Abstract
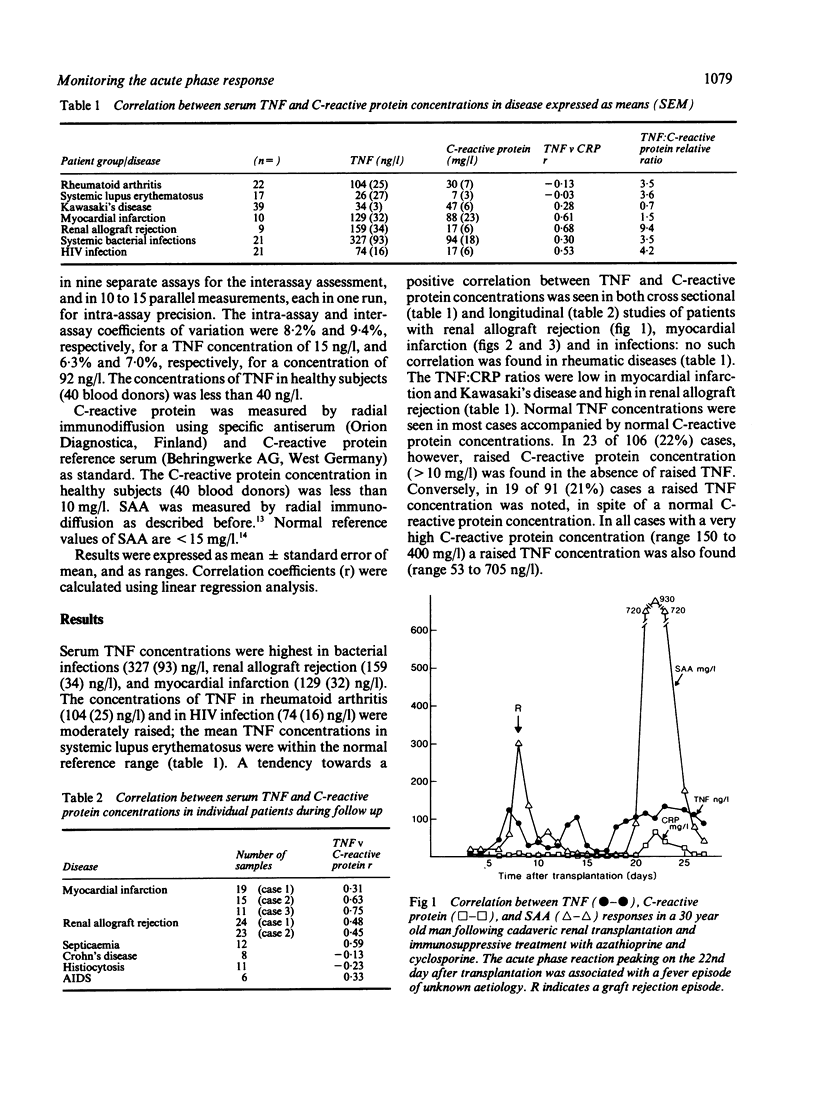
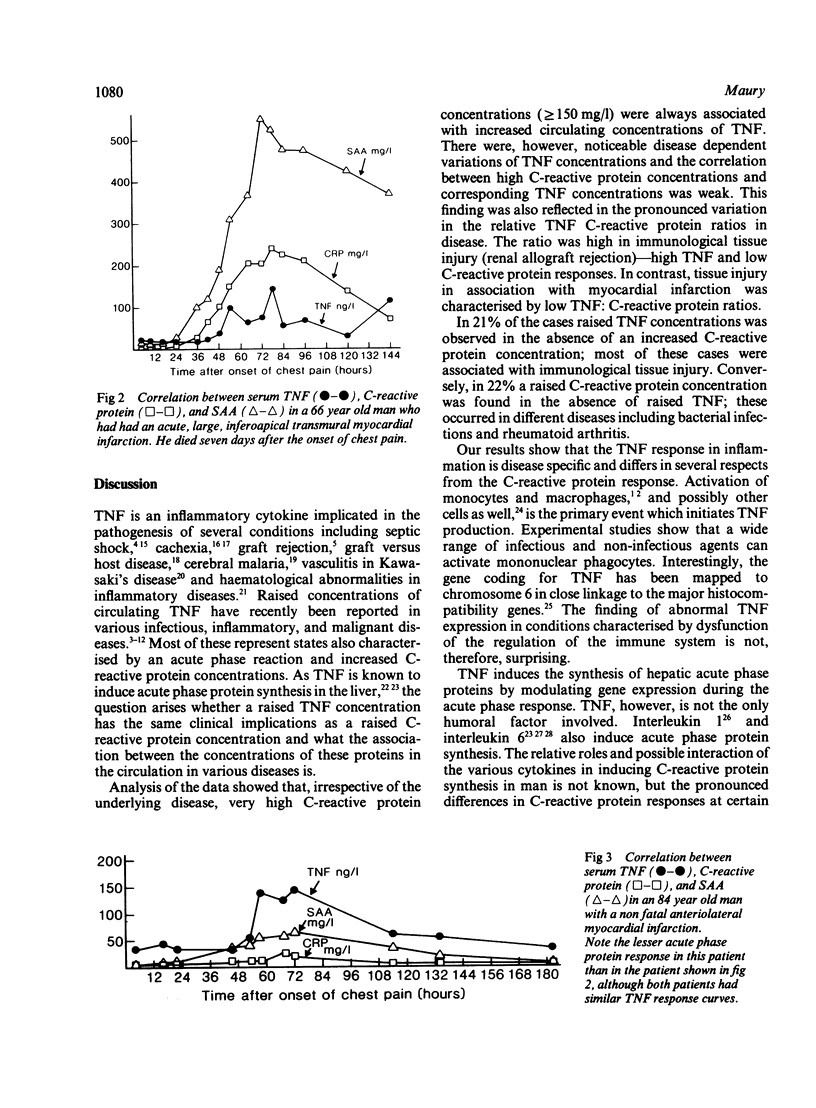
The relation between the inflammatory cytokine tumour necrosis factor-alpha (TNF or cachectin), which induces acute phase responses, and an established acute phase protein, C-reactive protein, was studied in various infectious and inflammatory diseases in man. All cases with very high serum concentrations of C-reactive protein (150 to 400 mg/l; normal reference value less than 10 mg/l) also had raised serum concentrations of TNF (53 to 705 ng/l; normal reference value less than 40 ng/l). In 19 out of 91 (21%) of the cases, however, a raised TNF concentration without correspondingly raised C-reactive protein concentration was also noted. Conversely, in 23 out of 106 (22%) cases raised C-reactive protein was observed in the absence of a raised TNF concentration. The ratios were high in allograft rejection and low in myocardial infarction and Kawasaki's disease. The highest mean concentration of circulating TNF was found in bacterial infections, graft rejection, and myocardial infarction. It is concluded that although high C-reactive protein concentrations are usually accompanied by raised TNF concentrations, there are pronounced relative variations in the serum concentrations of these proteins in various disease states, suggesting that there may be independent, disease specific regulatory pathways for TNF and C-reactive protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill F., Osborne R., Burke F., Naylor S., Talbot D., Durbin H., Tavernier J., Fiers W. Evidence for tumour necrosis factor/cachectin production in cancer. Lancet. 1987 Nov 28;2(8570):1229–1232. doi: 10.1016/s0140-6736(87)91850-2. [DOI] [PubMed] [Google Scholar]
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell J. V., Gómez-Lechón M. J., David M., Hirano T., Kishimoto T., Heinrich P. C. Recombinant human interleukin-6 (IL-6/BSF-2/HSF) regulates the synthesis of acute phase proteins in human hepatocytes. FEBS Lett. 1988 May 23;232(2):347–350. doi: 10.1016/0014-5793(88)80766-x. [DOI] [PubMed] [Google Scholar]
- Chapman P. B., Lester T. J., Casper E. S., Gabrilove J. L., Wong G. Y., Kempin S. J., Gold P. J., Welt S., Warren R. S., Starnes H. F. Clinical pharmacology of recombinant human tumor necrosis factor in patients with advanced cancer. J Clin Oncol. 1987 Dec;5(12):1942–1951. doi: 10.1200/JCO.1987.5.12.1942. [DOI] [PubMed] [Google Scholar]
- Ehnholm C., Teppo A. M., Ohisalo J. J., Maury C. P. Human high-density lipoprotein associated amyloid A protein. Structural characteristics, relation to apo A-I and A-II concentrations, and plasma clearance kinetics in the rat. Scand J Rheumatol. 1985;14(2):201–208. doi: 10.3109/03009748509165505. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Plasma clearance kinetics of the amyloid-related high density lipoprotein apoprotein, serum amyloid protein (apoSAA), in the mouse. Evidence for rapid apoSAA clearance. J Clin Invest. 1983 Apr;71(4):926–934. doi: 10.1172/JCI110847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Leung D. Y., Geha R. S., Newburger J. W., Burns J. C., Fiers W., Lapierre L. A., Pober J. S. Two monokines, interleukin 1 and tumor necrosis factor, render cultured vascular endothelial cells susceptible to lysis by antibodies circulating during Kawasaki syndrome. J Exp Med. 1986 Dec 1;164(6):1958–1972. doi: 10.1084/jem.164.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lähdevirta J., Maury C. P., Teppo A. M., Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988 Sep;85(3):289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Pettersson T. Detection of circulating tumour necrosis factor in systemic histiocytosis. Br J Haematol. 1989 Feb;71(2):293–295. doi: 10.1111/j.1365-2141.1989.tb04271.x. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Salo E., Pelkonen P. Elevated circulating tumor necrosis factor-alpha in patients with Kawasaki disease. J Lab Clin Med. 1989 May;113(5):651–654. [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M. Circulating tumour necrosis factor-alpha (cachectin) in myocardial infarction. J Intern Med. 1989 May;225(5):333–336. doi: 10.1111/j.1365-2796.1989.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M. Comparative study of serum amyloid-related protein SAA, C-reactive protein, and beta 2-microglobulin as markers of renal allograft rejection. Clin Nephrol. 1984 Dec;22(6):284–292. [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M., Raunio P. Control of the acute-phase serum amyloid A and C-reactive protein response: comparison of total replacement of the hip and knee. Eur J Clin Invest. 1984 Oct;14(5):323–328. doi: 10.1111/j.1365-2362.1984.tb01190.x. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M., Salaspuro M. P. Amyloid A fibril degrading activity in serum in liver disease--relation to serum acute phase and other protein levels. Clin Chim Acta. 1983 Jun 30;131(1-2):29–37. doi: 10.1016/0009-8981(83)90349-2. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M. Tumor necrosis factor in the serum of patients with systemic lupus erythematosus. Arthritis Rheum. 1989 Feb;32(2):146–150. doi: 10.1002/anr.1780320206. [DOI] [PubMed] [Google Scholar]
- Maury C. P. Tumour necrosis factor--an overview. Acta Med Scand. 1986;220(5):387–394. doi: 10.1111/j.0954-6820.1986.tb02785.x. [DOI] [PubMed] [Google Scholar]
- McAdam K. P., Elin R. J., Sipe J. D., Wolff S. M. Changes in human serum amyloid A and C-reactive protein after etiocholanolone-induced inflammation. J Clin Invest. 1978 Feb;61(2):390–394. doi: 10.1172/JCI108949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Allet B., Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987 Nov 1;166(5):1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. R., Olivecrona T., Pekala P. H. Regulation of lipoprotein lipase synthesis by recombinant tumor necrosis factor--the primary regulatory role of the hormone in 3T3-L1 adipocytes. Arch Biochem Biophys. 1986 Dec;251(2):738–746. doi: 10.1016/0003-9861(86)90384-x. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Van Damme J., Rieder H., Meyer zum Büschenfelde K. H. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol. 1988 Aug;18(8):1259–1264. doi: 10.1002/eji.1830180817. [DOI] [PubMed] [Google Scholar]
- Scuderi P., Sterling K. E., Lam K. S., Finley P. R., Ryan K. J., Ray C. G., Petersen E., Slymen D. J., Salmon S. E. Raised serum levels of tumour necrosis factor in parasitic infections. Lancet. 1986 Dec 13;2(8520):1364–1365. doi: 10.1016/s0140-6736(86)92007-6. [DOI] [PubMed] [Google Scholar]
- Spies T., Morton C. C., Nedospasov S. A., Fiers W., Pious D., Strominger J. L. Genes for the tumor necrosis factors alpha and beta are linked to the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8699–8702. doi: 10.1073/pnas.83.22.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Jung L. K., Walters J. A., Chen W., Wang C. Y., Fu S. M. Production of tumor necrosis factor/cachectin by human B cell lines and tonsillar B cells. J Exp Med. 1988 Nov 1;168(5):1539–1551. doi: 10.1084/jem.168.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppo A. M., Maury C. P. Radioimmunoassay of tumor necrosis factor in serum. Clin Chem. 1987 Nov;33(11):2024–2027. [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Gridley T., Huang S., Grandea A. G., 3rd, Gefter M. L. Single germline VH and V kappa genes encode predominating antibody variable regions elicited in strain A mice by immunization with p-azophenylarsonate. J Exp Med. 1987 Jul 1;166(1):1–11. doi: 10.1084/jem.166.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


