Significance
The first morphological sign of vertebrate eye formation is the appearance of eye vesicles on both sides of the ventral diencephalon, which primarily originate from a uniform Pax6-positive eye field. A key regulator for this process, which coincides with the establishment of the proximo-distal axis of the eye with eyestalk and retina, is the morphogen Shh. Shh induces the expression of pax2, which represses pax6 in the presumptive eyestalk region. Here, we report on the identification of the E3-ligase Mid1, which is induced by Shh and targets transcription factor Pax6 for proteasomal degradation. Our findings might provide insight into a mechanism, in which a morphogen initiates the degradation of a transcription factor to form sharp boundaries of gene expression.
Keywords: Xenopus, mid1, pax6, eye development, ubiquitin
Abstract
Pax6 is a key transcription factor involved in eye, brain, and pancreas development. Although pax6 is expressed in the whole prospective retinal field, subsequently its expression becomes restricted to the optic cup by reciprocal transcriptional repression of pax6 and pax2. However, it remains unclear how Pax6 protein is removed from the eyestalk territory on time. Here, we report that Mid1, a member of the RBCC/TRIM E3 ligase family, which was first identified in patients with the X-chromosome–linked Opitz BBB/G (OS) syndrome, interacts with Pax6. We found that the forming eyestalk is a major domain of mid1 expression, controlled by the morphogen Sonic hedgehog (Shh). Here, Mid1 regulates the ubiquitination and proteasomal degradation of Pax6 protein. Accordantly, when Mid1 levels are knocked down, Pax6 expression is expanded and eyes are enlarged. Our findings indicate that remaining or misaddressed Pax6 protein is cleared from the eyestalk region to properly set the border between the eyestalk territory and the retina via Mid1. Thus, we identified a posttranslational mechanism, regulated by Sonic hedgehog, which is important to suppress Pax6 activity and thus breaks pax6 autoregulation at defined steps during the formation of the visual system.
Pax6 is an evolutionary highly conserved transcription factor, playing key roles as a potent cell fate determinant in the development of the eye, brain, and pancreas (1–4). Pax6 is a member of the PAX family of transcription factors (5, 6). Pax6 autoregulation was suggested based on mouse genetic experiments (7) and studies regarding the quail, as well as human, promoters (8, 9). Thus, to maintain pax6 expression, Pax6 protein is required, which has been assumed already from studies of the Small eye (SEY) mutant mice (10, 11). In humans, heterozygous mutations in PAX6 cause a wide variety of ocular defects, whereas the homozygous loss results in anophthalmia (12). Not only does the reduction of Pax6 protein levels cause severe developmental defects in the eye, but transgenic mice carrying multiple copies of the human PAX6 gene also have similar ocular abnormalities as the small eye mice (13, 14). Moreover, in Drosophila and in vertebrates like Xenopus, overexpression of pax6 is able to induce the formation of ectopic eyes (15, 16). As a whole, Pax6 is one of the most important regulators of eye development and its function is critically dependent on a temporarily and quantitatively defined expression level (4).
In Xenopus, pax6 can be detected in all neuroblasts of the developing retina soon after gastrulation. In early tailbud stages, pax6 is expressed homogenously in all parts of the optic vesicle. In tadpoles, pax6 is limited to the lens epithelium and later to cells of the inner nuclear layer and the ganglion cell layer. However, pax6 transcripts are barely detectable in the outer nuclear layer containing photoreceptor cells but clearly visible in the ciliary marginal zone (17, 18). On the one hand, the sharp boundary between the optic cup with pax6 and the optic stalk region with pax2 is established by reciprocal transcriptional repression of these two pax genes (19, 20). On the other hand, it remains unclear how Pax6 protein is removed from the eyestalk territory on time. Some authors report the regulation of Pax6 activity by posttranslational modifications (21–23), and most interestingly, Tuoc et al. showed that in cortical progenitor cells, Pax6 protein is degraded by the proteasome mediated by Trim11 (24). However, the existence of similar mechanisms leading to the development of the visual system is not known.
The data of our present study show that Midline1 (Mid1) serves as one of these links. MID1, a member of the RBCC/TRIM E3 ligase family, was first identified in patients with the X-chromosome–linked Opitz BBB/G (OS) syndrome. Patients suffer from multiple malformations of the ventral midline as a consequence of mutations in the MID1 gene (25, 26). In previous studies, Mid1 has been described to regulate protein phosphatase 2A (PP2A) stability (27–30). PP2A/α4 is not the only known substrate, because recently it was shown that Mid1 interacts with the GLI regulator Fu, leading to a cytoplasmic retention of GLI3 in cancer cells (31). We show that Mid1 can physically interact with Pax6 leading to the ubiquitination and proteasomal degradation of Pax6 protein. We observe an overlapping expression of mid1 and pax6 in early stages of Xenopus development. In tadpole stages, mid1 transcripts are concentrated in the optic stalk territory in contrast to pax6. Overexpression of shh strongly induces mid1, whose expression expands to the whole remaining eye vesicle. Accordingly, when Mid1 levels are knocked down, Pax6 expression is expanded, and eyes are enlarged. Taken together, we provide evidence that Pax6 is a substrate for Mid1 and insights into how remaining or misaddressed Pax6 protein is cleared from the eyestalk region to properly set the border between the eyestalk territory and the retina at the right time.
Results
Mid1 Expression in Cells of the Forming Optic Stalk Is Under the Control of Shh.
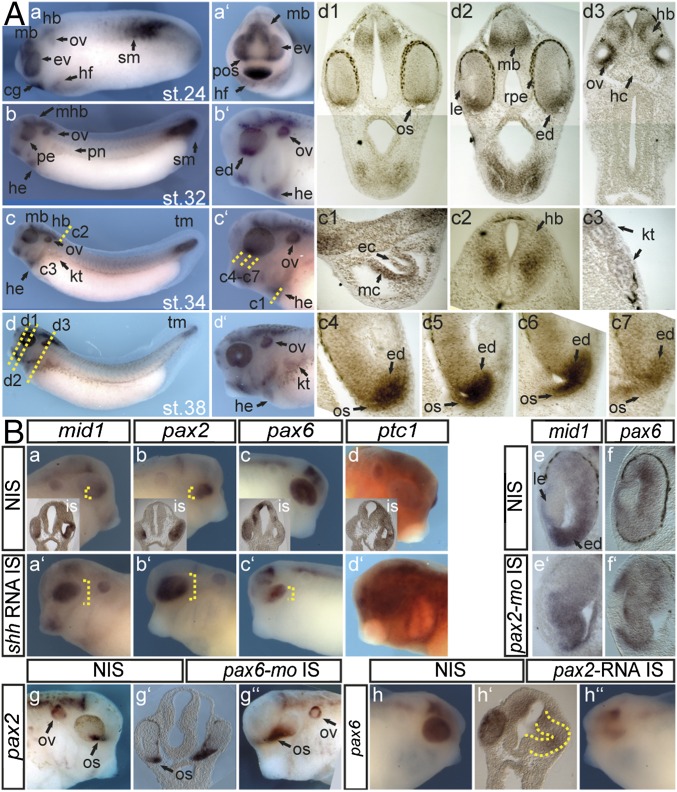
We analyzed the expression of mid1 during Xenopus laevis development by whole mount in situ hybridizations (Wmish) on embryos of Nieuwkoop and Faber (NF) stage 24–38 (Fig. 1A). While confirming the pattern described by Suzuki et al. (32), a closer look revealed that mid1 is expressed early in the whole eye vesicle. From the NF stage 32 onward, high levels of mid1 transcripts were detected in the ventral part of the forming eye and especially within the forming optic stalk (Fig. 1 A, c4–c7 and d1–d3). Here, the expression of mid1 appears similar, although not identical to those described for transcription factors vax1 or pax2, known targets of the hedgehog (hh) pathway (Fig. S1A) (33). To analyze whether Hedgehog also regulates mid1 expression, we injected synthetic shh RNA into a single cell of two-cell stage embryos and examined mid1 expression at tadpole stage. Misexpression of shh resulted in an up-regulation of the known target, the hh receptor ptc1 (Fig. 1 B, d–d′ and Fig. S1B, nptc1 = 20/27; nctrl = 6/60; P = 1.42E-11) in neural ectoderm. Moreover, mid1 expression was strongly induced but restricted to the optic cup (Fig. 1 B, a–a′; nmid1 = 66/81; nctrl = 3/44; P = 1.08E-15) similar to pax2 (Fig. 1 B, b–b′; npax2 = 74/83; nctrl = 3/42; P = 4.21E-18), whereas pax6 was repressed on shh RNA injection (Fig. 1 B, c–c′; npax6 = 86/106; nctrl = 4/51; P = 3.47E-18) (33). Interestingly, Hh-dependent regulation of mid1 is not mediated by pax2 because suppression of pax2 function did not impair mid1 expression (Fig. 1 B, e–e′; 8/11 eyes analyzed in section) but resulted in an enlargement of the retina and ectopic pax6 expression in the eye vesicle (Fig. 1B, f–f′; 13/16 eyes analyzed in section). Moreover, pax2 is under the control of pax6, because suppression of pax6 led to an expansion of pax2 expression into the remaining eye vesicle (Fig. 1 B, g–g″; npax2 = 7/17; nctrl = 4/47; P = 1.45E-08) and pax2 RNA injection impaired pax6 expression as in mice (Fig. 1 B, h–h″ and Fig. S1C; npax6 = 9/35; nctrl = 4/47; P = 1.5E-14) (20). Because Mid1 is an E3 ubiquitin ligase, we assume that Mid1 regulates Pax6 expression on a posttranslational level rather than on a transcriptional level like Pax2.
Fig. 1.
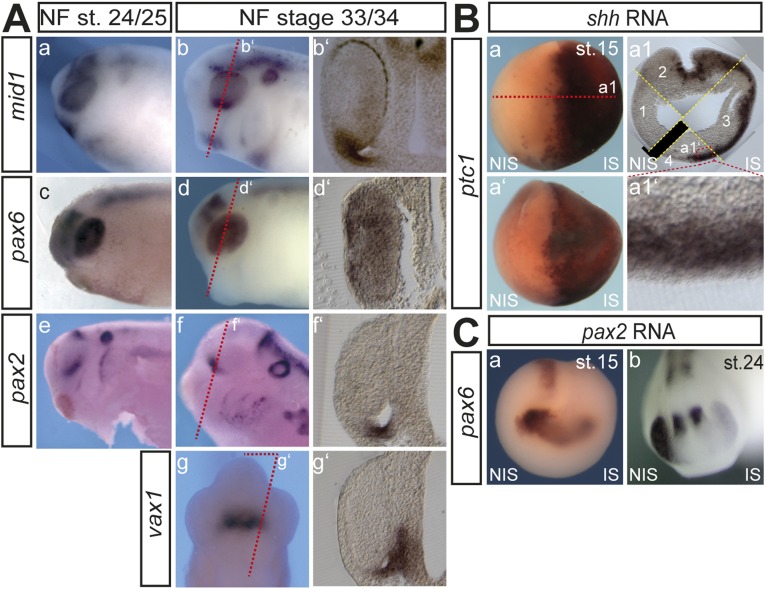
Mid1 is expressed in cells of the forming optic stalk and induced on shh RNA injection. (A) mid1 mRNA expression was assessed by Wmish on Xenopus laevis embryos at different NF stages. (a–d) Lateral view, (a′) cranial view, and (b′–d′) lateral view. Twenty-micrometer gelatin/albumin sections at the level of the tadpole’s head (d1–d3), heart (c1), neural tube (c2), and pronephric anlage (c3). cg, cement gland; ec, endocardium; ed, eye disk; ev, eye vesicle; hd, hindbrain; he, heart; hf, heart field; kt, kidney tubules; mb, midbrain; mc, myocardium; op, olfactory placode; os, optic stalk; ov, otic vesicle; pnt, pronephric tubule; pos, presumptive os; sm, somites. (c4–c7) mid1 expression in eye stalk region in serial sections. (B) Injections were done into one cell of two-cell stage embryos. (a–d′) Synthetic shh-RNA (500 pg) was injected and Wmish against mid1, pax2, pax6, and ptc1 was performed as indicated. (e–f′) pax2-mo (2.5 pmol) was injected, and expression of mid1 and pax6 was monitored. Shown are transversal sections of the eye at the level of the lens. (g–g″) pax6-mo (2.5 pmol) was injected, and pax2 was monitored. Shown are lateral views of the head region and a transversal section of the head region at the level of the optic vesicles (g′). (h–h″) pax2 RNA (100 pg) was injected and expression of pax6 was analyzed.
Fig. S1.
(A) a–f, Lateral view of the head region; g, ventral view of the head region; b′–g′, sections at the level of the forming eye stalk as indicated by the red dashed lines. Though pax2, vax1, and mid1 are clearly expressed within the forming eye stalk, their pattern of expression in this part is not identical and appears to follow a proximodistal axis with mid1 more distally, pax2 centrally, and vax1 proximally located. (B) a, dorsal view; a′, anterior view; a1, transversal section as indicated in a. The misexpression of shh upon the microinjection of synthetic 500 pg shh RNA induced the expression of ptc1 strongly at the open neural tube stage (NF stage 15) within the sensorial layer of the ectoderm (a1′) and this induction of ptc1 is maintained though less intense at tadpole stage (see also Fig. 1 B, d–d′). (C) a and b, anterior view. Misexpression of pax2 upon the microinjection of synthetic 100 pg pax2 RNA repressed the expression of pax6 already at the open neural tube stage (NF stage 15) and this suppression of pax6 is maintained at tadpole stage (see also Fig. 1 B, h–h′).
Mid1 Interacts with Pax6.
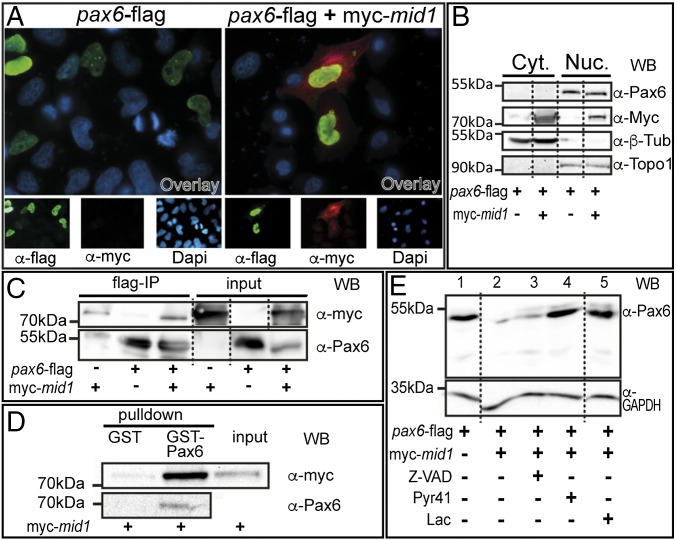
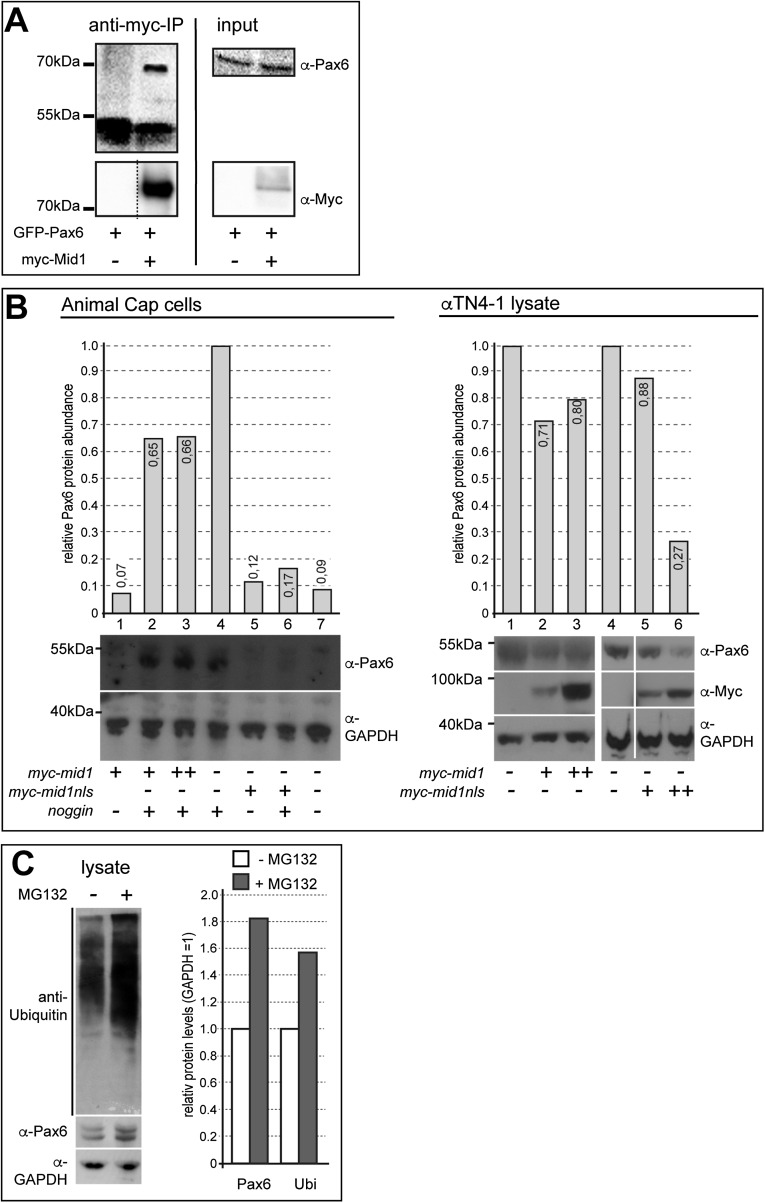
To investigate whether Mid1 and Pax6 interact physically, we expressed pax6 alone or together with mid1 in cell lines. We observed Pax6 within the nucleus and Mid1 predominantly in the cytoplasm (Fig. 2A). A physical interaction requires at least a temporary localization in the same cellular compartment. Thus, we generated cytoplasmic and nuclear fractions from transfected HEK293 cells. Western blot revealed that major fractions of Pax6 reside in the nucleus and of Mid1 in the cytoplasm. However, a minor amount of Mid1 protein was detected in the nucleus (Fig. 2B). This result suggests that Mid1 and Pax6 are able to interact in vivo in the nucleus. To demonstrate a direct interaction, we performed coimmunoprecipitation experiments. Cotransfection of HEK293 with myc-mid1 and pax6-flag revealed that Pax6 precipitates Mid1 (Fig. 2C). Similarly, Mid1 was able to precipitate Pax6 (Fig. S2A). To further confirm that Mid1 binds to Pax6, we performed a GST pull-down assay using purified GST-fused Pax6 and lysates of myc-mid1–transfected HEK293 cells. As shown in Fig. 2D, Mid1 physically interacts with GST-Pax6. To show that Mid1 primes Pax6 for degradation, we cotransfected HEK293 cells with pax6-flag and myc-mid1. Lysates of these cells show a clear reduction of the Pax6 protein level when Mid1 was present (Fig. 2E, lanes 1 and 2). To determine whether the lower Pax6 content is due to proteasomal degradation, we compared Pax6 expression levels in HEK293 cells in the presence or absence of two inhibitors of the proteasomal pathway. Both treatments restored Pax6 in the presence of Mid1, whereas the apoptotic inhibitor Z-VAD-FMK did not, as a control for Mid1 activity in the presence of an arbitrary inhibitor (Fig. 2E, lanes 3–5). In addition, overexpression of Mid1 in αTN4-1 cells or in neuralized animal cap explants, which both express Pax6 endogenously, led to a reduction of Pax6 protein level, in particular when Mid1 was forced to enter the nucleus by the fusion of a nuclear localization signal (mid1nls; Fig. S2B). These results strongly suggest that Mid1 mediates proteasomal degradation of Pax6.
Fig. 2.
Mid1 physically interacts with Pax6. (A) HeLa cells were transfected with pax6-flag alone or combined with myc-mid1 and analyzed by immunohistochemistry. Nuclei were stained with DAPI. Pictures are shown in false colors: green for Pax6, red for Mid1, blue for nuclei (Bottom). (B) HEK293 cells were transfected as in A. Cytosolic proteins were extracted with hypotonic buffer. Soluble nuclear proteins were extracted from the remaining fraction with high salt buffer. Blot shows Mid1 and Pax6 in the cytosolic (Cyt.) and nuclear (Nuc.) fractions, as well as the reprobes with anti–β-tubulin or anti-topoisomerase 1 antibody to control purity. (C) Interaction of Mid1 with Pax6 was assessed by coimmunoprecipitation performed in HEK293. (Left) Eluted myc-tagged proteins after immunoprecipitation (upper blot) and precipitated Pax6 (lower blot, reprobed with anti-Pax6 antibody). (Right) Blots for the input proteins. (D) Interaction of Mid1 and Pax6 was verified by GST using purified GST-Pax6 or purified GST as a control and lysates of HEK293 cells transfected with myc-mid1. (Left) Eluted myc-tagged proteins after GST pull down (upper blot) and precipitated Pax6 (lower blot, reprobed with anti-Pax6 antibody). (Right) Input protein of the lysate. (E) Pax6-flag was expressed alone or together with myc-Mid1 in HEK293 cells in the presence or absence of the proteasome inhibitor lactacystin (Lac), the E1 activating enzyme inhibitor Pyr41, or the caspase inhibitor Z-VAD-FMK (Z-VAD) as indicated. For loading control, blots were reprobed with an anti-GAPDH antibody.
Fig. S2.
Mid1 reduces endogenous Pax6 protein. (A) Mid1 physically interacted with Pax6. HEK293 cells were transfected with GFP-pax6 + myc-mid1 or myc-mid1 alone. The left panels show eluted Pax6 protein after immunoprecipitation with an anti-Myc antibody (upper blot) and precipitated myc-Mid1 (lower blot, reprobe with anti-Myc antibody). The right panels show blots with the respective lysates for the input proteins. (B) Xenopus animal cap explants expressed Pax6 upon neuralization by microinjection of 400 μg synthetic RNA of the BMP4 inhibitor noggin. Approximately 30 animal cap explants were separated in each lane. Coinjection of Xenopus mid1 RNA (1 ng) led to a reduction of Pax6 protein abundance. The reduction of Pax6 protein levels was considerably stronger, when Mid1 protein was forced to enter the nucleus by the fusion of a nuclear localization signal (mid1nls). The mouse lens epithelial cell line αTN4-1 expresses pax6 endogenously. Transfection of mid1 and of mid1nls led to a reduction of endogenous Pax6 protein. Corresponding Western blots were quantified with the help of ImageJ and normalized against GAPDH. (C) Pax6 levels increased upon proteasome inhibition. αTN4-1 cells were grown in DMEM medium (10% FCS) and were treated with DMSO (−) or with MG132 (5 μM) (+). Pax6 levels increased upon MG132 treatment (+), as well as polyubiquitinated proteins. Anti-GAPDH was used as a loading control. Densitometry was performed using ImageJ (right graph).
Mid1 Modulates Abundance of Pax6 Protein and Induces Ubiquitination.
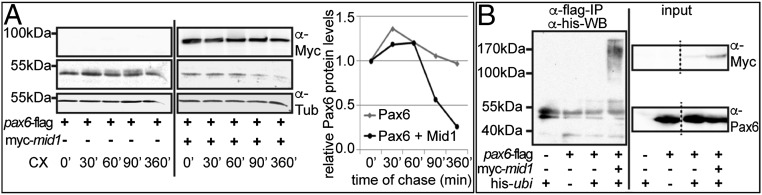
To further support our finding that Mid1 controls Pax6 degradation, we analyzed the abundance of Pax6 protein in HEK293 cells transfected with pax6-flag and either myc-mid1 or empty vector (EV), using a cycloheximide-chase approach (Fig. 3A). Quantification of Pax6 protein relative to β-tubulin contents indicates that the level of Pax6 protein is considerably reduced in cells overexpressing Mid1 (Fig. 3A, diagram). Next, we tested whether Pax6 could serve as a substrate for the ubiquitin ligase activity of Mid1. We looked for ubiquitinated Pax6 in HEK293 cells, overexpressing pax6-flag alone or in combination with ubiquitin (his-ubi) and myc-mid1 in the presence of the proteasome inhibitor, MG132. Pax6 was immunoprecipitated from RIPA cell lysates and was analyzed for Ubi-conjugated forms (Fig. 3B). Considerable amounts of ubiquitinated protein were detected only with immunoprecipitates from cells transfected with all three components, suggesting that Mid1 mediates ubiquitination of Pax6.
Fig. 3.
Mid1 induces ubiquitination and reduces the abundance of Pax6 protein. (A) Blots show levels of Pax6, Mid1, and β-tubulin in HEK293 cells after cotransfection on pax6-flag expression plasmid and either EV or myc-mid1 at different time points (min) on cycloheximide block of protein synthesis. Diagram shows the quantified values of Pax6 protein relative to β-tubulin levels in Western blot. Amounts of Pax6 protein before the cycloheximide treatment are designated as 1 (100%). (B) In vivo ubiquitination of Pax6 was analyzed by transfection of HEK293 cells with plasmids coding for pax6-flag, myc-mid1, and his-ubiquitin as indicated and anti-his Western blot of proteins eluted after an anti-flag-IP (Left). Input of the proteins was verified by Western blot of the corresponding lysates with anti-Myc and anti-Pax6 antibodies, respectively (Right).
Knockdown of mid1 Leads to Enlarged Eyes and Overexpression of Pax6 in Vivo.
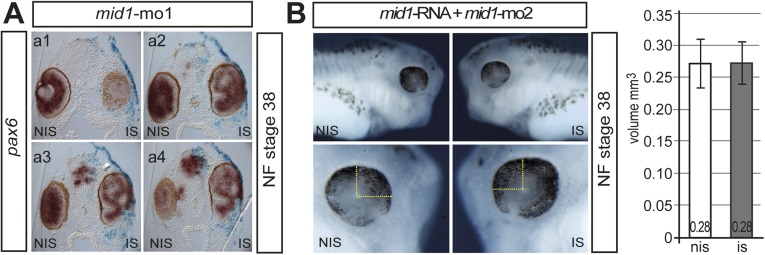
Because Mid1 interacts with Pax6 and controls Pax6 levels in cell culture, we wanted to know if this holds true in Xenopus embryos. To suppress mid1 function, we injected a mid1 antisense morpholino (mid1-mo1) into one cell at the two-cell stage. At NF stage 38, the eyes on the injected sides (IS) appeared larger than on the noninjected side (NIS) and often showed aberrant retinal folds, accompanied by a loss of sharp boundaries of retinal layers (n = 54/59; Fig. 4A and Fig. S3A). In transversal sections of the affected eyes, the outer and inner layers of the retina are morphologically not clearly defined and still express pax6. (Fig. 4 B, a–a′). We conclude that Mid1 is necessary for proper eye development, confining the area of Pax6 expression. Additionally we investigated the effect of Mid1 suppression on retinal cell fate specification and therefore analyzed marker gene expression of all retinal layers. We found that injection of mid1-mo1 interferes with the ordered expression of all retinal marker genes analyzed. The expression pattern of the ganglion cell marker (brn3.0), bipolar (vsx1), and horizontal (prox1) genes were irregular, whereas the expression of rhodopsin expression was reduced slightly (Fig. 4 B, b–e′). Repeating the experiment using a second morpholino (mid1-mo2) that targets only mid1, allowed to exclude the possibility that the observed increase in eye size is due to knockdown of mid2, a close homolog of mid1, which is potentially affected by the morpholino used thus far (32). Again, all of the mid1-mo2 injected embryos (n = 212) displayed a larger eye on the injected side (Fig. 4C). These eyes had an ∼30% larger volume compared with those on the noninjected side (n = 25; P = 0.001; Fig. 4 C, c). To test whether the increased eye size on suppression of mid1 function was the result of a higher rate of proliferative cells, we compared the number of phospho-histone H3 (pH3)-positive cells in the injected eye area of sections to those of the noninjected side. Remarkably, we counted almost twice as much pH3-positive cells in eyes of mid1-mo2 injected embryos compared with the control side (ctrl-mo: n = 6; mean 14.4 pH3+ cells per section; mid1-mo2: n = 8; mean 27.2 pH3+ cells per section; P = 0.0006; Fig. 4 C, d). To rule-out off target effects of the second morpholino, we tested the specificity by coinjecting synthetic mid1-RNA (1–2 ng), which cannot be targeted by the morpholino, along with mid1-mo2. As a result, mid1-mo2 injected embryos were rescued with equal volumes of the eyes on both sides (Fig. S3B). To take a closer look on the effect of the mid1 knockdown on Pax6 protein in retinal cells, we performed immunohistology of cryosections from morphant embryos at NF stage 38. Using an anti-Pax6 antibody, Pax6 was found in the inner part of the inner nuclear layer (INL) and in the ganglion cells of the control side (NIS). However, in mid1 morphant eyes, Pax6 expression was strongly expanded within the neural retina (Fig. 4D). The number of Pax6-expressing cells increased from an average of 67 cells per section on the noninjected side to 130 cells per section on the injected side (n = 3; *P = 0.03; Fig. 4 D, c). Because the retina was enlarged on the injected side, we normalized the number of Pax6-positive cells to the retinal area, and a higher ratio compared with the noninjected side was still measured (Fig. 4 D, d). These results indicate that an enhanced expression of Pax6 protein in the enlarged eyes is accompanied by a higher rate of cell proliferation in the retinal region on suppression of mid1 function.
Fig. 4.
Mid1 loss of function interferes with eye development. (A and B) 2.5 pmol of mid1-mo1 was injected into one cell at the two-cell stage and analyzed by Wmish at NF stage 38. (Upper) Lateral views of head region of noninjected (a; NIS) or injected side (a″; IS), dorsal view (a′). (B) Analysis of retinal stratification on mid1 suppression in transversal sections at the level of the lens using probes against pax6, brn3.0, vsx1, prox1, and rhodopsin. For better comparison, all images are oriented with the lens to the left. (C) Lateral view (a and b) of NF stage 38 embryos injected with mid1-mo2 into one cell at the two-cell stage and 10× enlarged view of the eye region of a′ and b′ (Lower). All mid1-mo2–injected embryos showed an increase in eye size (n = 212 mid1-mo2 and n = 126 ctrl-mo–injected embryos). The graph shows the mean values for 25 embryos (c; **P = 0.001). Analysis of cell proliferation of mid1-mo2–injected embryos. The number of phospho-histone H3 (pH3) positive cells were counted and compared. (d; ctrl-mo: six embryos, mean 14.4 pH3+ cells per section; mid1-mo2: eight embryos, mean 27.2 pH3+ cells per section; ***P = 0.0006). (D) Pax6 immunoreactivity in cryosections on mid1-mo1 injection. The number of Pax6-positive cells per sections within the retinal region was counted for both sides of two embryos, and the area of the total retina was estimated. The numbers of Pax6-positive cells from sections of three mid1-mo1–injected embryos were counted (c; *P = 0.03); the numbers of Pax6-positive cells relative to the area of the retina is shown in the right diagram (d). The value for the noninjected side was set to 1.
Fig. S3.
(A) a1–a4 show a series of transversal sections at the level of the eye of the mid1 injected tadpole (NF stage 38) shown in Fig. 1 A, a. (B) Rescue of the mid1 knockdown eye phenotype. Lateral view of NF stage 38 embryos injected with mid1-mo2 (1.25 pmol) along with synthetic human mid1 RNA (1–2 ng) into one cell of a 2-cell stage embryo. The lower panel shows a 10× enlarged view of the respective eye region. Considering the eye to be an oblate spheroid, the mean radius was estimated by measuring the drawn distances and total volume was calculated. The graph shows the mean values for 20 embryos. The specificity of the pax2 and pax6 morpholino had been tested already by rescue experiments in refs. 53 and 54, respectively.
Targeted Overexpression of mid1 in Retinal Precursor Cells Shifts the Ratio of Bipolar and Photoreceptor Cells.
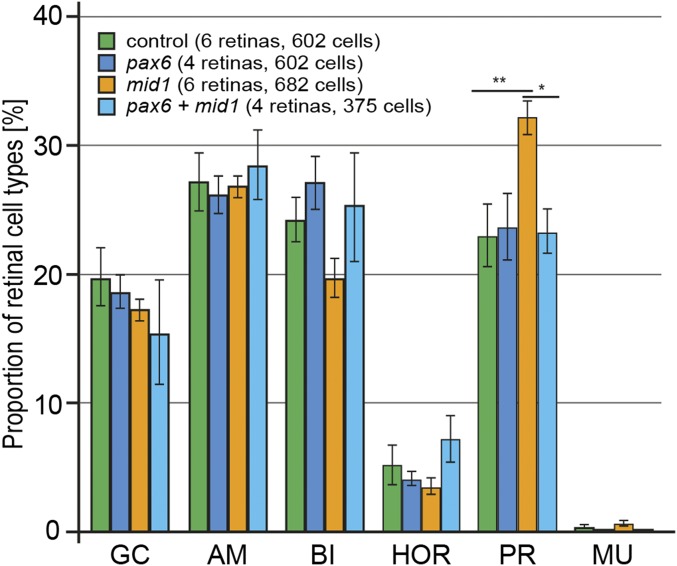
Because Pax6 has been shown to be required for retinal cell-fate determination (4), we investigated direct cell autonomous effects of Mid1 in a clonal analysis of the progeny of human or Xenopus tropicalis mid1 transfected cells following in vivo lipofection experiments. Compared with control embryos, which were lipofected with GFP alone, mid1-lipofected embryos showed a significant increase in the proportion of photoreceptor cells (human mid1, P < 0.1; X. tropicalis mid1, P < 0.01). In the same time, the proportion of bipolar cells was decreased by almost 30% (P < 0.01), whereas the proportion of ganglion, amacrine, horizontal, and Müller cells was not significantly changed (Fig. 5B and Fig. S4). Moreover, colipofection of pax6 and mid1 rescued the mid1 induced change in the proportion of retinal cell types (Fig. 5B). These data suggest that the increased proportion of photoreceptors observed upon Mid1 lipofection is due to a decrease in Pax6 expression.
Fig. 5.
Targeted overexpression of mid1 affects fate of retinal precursor cells, which can be reversed by pax6 coexpression. Cell fate analysis at stage 41 following overexpression of the indicated constructs by in vivo lipofection at the neurula stage. GFP was used as a tracer to visualize transfected cells. The error bars represent SEM. AM, amacrine cells; BI, bipolar cells; GC, ganglion cells; HOR, horizontal cells; MU, Müller cells; PR, photoreceptor cells.
Fig. S4.
Human and Xenopus mid1/Mid1 affects fate of retinal precursor cells similarly. (A) Schematic of the retina showing the different cellular layers and retinal cell types. The picture on the right illustrates a clone of GFP-positive cells following lipofection. Cell types are identified according to their position in the layers and their morphology. (B) Human or Xenopus mid1 expression plasmids together with GFP or GFP plasmid alone were lipofected into the area of the eye prospective retinal field at NF stage 17. At NF stage 41, cryosections were done, and the fluorescent cells in the central retina were counted. The different cell types were identified according to their morphology. The diagram shows the percentages of the different cell types for GFP-lipofected embryos (green, n = 40), human Mid1 (light blue, n = 24), and Xenopus mid1 (dark blue, n = 17); error bars represent SEM. AM, amacrine cells; BI, bipolar cells; GC, ganglion cells; HOR, horizontal cells; MU, Müller cells; PR, photoreceptor cells.
Discussion
In this study, we examined whether the E3-Ligase Mid1 can lower Pax6 protein levels. We provide evidence supported by gain- and loss-of-function experiments that Mid1 mediates poly-ubiquitination of Pax6. We also demonstrate that mid1 expression during eye development is in part mediated by the morphogen Shh, implicating a further level of control conducted by Shh on the development of the visual system.
Recent studies reveal new insights into mechanisms that posttranscriptionally regulate spatiotemporal Pax6 protein levels beyond the well-characterized genetic regulatory circuits (6, 22, 34). Tuoc et al. (24) reported that in the developing cortex Pax6 protein is specifically degraded by the proteasome after ubiquitination by Trim11 and Pax6 regulates trim11 transcription, a RING finger E3 ligase, which is not present in the Xenopus genome. This dual mechanism ensures the adjustment of a physiological level of Pax6 protein within tight limits, which is likewise important for eye development. Initially, Pax6 is required for the specification of the eye prospective retinal field, including the lens placode (8, 10). Later, Pax6 needs to be removed from various territories of the forming visual system, e.g., for a proper formation of the eyestalk and the differentiation of retinal precursor cells. Moreover, Pax6 can act autonomously in the lens surface ectoderm and in the optic vesicle (10, 35). Thus, concerning timing and level of expression, Pax6 is a tightly controlled transcription factor for the early and late stages of retina and lens development (4, 35–37). Our present findings provide insights into how Pax6 protein can be removed specifically from the eyestalk territory. Several lines of evidence point to Mid1 (Trim18), which mediates the proteasomal degradation of Pax6 in time and space. We were able to show that Pax6 protein levels are reduced in those cells, which concordantly express Mid1. The degradation of Pax6, mediated by Mid1, can be suppressed by treatment of the cells with proteasome inhibitors. In the cell, the majority of either Mid1 or Pax6 protein is present in different cellular compartments. Mid1 is mainly located at microtubules in the cytoplasm and Pax6 reside in the nucleus (38). We show that a minor fraction of Mid1 protein is in the nucleus allowing Mid1 and Pax6 to interact physically as indicated by coimmunoprecipitation and GST-pull-down experiments.
In 1997, MID1 was identified as the causative gene for X-linked Opitz G/BBB syndrome (OS) (26). Mutations in MID1 led to defects in the development of midline derived structures with a wide range of anomalies. The major activity of MID1 was related to ubiquitination and proteasome dependent degradation of PP2A and/or α4 protein (27). Recent studies revealed that additional proteins interact with Mid1 in vitro, which are involved in mRNA transport and translation or microtubule dynamics and might be part of the syndrome, affecting patients with OS (28, 29, 32). Our studies present evidence that part of Mid1 function in vertebrates is the control of Pax6 protein levels during visual system development. We could show that in Xenopus, early mid1 expression overlaps with early pax6 expression (optic vesicle). Shortly after, expression of pax6 and of mid1 appears to be almost exclusive with mid1 in the forming eyestalk and pax6 in the retina. In mouse embryos, mid1 is expressed in the outer nuclear layer (39), whereas pax6 expression becomes mainly restricted to the inner nuclear layer of the eyecup. When we suppressed mid1 function by injecting anti-mid1 morpholinos into embryos, we observed a significant increase in the size of the embryonic eyes due to a higher rate of cell proliferation. The enlargement of the optic vesicles led to folds of nonregular stratified retina with ectopic pax6 expression. In line with these results, the inhibition of pax2 led to a similar, mid1-like eye phenotype, whereas suppression of pax6 led to smaller eyes but enlarged pax2 domains of expression. Of note, Mid1 and Pax6 were retrieved together in a group of genes associated with diseases leading to abnormal corpus callosum (40). Thus, suppression of mid1 function allows to maintain pax6 expression in a region where normally Mid1 restricts Pax6.
Little is known about the regulation of the MID1 gene. An apparent regulator of ventral mid1 expression during development is the secreted factor Sonic hedgehog. It was shown that Shh induces the expression of pax2 and vax1 and thus indirectly allows pax6 expression only in the developing retina of Xenopus and zebrafish embryos (19, 33). Mid1 expression was therefore examined in Hedgehog-injected embryos. Ectopic Shh expands mid1 expression into the entire optic vesicle but also in the prospective forebrain. Therefore, the ventralization of these territories by Shh may partially depend on the rapid degradation of distal (eye) or dorsal (brain) determinants. Interestingly, Shh was also described to repress mid1 expression and mid1 can act upstream of SHH, most likely due to the induction of bmp4 expression in Hensen’s node of the chicken (31, 41). Recently it has been reported that human Fu, a Ser/Thr kinase important for Hh signaling in Drosophila, permits nuclear localization of GLI3. In a cancer cell line, MID1 marks Fu by ubiquitination for subsequent cleavage leading to the cytosolic retention of GLI3 (31) and thus blocks Hh signaling. In the eye, gli3 was shown to interact with other transcription factors to define retinal stem cells and gli3 may cooperate with pax6 during eye morphogenesis (42, 43). Thus, a feedback loop may exist, which regulates a balance between mid1 expression with medium levels of Shh that induce mid1 and lower levels that suppress mid1 expression. We show that in prospective brain areas close to the Shh source mid1 was not expressed, whereas the highest levels of mid1 transcripts were found in the eyestalk region but faded away in the dorsal retina (Fig. 1 A, d2 and c5). Even after misexpression of shh, mid1 was not found in central parts of the prospective brain, but distally in the remaining eye vesicle (Fig. 1B). Moreover, we show that pax2 and mid1 expression is up-regulated on misexpression of shh in the forming eye and that Pax2 protein is not necessary for mid1 expression. These results suggest that pax2 and mid1 expression are regulated by the Hh pathway independently and act in concert to restrict Pax6 activity.
Pax6 is expressed in all cells of the forming optic cup in mice initially. When differentiation proceeds, higher Pax6 expression is maintained in the peripheral optic cup, where cells differentiate later, and in centrally located retinal progenitors cells (RPCs) with lower levels of Pax6 (44). Pax6 is required in RPCs for both proliferation and cell-fate acquisition (4). However, different activities of Pax6 have been reported in RPCs according to their location along the central-to-peripheral axis (36, 45). Interestingly, in the chick and Xenopus retina, decreasing Pax6 activity increases cone cell genesis (46, 46), whereas nonphotoreceptor neurons increase on Pax6 overexpression (48). It has thus been proposed that Pax6 inhibits the differentiation of photoreceptor cells. Consistent with this, in mouse peripheral retina, Pax6 was shown to play a role in suppressing the expression of Crx, a transcription factor essential for photoreceptor cell differentiation (4). We observed a bias toward a photoreceptor fate following Mid1 overexpression in the retina and a reduction of rhodopsin on Mid1 knockdown. We thus propose that such abnormal cell type distribution in clones generated from Mid1-lipofected RPCs may be due to Mid1-dependent Pax6 degradation.
We conclude that in addition to transcriptional and posttranscriptional mechanisms (37, 44, 49, 50), the specific and fast regulation of Pax6 protein levels by protein degradation is essential for the normal formation of the visual system. Our results reveal an important aspect of E3-ligases, which are often considered as general negative regulators of protein abundance. The tight control of mid1 expression by Shh, which then further targets Pax6 for degradation, might serve as an important example of how a morphogen is able to accelerate the switch from one cell fate to another above the pure genetic regulation of transcription factors when time becomes limited during development.
Materials and Methods
Xenopus Embryos.
Production and rearing, whole mount in situ hybridization, and morpholino and RNA microinjections were done as previously described (51). All procedures were performed according to guidelines set by the German animal use and care laws (Tierschutzgesetz) and approved by the German state administration Saxony-Anhalt (Projekt/AZ: 42502–3-600 MLU). Details are presented in SI Materials and Methods.
Lipofection.
The retinoblasts-targeted lipofection was performed with NF stage 17/18 Xenopus embryos according to the protocol from Ohnuma et al. with minor modifications (52). Details are presented in SI Materials and Methods.
Plasmids, Morpholinos, Antibodies, and Chemicals.
Description of morpholinos (Table S1), plasmids (Table S2), antibodies (Table S3) can be found in SI Materials and Methods. Morpholino specificity has been addressed (Fig. S2). If not otherwise stated, drugs and chemicals were purchased from the Carl Roth GmbH.
Table S1.
Morpholinos were obtained from Gene Tools
Table S2.
Plasmids for transfection, lipofection, mRNA, and WMISH probe preparation
| Plasmid | Source |
| mid1 in pCMV-Sport6 | RZPD, CF239162 |
| mid1nls in pCS2+ | Cloned PCR fragment |
| Human Mid1 in pCMV-Sport6 | ImaGenes, BC053626 |
| mid1mo2bs-gfp-reporter in pCS2+ | Cloned PCR fragment |
| mid1mo2mut-gfp-reporter in pCS2+ | Cloned PCR fragment |
| mid1 in pBsk(−) | ImaGenes, BJ087663 |
| Gfp in pCS2+ | Gift from David Turner, Fred Hutchinson Cancer Research Center, Seattle |
| rhodopsin in pGEM-T | Cloned PCR fragment |
| brn3.0 in pBlueScript | Gift from Rob Grainger, University of Virginia, Charlottesville, VA |
| vsx1 in pGEM-T | Cloned PCR fragment |
| prox1 in pGEM-T | Cloned PCR fragment |
| pax2 in pGEM-T | Cloned PCR fragment |
| pax2 in pCS2+-MT | Gift from Dietmar Gradl, Karlsruhe Institute of Technology, Karlsruhe, Germany |
| pax2mo1bs-gfp-reporter in pCS2+ | Cloned PCR fragment |
| pax2mo1mut-gfp-reporter in pCS2+ | Cloned PCR fragment |
| pax6 in pCS2+ | Gift from Nicolas Hirsch, University of Virginia, Charlottesville, VA |
| pax6mo1bs-gfp-reporter in pCS2+ | Cloned PCR fragment |
| pax6mo1mut-gfp-reporter in pCS2+ | Cloned PCR fragment |
| Mouse Pax6-flag in pCS2+ | Gift from Anastassia Stoykova, Max Planck Institute for Biophysical Chemistry, Göttingen, Germany |
| ptc1 in pCS2+ | Cloned PCR fragment |
| his-Ubiquitin in pDNA3.1 | Gift from Dirk Bohmann, University of Rochester Medical Center, Rochester, NY |
| myc-human Mid1 in pCS2+ | Cloning of hsMid1 PCR product into pCS2+ MT |
| Gfp-pax6 in peGFP-N | Cloning of pax6 PCR product into peGFPN |
| GST-pax6 in pGex-4-T1 | Cloning of pax6 PCR product into pGex-4-T1 |
Table S3.
Antibodies
| Antibody | Purpose | Source |
| anti-Pax6, rabbit | IHC | Covance |
| anti-Pax6, mouse | IHC, WB | Merck Millipore |
| anti-Pax6, rabbit | WB | ThermoFisher |
| anti-GAPDH, mouse | WB | Sigma-Aldrich |
| anti-Ubiquitin, rabbit | WB | Santa-Cruz BT |
| anti-Rhodopsin, rabbit | IHC | Merck Millipore |
| anti-myc, rabbit | IP, IHC | Sigma-Aldrich |
| anti-myc, mouse | WB | Sigma-Aldrich |
| anti-his-HRP coupled | WB | Merck Millipore |
| anti-his, mouse | WB | Sigma-Aldrich |
| anti-flag, rabbit | IP | Sigma-Aldrich |
| anti-flag, mouse | IHC, WB | Sigma-Aldrich |
| anti-b-tubulin, mouse | WB | Merck Millipore |
| anti-Topoisomerase 1, rabbit | WB | Merck Millipore |
| Peroxidase-conj. goat anti-rabbit IgG | WB | Sigma-Aldrich |
| Alexa 488 goat anti-mouse | IHC | Invitrogen, Life Technologies |
| Alexa 594 goat anti-rabbit | IHC | Invitrogen, Life Technologies |
Cell Cultures and Transfections.
HeLa, αTN4-1, and HEK29 cells were maintained in DMEM supplemented with 10% (vol/vol) FCS. Details are presented in SI Materials and Methods.
Immunohistochemistry.
HeLa cells were seeded on glass coverslips 24 h prior to transfection and fixed in paraformaldehyde 48 h after transfection. To detect Pax6 in Xenopus embryos, cryostat sections were taken. Fluorescence images were documented with a microscope Nikon Eclipse E600. Details are presented in SI Materials and Methods.
Coimmunoprecipitation and GST pull down.
GST pull down and coimmunoprecipitation assays were done according to standard procedures. The details of these assays are provided in SI Materials and Methods.
Protein Expression, in Vivo Ubiquitination Assay, and Pax6 Protein Levels over Time.
HEK293 cells were cotransfected with pax6-flag with either myc-mid1 or EV. Inhibitors were applied after 24 h for 24 h (MG132, 20 µM, lactacystin; Sigma; 1 µM) or 32 h after transfection for an additional 16 h (Pyr 41; Boston Biochem; 20 µM; Z-VAD-FMK; Sigma; 20 µM) before harvesting. Lysates were separated by SDS/PAGE and probed for Pax6 protein. Details of these assays are provided in SI Materials and Methods.
Nuclear and Cytoplasmic Fractionation.
To separate cytosol, soluble nuclear proteins, and the insoluble membrane/DNA fraction, transfected HEK293 cells were collected in PBS. The pellets and nuclei were sedimented by centrifugation following standard procedures. Details are presented in SI Materials and Methods.
Statistical Analysis.
Results are presented as means ± SD. Statistical differences were evaluated using Student t test or χ2 test. Cell culture, pull down, Western blot, and PCR experiments were repeated at least three times, and a representative experiment is shown. Quantification of Western blots was done using ImageJ software (NIH).
SI Materials and Methods
Animals, Microinjections, and Animal Cap Explants.
Pigmented and albino Xenopus laevis were obtained from Nasco. Production, rearing, and staging of embryos was as previously described (33). In case of loss of function experiments, antisense-morpholino-oligonucleotides (mo) were designed and ordered from GeneTools. For control injections, the standard control morpholino from GeneTools are listed in Table S1. Sequences are listed in the supplement. All morpholinos were dissolved in DEPC-H2O and stored in aliquots at −20 °C. For all experiments, 2.5 pmol morpholinos or synthetic RNA (500 pg shh; 100 pg pax2; 100 pg noggin; 1 or 2 ng mid1) RNA was injected unilaterally into one blastomere at the two-cell stage (6). Synthetic β-gal (100 pg) RNA was coinjected as a lineage tracer in all experiments. Capped human mid1 and Xenopus tropicalis mid1, Xenopus laevis pax2, and noggin mRNA was generated using the mMESSAGE mMACHINE kit (Ambion). NotI linearized pCS2+ containing the gene was used as a template for SP6 transcription, and 5 nl capped mRNA was injected along with the morpholino into one blastomere of a two-cell stage embryo. The noninjected side served as internal control. Animal caps were excised with the help of a gastromaster (XENOTEK) at stage 8.5 in 0.5× Modified Barth's saline (MBS), and sets of 30–40 caps were cultured for 24 h at 18–22 °C in 0.5× MBS supplemented with penicillin/streptomycin.
Lipofection.
The retinoblasts-targeted lipofection was performed with NF stage 17/18 Xenopus embryos according to the protocol from Ohnuma et al. (52). Plasmids were mixed with 3 µL N-(2,3-dioleoyloxy-1-propyl)trimethylammonium methyl sulfate (DOTAP) (Roche)/µg DNA directly before loading the mixture into a glass needle (same as microinjection). A plasmid coding for GFP was either mixed with Mid1 coding plasmids as lineage marker to identify lipofected cells or alone as negative control. Embryos were arranged with the anterior side upward, the opened tip of the needle was introduced into the embryo’s retinal area just underneath the epidermis, and 4–10 nL lipofection mixture was injected using several pulses. After injection, embryos were kept in a 0.1× MBS solution until NF stage 41–42, fixed with 4 g/100 ml (4%) paraformaldehyde, and embedded for cryostat sections (Microm HM500 OM).
Whole Mount in Situ Hybridization.
To analyze the spatiotemporal expression of the respective marker genes, Xenopus embryos were fixed at different developmental stages with paraformaldehyde, and whole-mount in situ hybridization was carried out as previously described (32). For a more detailed analysis of gene expression, we performed gelatin/albumin sections of stained embryos with a thickness of 25 µm using a vibratome (Leica). Digoxigenin (DIG) labeled antisense probes of mid1, pax2, pax6, ptc1, brn3.0, vsx1, prox1, and rhodopsin were made from plasmids brn3.0/pBlueScript (BamH1/T7), vs.x1/pGEM-T (Not1/T7), prox1/pCR2.1 (BamH1/T7), rhodopsin/pGEM-T (Not1/T7), mid1/pCS2+ (ClaI/T3), ptc1/pGEM-T (Not1/T7), pax6/pCS2+ (HindIII/T7), and pax2/pCS2+ (HindIII/T7), respectively. X-gal staining was carried out before whole mount in situ hybridization.
Plasmids, Antibodies, and Chemicals.
Description of plasmids (Table S2) and antibodies (Table S3) can be found in the supplemental material. If not otherwise stated, drugs and chemicals were purchased from the Carl Roth GmbH.
Cell Cultures and Transfections.
HeLa, αTN4-1, and HEK29 cells were maintained in DMEM supplemented with 10 ml/100 ml (10%) FCS. All media and sera were purchased from PAA Laboratories. Transfections were performed using Lipofectamin 2000 (Invitrogen) according to the manufacturer’s protocol, except for HEK293 cells, which were transfected using polyethylenimin (25 kDa, branched; Sigma-Aldrich) with 2.5 μL 10 mM Poly(ethyleneimine) (PEI)/μg DNA in PBS. Empty vector pCS2+ was used to obtain equal amounts of transfected DNA and as a negative (EV) control. If not otherwise stated, cells were harvested 48 h after transfection. To detect Pax6 in lysates of animal caps, ∼30 explants were used per lane.
Immunohistochemistry.
HeLa cells were seeded on glass coverslips 24 h prior to transfection, fixed in paraformaldehyde 48 h after transfection, permeabilized with 0.2% Triton X-100, and blocked with 2 g/100 ml (2%) BSA. Following the incubation with the primary antibodies (anti-flag, mouse, 1:800 + anti-myc, rabbit, 1:400), cells were blocked again and incubated with the secondary antibodies (anti-mouse-Alexa 488, anti–rabbit-Alexa 594, 1:400). Cells were washed three times with PBS after each step except blocking and were finally desalted in H2O, dehydrated with 100% ethanol, and mounted on superfrost glass slides with Mowiol containing DAPI (1:1,000). To detect Pax6-positive cells in the eye region of Xenopus embryos, cryostat sections were rehydrated in PBS, permeabilized, and blocked with permeabilization solution. Thereafter, the first antibody (anti-Pax6, rabbit; Covance) diluted in antibody buffer (1:50) was applied. Afterward, the sections were intensively washed three times with PBS, and the secondary antibody (anti–rabbit-Alexa594) was applied with a dilution of 1:1,000 in PBS. Sections were washed again and mounted with Mowiol containing DAPI (1:1,000). Fluorescence images were documented with a microscope Nikon Eclipse E600 installed with a camera Vosskühler CCD-1300QLN. Sixteen-bit grayscale images with a size of 1,388 × 1,040 pixels were acquired using the AxioVision software under automated exposure settings for all channels. Images were exported as JPG and TIF files either separately for each channel or with combined channels.
Coimmunoprecipitation and GST-Pulldown.
HEK293 cells were transfected with myc-tagged mid1 and harvested in PBS, and proteins were extracted with IP lysis buffer containing protease inhibitors. Fifty micrograms of bacterial expressed and purified GST or GST-Pax6 was added to 1 mL (1 mg protein) lysate and after 1 h at 4 °C under continuous rotation, 50 µL GSH-Sepharose beads were added to the mixture. After 60 min of incubation, the beads were washed three times with IP lysis buffer and once with 0.1 mM Tris, pH 7.4. Proteins were eluted with 2× Laemmli buffer, separated by SDS/PAGE, and analyzed by Western blotting. For coimmunoprecipitation experiments, HEK293 cells were transfected with pax6-flag and myc-mid1 or pax6-flag and myc-mid1 alone, respectively. Cells were harvested in PBS and lysed in IP lysis buffer (with protease inhibitors + 20 µM MG132), and proteins were immunoprecipitated with 2 µg anti-flag antibody and 30 µL Protein A Sepharose per sample. After 2 h at 4 °C under continuous rotation, the beads were washed three times with IP lysis buffer and once with 0.1 mM Tris, pH 7.4. Proteins were eluted with 2× Laemmli buffer. For Western blot analyses, input and eluted proteins were separated by SDS/PAGE and probed with the corresponding antibodies.
Protein Expression, in Vivo Ubiquitination Assay, and Pax6 Protein Levels over Time.
To examine protein expression, αTN4-1 (expresses Pax6 endogenously) or HEK293 cells were transfected with pax6-flag with either myc-mid1 or EV as indicated. Inhibitors were applied 24 h after transfection for an additional 12 or 24 h (MG132, 20 µM; lactacystin; Sigma; 1 µM) or 32 h after transfection for an additional 16 h (Pyr 41; Boston Biochem; 20 µM, Z-VAD-FMK; Sigma; 20 µM) before harvesting. Lysates of harvested cells were separated by SDS/PAGE and probed for Pax6 protein by Western blotting using an anti-Pax6 antibody. As a control for equal loading, blots were subsequently reprobed with anti–β-tubulin antibody. For analysis of Pax6 ubiquitination, HEK293 cells were transfected with plasmids for his-ubi, myc-mid1, and pax6-flag as indicated. Twenty-four hours after transfection, MG132 (20 µM) was added, and cells were incubated for an additional 8 h, harvested in PBS, and lysed in RIPA buffer [with protease inhibitors, 20 µM MG132, 10 mM N-ethylmaleimide (NEM); Sigma-Aldrich], and proteins were immunoprecipitated with 3 µg anti-flag antibody and 50 µL protein A Sepharose per sample. After 2 h at 4 °C under continuous rotation, the beads were washed three times with RIPA buffer and once with 0.1 mM Tris, pH 7.4. Proteins were eluted with 2× Laemmli buffer and were separated by SDS/PAGE and probed with the corresponding antibodies for Western blot analysis. To determine Pax6 protein abundance over time, cycloheximide (40 µg/mL; Sigma-Aldrich) was added to HEK293 cells 24 h after transfection with the indicated combination of plasmids. At different time points, cells were collected in PBS and lysed in RIPA buffer (with protease inhibitor). Five to 20 µg total protein per sample was separated by SDS/PAGE probed with the corresponding antibodies in Western blot analyses. The relative amount of Pax6 protein compared with β-tubulin as loading control was quantified densitometrically using ImageJ software (NIH).
Nuclear and Cytoplasmic Fractionation.
To separate cytosol, soluble nuclear proteins, and the insoluble membrane/DNA fraction, transfected HEK293 cells were collected in PBS. The pellet was resuspended in 5× the volume hypotonic buffer (10 mM Hepes, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5% Nonidet P-40) and incubated for 5 min on ice. Nuclei were sedimented by centrifugation, and the supernatant containing the cytoplasmic fraction was removed. The nuclei were washed two times with PBS and incubated with high salt buffer [20 mM Hepes, pH 7.9, 25 ml/100 ml (25%) glycerol, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA], using 5× the volume of the pellet, for 30 min under continuous rotation at 4 °C to extract the soluble nuclear proteins, which were in the supernatant after centrifugation. The final pellet contained the insoluble membrane/DNA fraction with proteins still bound.
Acknowledgments
We thank J. Herfurth for excellent technical help; Z. Kozmik for aTN4-1 cells; and D. Gradl, A. Brändli, and S. C. Ekker for plasmids. S.R. received a travel grant from the Deutsche Akademische Austauschdienst. This work was supported by the University of Halle and by the Deutsche Forschungsgemeinschaft (HO 1879/3-3). M.P.’s laboratory is supported by grants from L'Agence nationale de la recherche, Retina France, Association Valentin Haüy, and Fondation pour la recherché médicale.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600770113/-/DCSupplemental.
References
- 1.Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113(4):1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 2.Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992;2(3):232–239. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- 3.Gosmain Y, Cheyssac C, Heddad Masson M, Dibner C, Philippe J. Glucagon gene expression in the endocrine pancreas: The role of the transcription factor Pax6 in α-cell differentiation, glucagon biosynthesis and secretion. Diabetes Obes Metab. 2011;13(Suppl 1):31–38. doi: 10.1111/j.1463-1326.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- 4.Shaham O, Menuchin Y, Farhy C, Ashery-Padan R. Pax6: A multi-level regulator of ocular development. Prog Retin Eye Res. 2012;31(5):351–376. doi: 10.1016/j.preteyeres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Cvekl A, Piatigorsky J. Lens development and crystallin gene expression: Many roles for Pax-6. BioEssays. 1996;18(8):621–630. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- 6.Davis N, et al. Pax6 dosage requirements in iris and ciliary body differentiation. Dev Biol. 2009;333(1):132–142. doi: 10.1016/j.ydbio.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121(5):1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- 8.Plaza S, Dozier C, Turque N, Saule S. Quail Pax-6 (Pax-QNR) mRNAs are expressed from two promoters used differentially during retina development and neuronal differentiation. Mol Cell Biol. 1995;15(6):3344–3353. doi: 10.1128/mcb.15.6.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinjan DA, Seawright A, Childs AJ, van Heyningen V. Conserved elements in Pax6 intron 7 involved in (auto)regulation and alternative transcription. Dev Biol. 2004;265(2):462–477. doi: 10.1016/j.ydbio.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14(21):2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grindley JC, Hargett LK, Hill RE, Ross A, Hogan BL. Disruption of PAX6 function in mice homozygous for the Pax6Sey-1Neu mutation produces abnormalities in the early development and regionalization of the diencephalon. Mech Dev. 1997;64(1-2):111–126. doi: 10.1016/s0925-4773(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 12.Glaser T, et al. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994;7(4):463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- 13.Schedl A, et al. Influence of PAX6 gene dosage on development: Overexpression causes severe eye abnormalities. Cell. 1996;86(1):71–82. doi: 10.1016/s0092-8674(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 14.Manuel M, Pratt T, Liu M, Jeffery G, Price DJ. Overexpression of Pax6 results in microphthalmia, retinal dysplasia and defective retinal ganglion cell axon guidance. BMC Dev Biol. 2008;8:59. doi: 10.1186/1471-213X-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267(5205):1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 16.Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126(19):4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- 17.Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998;199(2):185–200. doi: 10.1006/dbio.1998.8939. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch N, Harris WA. Xenopus Pax-6 and retinal development. J Neurobiol. 1997;32(1):45–61. [PubMed] [Google Scholar]
- 19.Macdonald R, et al. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121(10):3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz M, et al. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127(20):4325–4334. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- 21.Mikkola I, Bruun JA, Bjorkoy G, Holm T, Johansen T. Phosphorylation of the transactivation domain of Pax6 by extracellular signal-regulated kinase and p38 mitogen-activated protein kinase. J Biol Chem. 1999;274(21):15115–15126. doi: 10.1074/jbc.274.21.15115. [DOI] [PubMed] [Google Scholar]
- 22.Kim EA, et al. Phosphorylation and transactivation of Pax6 by homeodomain-interacting protein kinase 2. J Biol Chem. 2006;281(11):7489–7497. doi: 10.1074/jbc.M507227200. [DOI] [PubMed] [Google Scholar]
- 23.Yan Q, et al. Sumoylation activates the transcriptional activity of Pax-6, an important transcription factor for eye and brain development. Proc Natl Acad Sci USA. 2010;107(49):21034–21039. doi: 10.1073/pnas.1007866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuoc TC, Stoykova A. Trim11 modulates the function of neurogenic transcription factor Pax6 through ubiquitin-proteosome system. Genes Dev. 2008;22(14):1972–1986. doi: 10.1101/gad.471708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opitz JM. G syndrome (hypertelorism with esophageal abnormality and hypospadias, or hypospadias-dysphagia, or “Opitz-Frias” or “Opitz-G” syndrome)--perspective in 1987 and bibliography. Am J Med Genet. 1987;28(2):275–285. doi: 10.1002/ajmg.1320280203. [DOI] [PubMed] [Google Scholar]
- 26.Quaderi NA, et al. Opitz G/BBB syndrome, a defect of midline development, is due to mutations in a new RING finger gene on Xp22. Nat Genet. 1997;17(3):285–291. doi: 10.1038/ng1197-285. [DOI] [PubMed] [Google Scholar]
- 27.Trockenbacher A, et al. MID1, mutated in Opitz syndrome, encodes an ubiquitin ligase that targets phosphatase 2A for degradation. Nat Genet. 2001;29(3):287–294. doi: 10.1038/ng762. [DOI] [PubMed] [Google Scholar]
- 28.Berti C, Fontanella B, Ferrentino R, Meroni G. Mig12, a novel Opitz syndrome gene product partner, is expressed in the embryonic ventral midline and co-operates with Mid1 to bundle and stabilize microtubules. BMC Cell Biol. 2004;5:9. doi: 10.1186/1471-2121-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aranda-Orgillés B, et al. The Opitz syndrome gene product MID1 assembles a microtubule-associated ribonucleoprotein complex. Hum Genet. 2008;123(2):163–176. doi: 10.1007/s00439-007-0456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du H, et al. MID1 catalyzes the ubiquitination of protein phosphatase 2A and mutations within its Bbox1 domain disrupt polyubiquitination of alpha4 but not of PP2Ac. PLoS One. 2014;9(9):e107428. doi: 10.1371/journal.pone.0107428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schweiger S, et al. The E3 ubiquitin ligase MID1 catalyzes ubiquitination and cleavage of Fu. J Biol Chem. 2014;289(46):31805–31817. doi: 10.1074/jbc.M113.541219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki M, Hara Y, Takagi C, Yamamoto TS, Ueno N. MID1 and MID2 are required for Xenopus neural tube closure through the regulation of microtubule organization. Development. 2010;137(14):2329–2339. doi: 10.1242/dev.048769. [DOI] [PubMed] [Google Scholar]
- 33.Hallonet M, Hollemann T, Pieler T, Gruss P. Vax1, a novel homeobox-containing gene, directs development of the basal forebrain and visual system. Genes Dev. 1999;13(23):3106–3114. doi: 10.1101/gad.13.23.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Chevigny A, et al. miR-7a regulation of Pax6 controls spatial origin of forebrain dopaminergic neurons. Nat Neurosci. 2012;15(8):1120–1126. doi: 10.1038/nn.3142. [DOI] [PubMed] [Google Scholar]
- 35.Klimova L, Kozmik Z. Stage-dependent requirement of neuroretinal Pax6 for lens and retina development. Development. 2014;141(6):1292–1302. doi: 10.1242/dev.098822. [DOI] [PubMed] [Google Scholar]
- 36.Oron-Karni V, et al. Dual requirement for Pax6 in retinal progenitor cells. Development. 2008;135(24):4037–4047. doi: 10.1242/dev.028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farhy C, et al. Pax6 is required for normal cell-cycle exit and the differentiation kinetics of retinal progenitor cells. PLoS One. 2013;8(9):e76489. doi: 10.1371/journal.pone.0076489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweiger S, et al. The Opitz syndrome gene product, MID1, associates with microtubules. Proc Natl Acad Sci USA. 1999;96(6):2794–2799. doi: 10.1073/pnas.96.6.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinson L, et al. Embryonic expression of the human MID1 gene and its mutations in Opitz syndrome. J Med Genet. 2004;41(5):381–386. doi: 10.1136/jmg.2003.014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poot M, Badea A, Williams RW, Kas MJ. Identifying human disease genes through cross-species gene mapping of evolutionary conserved processes. PLoS One. 2011;6(5):e18612. doi: 10.1371/journal.pone.0018612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granata A, Quaderi NA. The Opitz syndrome gene MID1 is essential for establishing asymmetric gene expression in Hensen’s node. Dev Biol. 2003;258(2):397–405. doi: 10.1016/s0012-1606(03)00131-3. [DOI] [PubMed] [Google Scholar]
- 42.Zaki P, et al. Penetrance of eye defects in mice heterozygous for mutation of Gli3 is enhanced by heterozygous mutation of Pax6. BMC Dev Biol. 2006;6:46. doi: 10.1186/1471-213X-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinhardt R, et al. Sox2, Tlx, Gli3, and Her9 converge on Rx2 to define retinal stem cells in vivo. EMBO J. 2015;34(11):1572–1588. doi: 10.15252/embj.201490706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis-Silberman N, et al. Genetic dissection of Pax6 dosage requirements in the developing mouse eye. Hum Mol Genet. 2005;14(15):2265–2276. doi: 10.1093/hmg/ddi231. [DOI] [PubMed] [Google Scholar]
- 45.Marquardt T, et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105(1):43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 46.Zaghloul NA, Moody SA. Changes in Rx1 and Pax6 activity at eye field stages differentially alter the production of amacrine neurotransmitter subtypes in Xenopus. Mol Vis. 2007;13:86–95. [PMC free article] [PubMed] [Google Scholar]
- 47.Hsieh YW, Yang XJ. Dynamic Pax6 expression during the neurogenic cell cycle influences proliferation and cell fate choices of retinal progenitors. Neural Dev. 2009;4:32. doi: 10.1186/1749-8104-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toy J, Norton JS, Jibodh SR, Adler R. Effects of homeobox genes on the differentiation of photoreceptor and nonphotoreceptor neurons. Invest Ophthalmol Vis Sci. 2002;43(11):3522–3529. [PubMed] [Google Scholar]
- 49.Ashery-Padan R, Gruss P. Pax6 lights-up the way for eye development. Curr Opin Cell Biol. 2001;13(6):706–714. doi: 10.1016/s0955-0674(00)00274-x. [DOI] [PubMed] [Google Scholar]
- 50.Xu S, et al. The proliferation and expansion of retinal stem cells require functional Pax6. Dev Biol. 2007;304(2):713–721. doi: 10.1016/j.ydbio.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metikala S, Neuhaus H, Hollemann T. Suppression of vascular network formation by chronic hypoxia and prolyl-hydroxylase 2 (phd2) deficiency during vertebrate development. Angiogenesis. 2016;19(2):119–131. doi: 10.1007/s10456-015-9492-3. [DOI] [PubMed] [Google Scholar]
- 52.Ohnuma S, Mann F, Boy S, Perron M, Harris WA. Lipofection strategy for the study of Xenopus retinal development. Methods. 2002;28(4):411–419. doi: 10.1016/s1046-2023(02)00260-8. [DOI] [PubMed] [Google Scholar]
- 53.Koenig SF, et al. En2, Pax2/5 and Tcf-4 transcription factors cooperate in patterning the Xenopus brain. Dev Biol. 2010;340(2):318–328. doi: 10.1016/j.ydbio.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Thélie A, et al. Prdm12 specifies V1 interneurons through cross-repressive interactions with Dbx1 and Nkx6 genes in Xenopus. Development. 2015;142(19):3416–3428. doi: 10.1242/dev.121871. [DOI] [PMC free article] [PubMed] [Google Scholar]