Abstract
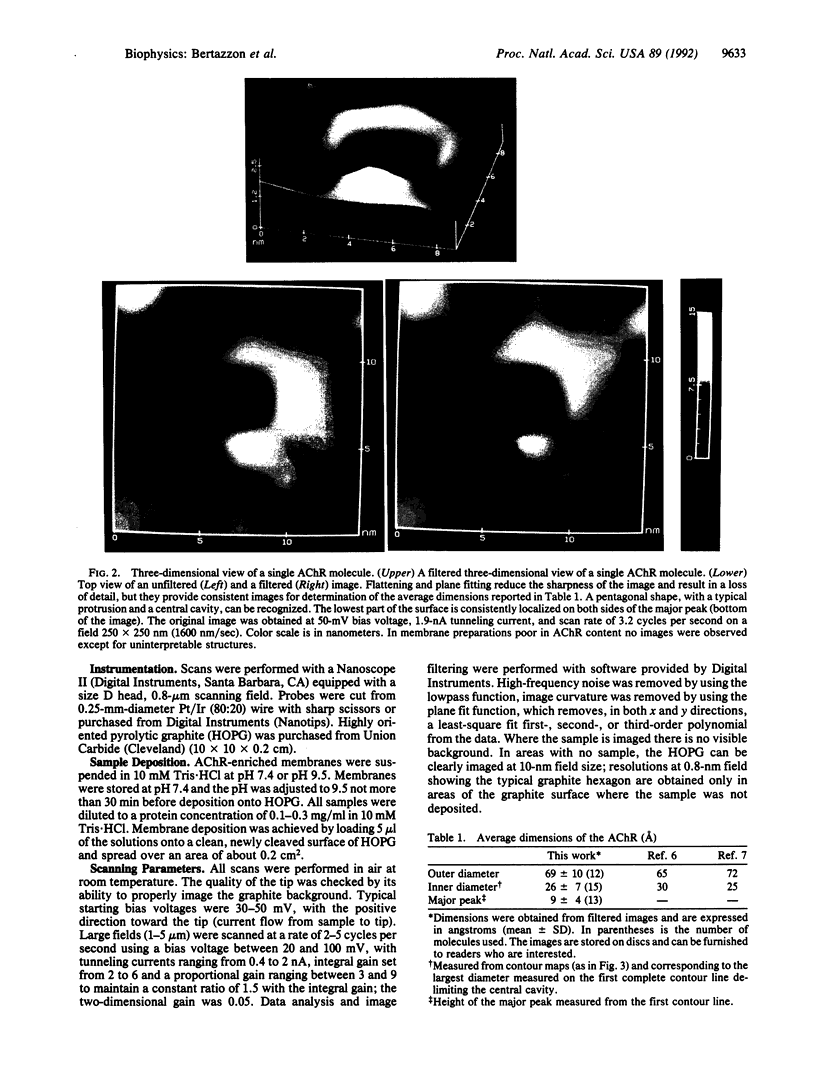
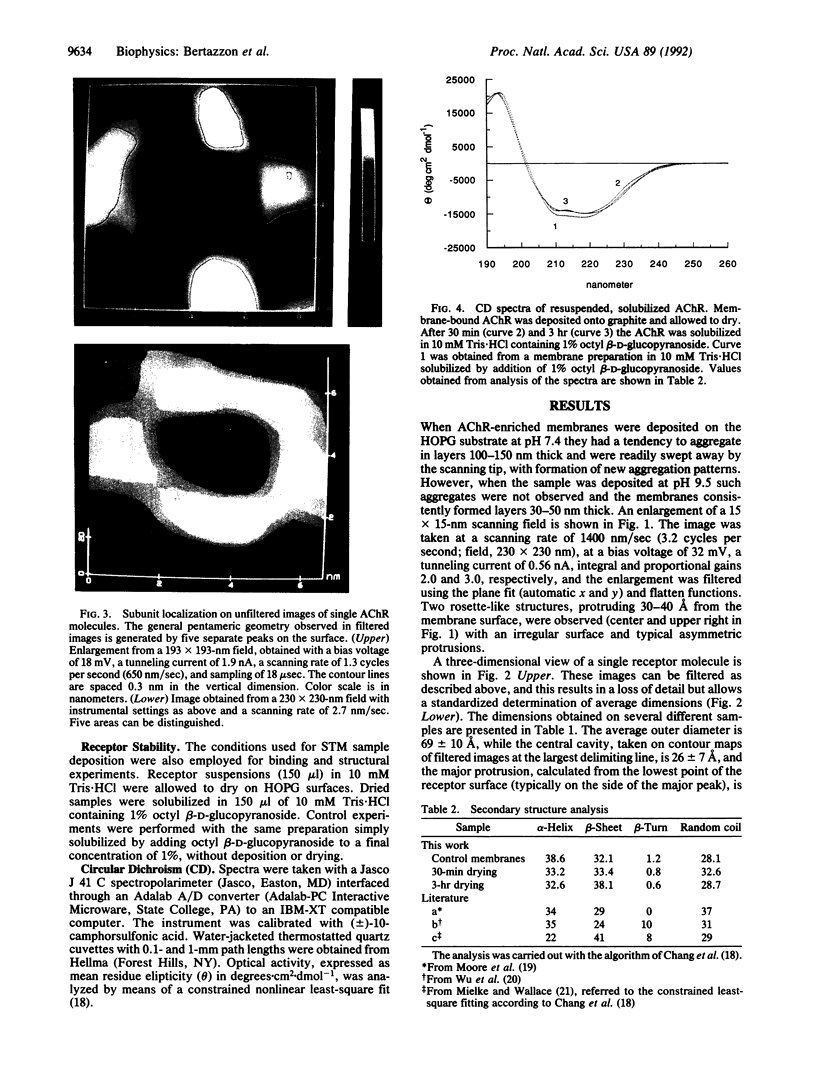
The synaptic surface of the acetylcholine receptor in membranes from Torpedo californica electric organ has been imaged by scanning tunneling microscopy. The molecule appears pentameric, with one major and four minor protrusions rising above the surface, and these protrusions encompass a large central cavity. The outer diameter of the molecule is 69 +/- 10 A, while the diameter of the cavity, measured at the widest complete contour line delimiting the opening, is 26 +/- 7 A. The images and dimensions obtained are consistent with the structure determined from hybrid density maps obtained by x-ray diffraction and electron microscopy. Thus, scanning tunneling microscopy can be used to obtain overall dimensions and low-resolution structural features of the surface of a membrane-embedded protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arscott P. G., Lee G., Bloomfield V. A., Evans D. F. Scanning tunnelling microscopy of Z-DNA. Nature. 1989 Jun 8;339(6224):484–486. doi: 10.1038/339484a0. [DOI] [PubMed] [Google Scholar]
- Blanchard S. G., Quast U., Reed K., Lee T., Schimerlik M. I., Vandlen R., Claudio T., Strader C. D., Moore H. P., Raftery M. A. Interaction of [125I]-alpha-bungarotoxin with acetylcholine receptor from Torpedo californica. Biochemistry. 1979 May 15;18(10):1875–1883. doi: 10.1021/bi00577a005. [DOI] [PubMed] [Google Scholar]
- Brisson A., Unwin P. N. Quaternary structure of the acetylcholine receptor. Nature. 1985 Jun 6;315(6019):474–477. doi: 10.1038/315474a0. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Wu C. S., Yang J. T. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978 Nov;91(1):13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- Clemmer C. R., Beebe T. P., Jr Graphite: a mimic for DNA and other biomolecules in scanning tunneling microscope studies. Science. 1991 Feb 8;251(4994):640–642. doi: 10.1126/science.1992517. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Gotti C. M., Hunkapiller M. W., Raftery M. A. Mammalian muscle acetylcholine receptor: a supramolecular structure formed by four related proteins. Science. 1982 Dec 17;218(4578):1227–1229. doi: 10.1126/science.7146904. [DOI] [PubMed] [Google Scholar]
- Cricenti A., Selci S., Felici A. C., Generosi R., Gori E., Djaczenko W., Chiarotti G. Molecular structure of DNA by scanning tunneling microscopy. Science. 1989 Sep 15;245(4923):1226–1227. doi: 10.1126/science.2781279. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Raftery M. A. Fractionation and partial characterization of membrane particles from Torpedo californica electroplax. Biochemistry. 1973 Sep 11;12(19):3593–3597. doi: 10.1021/bi00743a003. [DOI] [PubMed] [Google Scholar]
- Edstrom R. D., Meinke M. H., Yang X., Yang R., Evans D. F. Direct observation of phosphorylase kinase and phosphorylase b by scanning tunneling microscopy. Biochemistry. 1989 Jun 13;28(12):4939–4942. doi: 10.1021/bi00438a004. [DOI] [PubMed] [Google Scholar]
- Elliott J., Blanchard S. G., Wu W., Miller J., Strader C. D., Hartig P., Moore H. P., Racs J., Raftery M. A. Purification of Torpedo californica post-synaptic membranes and fractionation of their constituent proteins. Biochem J. 1980 Mar 1;185(3):667–677. doi: 10.1042/bj1850667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Dunn S. M., Blanchard S. G., Raftery M. A. Specific binding of perhydrohistrionicotoxin to Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2576–2579. doi: 10.1073/pnas.76.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky M. W., Stroud R. M. Immunospecific identification and three-dimensional structure of a membrane-bound acetylcholine receptor from Torpedo californica. J Mol Biol. 1979 Mar 5;128(3):319–334. doi: 10.1016/0022-2836(79)90091-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindsay S. M., Thundat T., Nagahara L., Knipping U., Rill R. L. Images of the DNA double helix in water. Science. 1989 Jun 2;244(4908):1063–1064. doi: 10.1126/science.2727694. [DOI] [PubMed] [Google Scholar]
- Mielke D. L., Wallace B. A. Secondary structural analyses of the nicotinic acetylcholine receptor as a test of molecular models. J Biol Chem. 1988 Mar 5;263(7):3177–3182. [PubMed] [Google Scholar]
- Miles M. J., Carr H. J., McMaster T. C., I'Anson K. J., Belton P. S., Morris V. J., Field J. M., Shewry P. R., Tatham A. S. Scanning tunneling microscopy of a wheat seed storage protein reveals details of an unusual supersecondary structure. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):68–71. doi: 10.1073/pnas.88.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A. K., McCarthy M. P., Stroud R. M. Three-dimensional structure of the nicotinic acetylcholine receptor and location of the major associated 43-kD cytoskeletal protein, determined at 22 A by low dose electron microscopy and x-ray diffraction to 12.5 A. J Cell Biol. 1989 Aug;109(2):755–774. doi: 10.1083/jcb.109.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. M., Holladay L. A., Puett D., Brady R. N. On the conformation of the acetylcholine receptor protein from Torpedo nobiliana. FEBS Lett. 1974 Sep 1;45(1):145–149. doi: 10.1016/0014-5793(74)80832-x. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numa S., Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Kikyotani S. Molecular structure of the nicotinic acetylcholine receptor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):57–69. doi: 10.1101/sqb.1983.048.01.008. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Vandlen R. L., Reed K. L., Lee T. Characterization of Torpedo californica acetylcholine receptor: its subunit composition and ligand-binding properties. Cold Spring Harb Symp Quant Biol. 1976;40:193–202. doi: 10.1101/sqb.1976.040.01.021. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., McCarthy M. P., Shuster M. Nicotinic acetylcholine receptor superfamily of ligand-gated ion channels. Biochemistry. 1990 Dec 18;29(50):11009–11023. doi: 10.1021/bi00502a001. [DOI] [PubMed] [Google Scholar]
- Wu C. S., Sun X. H., Yang J. T. Conformation of acetylcholine receptor in the presence of agonists and antagonists. J Protein Chem. 1990 Feb;9(1):119–126. doi: 10.1007/BF01024993. [DOI] [PubMed] [Google Scholar]
- Yang X. R., Miller M. A., Yang R., Evans D. F., Edstrom R. D. Scanning tunneling microscopic images show a laminated structure for glycogen molecules. FASEB J. 1990 Oct;4(13):3140–3143. doi: 10.1096/fasebj.4.13.2210158. [DOI] [PubMed] [Google Scholar]