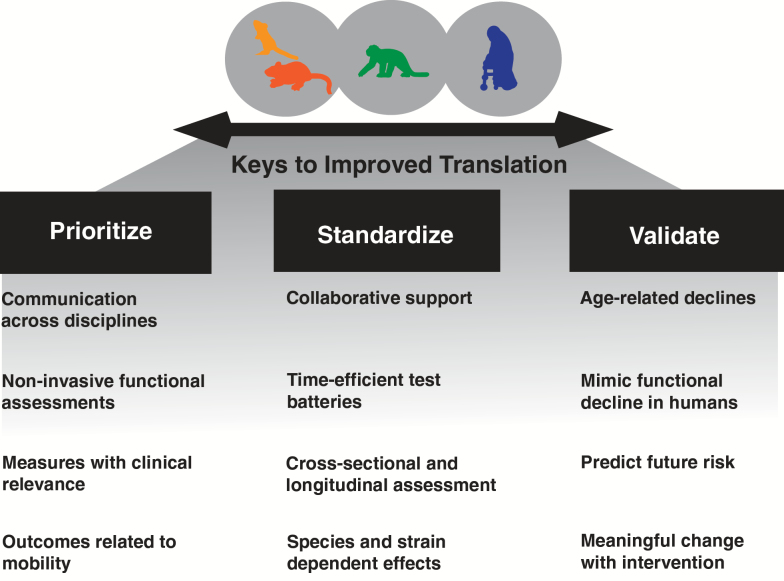
Figure 3.
Keys to improving translation of preclinical physical function outcomes. Essential elements to improve our ability to translate findings to clinical trials include (i) prioritizing communication and use of clinically relevant domains of function in animal models of aging, (ii) an interdisciplinary and collaborative effort to standardize tests of physical function and performance, and (iii) a structured set of validation steps to improve the relation to clinical outcomes.
Prioritizing and Refining Mobility Measures as the Prototypical Marker of Physical Function
The most easily assessed, and possibly most important biomarker, across mammalian models is locomotor activity or mobility. Several large-scale human observational studies show that walking speed is highly indicative of survival and other outcomes such as falls, hospitalizations, and overall health in older individuals. Defining gait speed thresholds to establish normative ranges within species will serve as a more refined predictor of health-related events. Other measures of mobility should also be considered. For instance, swimming speed in rats is highly predictive of mortality, although perhaps slightly more challenging given the requirement to swim. This task is highly motivating, using escape as the reinforcement for animals to engage in swimming. Exploiting this performance outcome may indeed represent an objective measure of mobility. However, many measures of mobility and physical function in rodent models are collected under duress which may be analogous to how fast a frightened person can run to safety, and not to usual walking speed. Application of technological advancements (eg, accelerometry) scaled to small animals will enhance investigators’ ability to collect large datasets on mobility under “usual” conditions and potentially aid in establishing thresholds for normality.
Developing and Standardizing Phenotypic Markers of Physical Function
There are immediate needs, both general and specific, in supporting the development of batteries for assessing functional decline using diverse animal models. Foremost, improved communication among investigators involved in clinical and basic biology of aging is needed to ensure that testing design and procedures of various functional domains in animals are clinically relevant in humans. This may also include standardization of nutrition, as most of the literature is based on studies of animals fed ideal nutrition, which does not resemble either the natural diet of the species or the higher fat diet of the human beings (eg, laboratory rodent diet #5001, monkey diet #5037; LabDiet, St. Louis, MO).
Secondly, testing across functional domains can be time and labor intensive. Currently, the most comprehensive predictive batteries in rodents and nonhuman primates take hours to days to perform. In humans, this barrier was broken through development of the SPPB, the wide use of gait speed, and the more recent availability of the NIH Toolbox motor domain assessments. Hence, there is a need to develop time and cost-effective batteries in mice and rats. However, over the years, these types of proposals have been often deemed descriptive and nonmechanistic. This is because initial forays into this science of behavior would, just as in the development of human functional batteries, require exhaustive testing of a variety of measures (cross-sectionally, longitudinally, and validated across laboratories). As such, funding agencies and other organizations that support aging research will need to provide opportunities specifically for such model development. Whether standardized batteries can be established using presently available information (eg, via a series of developmental workshops gathering experts from different backgrounds) or will require a prospective effort involving instrument development and validation as with the NIH Toolbox batteries, needs to be determined.
Validation of Phenotypic Markers of Physical Function
Developing and standardizing performance measures in aging animal models is but one piece of the puzzle—those measures also must demonstrate validity to the human condition to truly qualify as a model. For example, the measure should reproducibly decline with age rather than unreliably fluctuate over time. These age-related declines should mimic those observed in humans in terms of the life course of events (Figure 2). Furthermore, outcomes derived from functional tests must predict future adverse health outcomes as is observed in humans. This may mean adjusting models to either manipulate the manifestation of adverse outcomes or choose models where these outcomes emerge with natural aging in the laboratory.
Two final points regarding underlying biological mechanisms and the application of interventions deserve some special attention. The strength of the animal model is that we may gather, beyond functional outcomes, a wide variety of invasive measures including tissues from various organs and systems for analysis of genetic, cellular, and physiological mechanisms underlying the function declines. Ideally, interventions targeted at mechanisms underlying functional decline should actually mitigate functional decline. However, this can go both ways. If an intervention actually modifies a biological change hypothesized to be relevant to declining function, but there is no impact on the observed function, then rethinking of the contribution of the studied biological mechanism is needed. This approach prioritizes the functional measure, when validly mapped upon the human condition, as the driving force behind our understanding of these biological mechanisms. Finally, of great import is the need to communicate across disciplines and species, modeling based on clinical observation and functional decline in people for improved translation, and enhanced quality of research on aging.
Funding
This work was supported in part by the National Institute on Aging (training grant AG-000279-12 to J.N.J.).
References
- 1. Nagi SZ. Disability in America: toward a National agenda for prevention. In: Pope AM, Tarlov AR, eds. Disability Concepts Revisited: Implications for Prevention. Washington, DC: National Academy Press; 1991:309–327. [Google Scholar]
- 2. Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54:439–467. [PubMed] [Google Scholar]
- 3. Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q. 2009;87:842–862. doi:10.1111/j.1468-0009.2009.00581.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beard JR, Bloom DE. Towards a comprehensive public health response to population ageing. Lancet. 2015;385:658–661. doi:10.1016/S0140-6736(14)61461-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried TR, Bradley EH, Williams CS, Tinetti ME. Functional disability and health care expenditures for older persons. Arch Intern Med. 2001;161:2602–2607. [DOI] [PubMed] [Google Scholar]
- 6. Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135. doi:10.1056/NEJM198007173030304 [DOI] [PubMed] [Google Scholar]
- 7. Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol. 2013;48:1–5. doi:10.1016/j.exger.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Justice JN, Carter CS, Beck HJ, et al. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr). 2014;36:583–595. doi:10.1007/s11357-013-9589-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graber TG, Ferguson-Stegall L, Kim JH, Thompson LV. C57BL/6 neuromuscular healthspan scoring system. J Gerontol A Biol Sci Med Sci. 2013;68:1326–1336. doi:10.1093/gerona/glt032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shively CA, Willard SL, Register TC, et al. Aging and physical mobility in group-housed Old World monkeys. Age (Dordr). 2012;34:1123–1131. doi:10.1007/s11357-011-9350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci. 2002;57:B193–B197. [DOI] [PubMed] [Google Scholar]
- 12. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi:10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 14. Vellas B, Balardy L, Gillette-Guyonnet S, et al. Looking for frailty in community-dwelling older persons: the Gérontopôle Frailty Screening Tool (GFST). J Nutr Health Aging. 2013;17:629–631. doi:10.1007/s12603-013-0363-6 [DOI] [PubMed] [Google Scholar]
- 15. Mohler MJ, Fain MJ, Wertheimer AM, Najafi B, Nikolich-Žugich J. The Frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol. 2014;54:6–13. doi:10.1016/j.exger.2014.01.024 [DOI] [PubMed] [Google Scholar]
- 16. Applegate WB, Blass JP, Williams TF. Instruments for the functional assessment of older patients. N Engl J Med. 1990;322:1207–1214. doi:10.1056/NEJM199004263221707 [DOI] [PubMed] [Google Scholar]
- 17. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 18. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 19. Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80(11 suppl 3):S2–S6. doi:10.1212/WNL.0b013e3182872e5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rejeski WJ, Ip EH, Marsh AP, Miller ME, Farmer DF. Measuring disability in older adults: the International Classification System of Functioning, Disability and Health (ICF) framework. Geriatr Gerontol Int. 2008;8:48–54. doi:10.1111/j.1447-0594.2008.00446.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Najafi B, Armstrong DG, Mohler J. Novel wearable technology for assessing spontaneous daily physical activity and risk of falling in older adults with diabetes. J Diabetes Sci Technol. 2013;7:1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kowalski K, Rhodes R, Naylor PJ, Tuokko H, MacDonald S. Direct and indirect measurement of physical activity in older adults: a systematic review of the literature. Int J Behav Nutr Phys Act. 2012;9:148. doi:10.1186/1479-5868-9-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. [DOI] [PubMed] [Google Scholar]
- 24. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 25. Fusco O, Ferrini A, Santoro M, Lo Monaco MR, Gambassi G, Cesari M. Physical function and perceived quality of life in older persons. Aging Clin Exp Res. 2012;24:68–73. [DOI] [PubMed] [Google Scholar]
- 26. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi:10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reuben DB, Magasi S, McCreath HE, et al. Motor assessment using the NIH Toolbox. Neurology. 2013;80(11 suppl 3):S65–S75. doi:10.1212/WNL.0b013e3182872e01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McDonough AL, Batavia M, Chen FC, Kwon S, Ziai J. The validity and reliability of the GAITRite system’s measurements: a preliminary evaluation. Arch Phys Med Rehabil. 2001;82:419–425. doi:10.1053/apmr.2001.19778 [DOI] [PubMed] [Google Scholar]
- 29. Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17:68–74. [DOI] [PubMed] [Google Scholar]
- 30. Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. [DOI] [PubMed] [Google Scholar]
- 31. Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457. doi:10.1111/j.1532-5415.2007.01087.x [DOI] [PubMed] [Google Scholar]
- 32. Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. [DOI] [PubMed] [Google Scholar]
- 33. Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97:182–189. doi:10.1016/j.physio.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 34. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bohannon RW, Bubela DJ, Magasi SR, Wang YC, Gershon RC. Sit-to-stand test: performance and determinants across the age-span. Isokinet Exerc Sci. 2010;18:235–240. doi:10.3233/IES-2010-0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 37. Bischoff HA, Stähelin HB, Monsch AU, et al. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32:315–320. [DOI] [PubMed] [Google Scholar]
- 38. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. [DOI] [PubMed] [Google Scholar]
- 39. Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57:251–259. doi:10.1111/j.1532-5415.2008.02126.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cesari M, Onder G, Zamboni V, et al. Physical function and self-rated health status as predictors of mortality: results from longitudinal analysis in the ilSIRENTE study. BMC Geriatr. 2008;8:34. doi:10.1186/1471-2318-8-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12. doi:10.1097/JES.0b013e31823b5f13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ploutz-Snyder LL, Manini T, Ploutz-Snyder RJ, Wolf DA. Functionally relevant thresholds of quadriceps femoris strength. J Gerontol A Biol Sci Med Sci. 2002;57:B144–B152. [DOI] [PubMed] [Google Scholar]
- 43. Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58:728–733. [DOI] [PubMed] [Google Scholar]
- 44. Bohannon RW, Magasi SR, Bubela DJ, Wang YC, Gershon RC. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve. 2012;46:555–558. doi:10.1002/mus.23350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing. 1994;23:132–137. [DOI] [PubMed] [Google Scholar]
- 46. Rantanen T, Masaki K, He Q, Ross GW, Willcox BJ, White L. Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordr). 2012;34:563–570. doi:10.1007/s11357-011-9256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi:10.1136/bmj.c4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cooper R, Kuh D, Cooper C, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40:14–23. doi:10.1093/ageing/afq117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 2011;11:33. doi:10.1186/1471-2318-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. den Ouden ME, Schuurmans MJ, Arts IE, van der Schouw YT. Physical performance characteristics related to disability in older persons: a systematic review. Maturitas. 2011;69:208–219. doi:10.1016/j.maturitas.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 51. Melvin J, Hummer R, Elo I, Mehta N. Age patterns of racial/ethnic/nativity differences in disability and physical functioning in the United States. Demographic Research. 2014;31:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Murphy SL. Review of physical activity measurement using accelerometers in older adults: considerations for research design and conduct. Prev Med. 2009;48:108–114. doi:10.1016/j.ypmed.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi:10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 54. Harris AM, Lanningham-Foster LM, McCrady SK, Levine JA. Nonexercise movement in elderly compared with young people. Am J Physiol Endocrinol Metab. 2007;292:E1207–E1212. doi:10.1152/ajpendo.00509. 2006 [DOI] [PubMed] [Google Scholar]
- 55. Taraldsen K, Chastin SF, Riphagen II, Vereijken B, Helbostad JL. Physical activity monitoring by use of accelerometer-based body-worn sensors in older adults: a systematic literature review of current knowledge and applications. Maturitas. 2012;71:13–19. doi:10.1016/j.maturitas.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 56. Van Remoortel H, Giavedoni S, Raste Y, et al. Validity of activity monitors in health and chronic disease: a systematic review. Int J Behav Nutr Phys Act. 2012;9:84. doi:10.1186/1479-5868-9-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang CC, Hsu YL. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors (Basel). 2010;10:7772–7788. doi:10.3390/s100807772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lord S, Chastin SF, McInnes L, Little L, Briggs P, Rochester L. Exploring patterns of daily physical and sedentary behaviour in community-dwelling older adults. Age Ageing. 2011;40:205–210. doi:10.1093/ageing/afq166 [DOI] [PubMed] [Google Scholar]
- 59. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi:10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cesari M, Fielding RA, Pahor M, et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:181–190. doi:10.1007/s13539-012-0078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Working Group on Functional Outcome Measures for Clinical T, Bhasin S Espeland MA Evans WJ Ferrucci L Fried LP et al. . Indications, labeling, and outcomes assessment for drugs aimed at improving functional status in older persons: a conversation between aging researchers and FDA regulators. J Gerontol A Biol Sci Med Sci. 2009;64:487–491. doi:10.1093/gerona/gln042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lilamand M, Dumonteil N, Nourhashémi F, et al. Gait speed and comprehensive geriatric assessment: two keys to improve the management of older persons with aortic stenosis. Int J Cardiol. 2014;173:580–582. doi:10.1016/j.ijcard.2014.03.112 [DOI] [PubMed] [Google Scholar]
- 63. Tavassoli N, Guyonnet S, Abellan Van Kan G, et al. Description of 1,108 older patients referred by their physician to the “Geriatric Frailty Clinic (G.F.C) for Assessment of Frailty and Prevention of Disability” at the gerontopole. J Nutr Health Aging. 2014;18:457–464. doi:10.1007/s12603-014-0462-z [DOI] [PubMed] [Google Scholar]
- 64. Vanhooren V, Libert C. The mouse as a model organism in aging research: usefulness, pitfalls and possibilities. Ageing Res Rev. 2013;12:8–21. doi:10.1016/j.arr.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 65. Anastasi A. Psychological Testing. 5th ed. New York: Macmillan; 1982. [Google Scholar]
- 66. Prasad KN, Bondy SC. Evaluation of role of oxidative stress on aging in Caenorhabditis elegans: a brief review. Curr Aging Sci. 2013;6:215–219. [DOI] [PubMed] [Google Scholar]
- 67. Tissenbaum HA. Genetics, life span, health span, and the aging process in Caenorhabditis elegans . J Gerontol A Biol Sci Med Sci. 2012;67:503–510. doi:10.1093/gerona/gls088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cypser JR, Wu D, Park SK, et al. Predicting longevity in C. elegans: fertility, mobility and gene expression. Mech Ageing Dev. 2013;134:291–297. doi:10.1016/j.mad.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 69. Fisher AL. Of worms and women: sarcopenia and its role in disability and mortality. J Am Geriatr Soc. 2004;52:1185–1190. doi:10.1111/j.1532-5415.2004.52320.x [DOI] [PubMed] [Google Scholar]
- 70. Piazza N, Gosangi B, Devilla S, Arking R, Wessells R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS One. 2009;4:e5886. doi:10.1371/journal.pone.0005886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Carey JR, Papadopoulos N, Kouloussis N, et al. Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata . Exp Gerontol. 2006;41:93–97. doi:10.1016/j.exger.2005.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E. Functional senescence in Drosophila melanogaster . Ageing Res Rev. 2005;4:372–397. doi:10.1016/j.arr.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 73. Gilbert MJ, Zerulla TC, Tierney KB. Zebrafish (Danio rerio) as a model for the study of aging and exercise: physical ability and trainability decrease with age. Exp Gerontol. 2014;50:106–113. doi:10.1016/j.exger.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 74. Lucas-Sánchez A, Almaida-Pagán PF, Martinez-Nicolas A, Madrid JA, Mendiola P, de Costa J. Rest–activity circadian rhythms in aged Nothobranchius korthausae. The effects of melatonin. Exp Gerontol. 2013;48:507–516. doi:10.1016/j.exger.2013.02.026 [DOI] [PubMed] [Google Scholar]
- 75. Holmes DJ, Ottinger MA. Birds as long-lived animal models for the study of aging. Exp Gerontol. 2003;38:1365–1375. [DOI] [PubMed] [Google Scholar]
- 76. Austad SN. Candidate bird species for use in aging research. ILAR J. 2011;52:89–96. [DOI] [PubMed] [Google Scholar]
- 77. Austad SN. Introduction to animal models. Exp Gerontol. 2003;38:1327–1328. [DOI] [PubMed] [Google Scholar]
- 78. Waters DJ. Aging research 2011: exploring the pet dog paradigm. ILAR J. 2011;52:97–105. [DOI] [PubMed] [Google Scholar]
- 79. Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging. 2002;23:809–818. [DOI] [PubMed] [Google Scholar]
- 80. Hutchinson D, Sutherland-Smith J, Watson AL, Freeman LM. Assessment of methods of evaluating sarcopenia in old dogs. Am J Vet Res. 2012;73:1794–1800. doi:10.2460/ajvr.73.11.1794 [DOI] [PubMed] [Google Scholar]
- 81. Adams B, Chan A, Callahan H, Milgram NW. The canine as a model of human cognitive aging: recent developments. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:675–692. [DOI] [PubMed] [Google Scholar]
- 82. Smolders LA, Kingma I, Bergknut N, et al. Biomechanical assessment of the effects of decompressive surgery in non-chondrodystrophic and chondrodystrophic canine multisegmented lumbar spines. Eur Spine J. 2012;21:1692–1699. doi:10.1007/s00586-012-2285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wolf NS, Austad S. Introduction: lifespans and pathologies present at death in laboratory animals. In: Woldf NS, ed. The Comparative Biology of Aging. New York, NY: Springer Science+Business Media; 2010:1–26. [Google Scholar]
- 84. Arias E. United States life tables, 2009. Natl Vital Stat Rep. 2014;62:1–63. [PubMed] [Google Scholar]
- 85. Forster MJ, Lal H. Estimating age-related changes in psychomotor function: influence of practice and of level of caloric intake in different genotypes. Neurobiol Aging. 1999;20:167–176. [DOI] [PubMed] [Google Scholar]
- 86. Ingram DK, Reynolds MA. Assessing the predictive validity of psychomotor tests as measures of biological age in mice. Exp Aging Res. 1986;12:155–162. doi:10.1080/03610738608259454 [DOI] [PubMed] [Google Scholar]
- 87. Campbell BA, Gaddy JR. Rate of aging and dietary restriction: sensory and motor function in the Fischer 344 rat. J Gerontol. 1987;42:154–159. [DOI] [PubMed] [Google Scholar]
- 88. Altun M, Bergman E, Edström E, Johnson H, Ulfhake B. Behavioral impairments of the aging rat. Physiol Behav. 2007;92:911–923. doi:10.1016/j.physbeh.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 89. de Fiebre NC, Sumien N, Forster MJ, de Fiebre CM. Spatial learning and psychomotor performance of C57BL/6 mice: age sensitivity and reliability of individual differences. Age (Dordr). 2006;28:235–253. doi:10.1007/s11357-006-9027-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liu H, Graber TG, Ferguson-Stegall L, Thompson LV. Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci. 2013;69:1485–1491. doi:10.1093/gerona/glt188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sumien N, Sims MN, Taylor HJ, Forster MJ. Profiling psychomotor and cognitive aging in four-way cross mice. Age (Dordr). 2006;28:265–282. doi:10.1007/s11357-006-9015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi:10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fahlström A, Zeberg H, Ulfhake B. Changes in behaviors of male C57BL/6J mice across adult life span and effects of dietary restriction. Age (Dordr). 2012;34:1435–1452. doi:10.1007/s11357-011-9320-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA. 1996;93:4765–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Meek TH, Lonquich BP, Hannon RM, Garland T., Jr Endurance capacity of mice selectively bred for high voluntary wheel running. J Exp Biol. 2009;212:2908–2917. doi:10.1242/jeb.028886 [DOI] [PubMed] [Google Scholar]
- 96. Zombeck JA, Deyoung EK, Brzezinska WJ, Rhodes JS. Selective breeding for increased home cage physical activity in collaborative cross and Hsd:ICR mice. Behav Genet. 2011;41:571–582. doi:10.1007/s10519-010-9425-2 [DOI] [PubMed] [Google Scholar]
- 97. Spruijt BM, DeVisser L. Advanced behavioural screening: automated home cage ethology. Drug Discov Today Technol. 2006;3:231–237. doi:10.1016/j.ddtec.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 98. Durrant JR, Seals DR, Connell ML, et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587(Pt 13):3271–3285. doi:10.1113/jphysiol.2009.169771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Carter CS, Marzetti E, Leeuwenburgh C, et al. Usefulness of preclinical models for assessing the efficacy of late-life interventions for sarcopenia. J Gerontol A Biol Sci Med Sci. 2012;67:17–27. doi:10.1093/ gerona/glr042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lawler JM, Powers SK, Hammeren J, Martin AD. Oxygen cost of treadmill running in 24-month-old Fischer-344 rats. Med Sci Sports Exerc. 1993;25:1259–1264. [PubMed] [Google Scholar]
- 101. Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi:10.1093/gerona/glp076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cesari M, Onder G, Russo A, et al. Comorbidity and physical function: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study). Gerontology. 2006;52:24–32. doi:10.1159/ 000089822 [DOI] [PubMed] [Google Scholar]
- 103. Kemnitz JW. Obesity in macaques: spontaneous and induced. Adv Vet Sci Comp Med. 1984;28:81–114. [DOI] [PubMed] [Google Scholar]
- 104. Colman RJ, McKiernan SH, Aiken JM, Weindruch R. Muscle mass loss in Rhesus monkeys: age of onset. Exp Gerontol. 2005;40:573–581. doi:10.1016/j.exger.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 105. Wagner JE, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ, Jr, Kaplan JR. Old world nonhuman primate models of type 2 diabetes mellitus. ILAR J. 2006;47:259–271. [DOI] [PubMed] [Google Scholar]
- 106. Shively CA, Clarkson TB, Miller LC, Weingand KW. Body fat distribution as a risk factor for coronary artery atherosclerosis in female cynomolgus monkeys. Arteriosclerosis. 1987;7:226–231. [DOI] [PubMed] [Google Scholar]
- 107. Shively CA, Clarkson TB, Kaplan JR. Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis. 1989;77:69–76. [DOI] [PubMed] [Google Scholar]
- 108. Shively CA, Kaplan JR, Clarkson TB. Carotid artery atherosclerosis in cholesterol-fed female cynomolgus monkeys. Effects of oral contraceptive treatment, social factors, and regional adiposity. Arteriosclerosis. 1990;10:358–366. [DOI] [PubMed] [Google Scholar]
- 109. Carlson CS, Loeser RF, Jayo MJ, Weaver DS, Adams MR, Jerome CP. Osteoarthritis in cynomolgus macaques: a primate model of naturally occurring disease. J Orthop Res. 1994;12:331–339. doi:10.1002/jor.1100120305 [DOI] [PubMed] [Google Scholar]
- 110. Emborg ME, Ma SY, Mufson EJ, et al. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- 111. Kastman EK, Willette AA, Coe CL, et al. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J Neurosci. 2010;30:7940–7947. doi:10.1523/JNEUROSCI.0835-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ingram DK, Young J, Mattison JA. Calorie restriction in nonhuman primates: assessing effects on brain and behavioral aging. Neuroscience. 2007;145:1359–1364. doi:10.1016/j.neuroscience.2006.10.031 [DOI] [PubMed] [Google Scholar]
- 113. Yamada Y, Colman RJ, Kemnitz JW, et al. Long-term calorie restriction decreases metabolic cost of movement and prevents decrease of physical activity during aging in rhesus monkeys. Exp Gerontol. 2013;48:1226–1235. doi:10.1016/j.exger.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Walton A, Branham A, Gash DM, Grondin R. Automated video analysis of age-related motor deficits in monkeys using EthoVision. Neurobiol Aging. 2006;27:1477–1483. doi:10.1016/j.neurobiolaging.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 115. Choi SJ, Shively CA, Register TC, et al. Force-generation capacity of single vastus lateralis muscle fibers and physical function decline with age in African green vervet monkeys. J Gerontol A Biol Sci Med Sci. 2013;68:258–267. doi:10.1093/gerona/gls143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Plate JF, Bates CM, Mannava S, et al. Age-related degenerative functional, radiographic, and histological changes of the shoulder in nonhuman primates. J Shoulder Elbow Surg. 2013;22:1019–1029. doi:10.1016/j.jse.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kalinin S, Willard SL, Shively CA, et al. Development of amyloid burden in African Green monkeys. Neurobiol Aging. 2013;34:2361–2369. doi:10.1016/j.neurobiolaging.2013.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]