Abstract
BACKGROUND
We compared the cost-effectiveness of hypertension treatment in non-Hispanic blacks and non-Hispanic whites according to 2014 US hypertension treatment guidelines.
METHODS
The cardiovascular disease (CVD) policy model simulated CVD events, quality-adjusted life years (QALYs), and treatment costs in 35- to 74-year-old adults with untreated hypertension. CVD incidence, mortality, and risk factor levels were obtained from cohort studies, hospital registries, vital statistics, and national surveys. Stage 1 hypertension was defined as blood pressure 140–149/90–99mm Hg; stage 2 hypertension as ≥150/100mm Hg. Probabilistic input distribution sampling informed 95% uncertainty intervals (UIs). Incremental cost-effectiveness ratios (ICERs) < $50,000/QALY gained were considered cost-effective.
RESULTS
Treating 0.7 million hypertensive non-Hispanic black adults would prevent about 8,000 CVD events annually; treating 3.4 million non-Hispanic whites would prevent about 35,000 events. Overall 2014 guideline implementation would be cost saving in both groups compared with no treatment. For stage 1 hypertension but without diabetes or chronic kidney disease, cost savings extended to non-Hispanic black males ages 35–44 but not same-aged non-Hispanic white males (ICER $57,000/QALY; 95% UI $15,000–$100,000) and cost-effectiveness extended to non-Hispanic black females ages 35–44 (ICER $46,000/QALY; $17,000–$76,000) but not same-aged non-Hispanic white females (ICER $181,000/QALY; $111,000–$235,000).
CONCLUSIONS
Compared with non-Hispanic whites, cost-effectiveness of implementing hypertension guidelines would extend to a larger proportion of non-Hispanic black hypertensive patients.
Keywords: blood pressure, cost-effectiveness, hypertension, race.
Hypertension awareness and treatment improved in the United States over the last 2 decades, contributing to a nearly 50% improvement in the proportion of hypertensive patients with controlled blood pressure (BP).1 Hypertension treatment, control, and awareness improved in non-Hispanic blacks, but hypertension prevalence and the proportion of uncontrolled BP in non-Hispanic black hypertensive patients remain higher compared with non-Hispanic whites. Rates of deaths attributable to hypertension have increased over time in non-Hispanic black men, in whom mortality rates are double that of non-Hispanic white men.2 Hypertension awareness and treatment also continue to be lowest in young adults,1 and hypertension screening and control have been especially difficult to implement in young non-Hispanic black men.3,4
Our recent cost-effectiveness modeling study found that implementing 2014 US hypertension guidelines would be cost saving overall in untreated hypertensive adults aged 35–74 years old, but cost-effectiveness did not extend to primary of 35- to 44-year-old women with stage 1 hypertension for primary cardiovascular disease (CVD) prevention.5 Given their higher CVD risk, we hypothesized that hypertension treatment would be more cost-effective in young and middle-aged non-Hispanic blacks compared with same-aged non-Hispanic whites. We therefore compared the cost-effectiveness of implementing 2014 hypertension guidelines in untreated non-Hispanic black and non-Hispanic white patients overall and by age, sex, hypertension stage, and CVD, diabetes, and chronic kidney disease (CKD) status.
METHODS
CVD policy model
The CVD policy model is a computer-simulation, state-transition model of incidence, prevalence, mortality, and costs of CVD in the United States (Supplementary Figure). The primary study population included non-Hispanic black and white men and women aged 35–74 in 2014 who were not receiving pharmacotherapy for hypertension. We restricted our analysis to currently untreated hypertensive patients because we were not able to discern among different causes of uncontrolled BP in already treated patients (financial obstacles, nonadherence, side effects, or contraindications due to comorbidities). Adults aged 75 years and older were excluded from this analysis due to highly variable rates of medication-related adverse events.6 We were unable to analyze Hispanic Americans or Asian Americans at this time due to insufficient contemporary CVD incidence data for these heterogeneous groups.
Means or proportions and joint distributions of risk factors, including systolic and diastolic BP, cholesterols, and hypertension medication use, smoking, diabetes, and CKD status, were estimated from pooled National Health and Nutrition Examination Surveys (NHANES) 2007–2010 by 10-year age categories, sex, and race/ethnic group. NHANES 2007–2010 systolic BP was categorized into 3 main categories: <140, 140–159 (stage 1 hypertension), and ≥160mm Hg (stage 2 hypertension). To assess 2014 guideline treatment recommendations in older adults (ages 60–74 years), stage 1 systolic BP was further divided into the intervals 140–149 and 150–159mm Hg. Diastolic BP categories were <90, 90–99 (stage 1), and ≥100mm Hg (stage 2). Hypertensive patients were considered untreated if they answered no to both NHANES questions: “Because of your high blood pressure/hypertension, have you ever been told to take prescribed medicine?” and “Are you currently taking medication to lower your blood pressure?”
Default multivariate stroke and coronary heart disease incidence functions were estimated in an original Framingham Heart Study analysis. The CVD policy model simulated CVD events, non-CVD deaths, quality-adjusted life years (QALYs), and hypertension and CVD treatment costs of implementing 2014 US treatment guidelines in untreated hypertensive non-Hispanic black and white adults aged 35–74 years compared with no treatment.
Race/ethnic-specific CVD policy models
While the structure of both the non-Hispanic black and non-Hispanic white CVD policy model versions is identical to that of the national model, several inputs were re-populated with data specific to non-Hispanic blacks and non-Hispanic whites (for details, see Supplementary Text).
Population projections for the years 2014–2024 were obtained from 2010 US Census Bureau. National Health Interview Survey data were used to estimate the proportion of adults with known coronary heart disease, stroke, or both these conditions. All beta coefficients for the effect of non-BP risk factors on incident stroke (diabetes, CKD, and smoking) and on incident coronary heart disease (cholesterols, diabetes, CKD, and smoking) were estimated from Framingham Heart Study data. Numbers of 2010 CVD deaths (stroke (ICD-10 codes I60–I69), coronary heart disease (I20–I25 and two-thirds of I49, I50, and I51), and hypertensive heart disease deaths (I11.0, I11.9)) and non-CVD deaths (remainder of ICD codes) reported by the Centers for Disease Control (CDC WONDER) for 2010 were used as model calibration targets.
Hospitalized stroke incidence in non-Hispanic whites was based on rates observed in the 2010 National Hospital Discharge Survey (NHDS). Since NHDS is missing over 15% of data on self-reported race/ethnicity, we were unable to similarly determine the number of total stroke events in non-Hispanic blacks. For non-Hispanic black stroke incidence rates, we first adjusted national NHDS-based stroke incidence rates for risk factor differences between the United States and racial subpopulations and the stronger association between BP and stroke in non-Hispanic blacks.7 Stroke incidence rate inputs for both groups were calibrated until simulated stroke deaths predicted 2010 national stroke mortality numbers within 1% (Supplementary Table 1). The resulting incidence of hospitalized stroke approximated age- and sex-specific stroke incidence rates observed in stroke cohorts and surveillance studies of non-Hispanic blacks and whites.8–13
Non-Hispanic white age-range/gender-specific coronary heart disease incidence rates were estimated by calibrating incidence to match with cause-specific coronary heart disease mortality in 2010. Coronary heart disease incidence for non-Hispanic blacks was estimated by multiplying white age and sex incidence rates by black/white incidence ratios observed during the 2005–2011 interval in the Atherosclerosis Risk in Communities (ARIC) surveillance study. Coronary heart disease incidence rate inputs for both groups were calibrated until simulated coronary heart disease deaths predicted 2010 national mortality numbers within 1% (Supplementary Table 1).
Thirty-day acute myocardial infarction case fatality was assumed to be similar for non-Hispanic blacks and whites in 2010.14 Stroke 30-day case fatality rates for non-Hispanic blacks were assumed to be higher than national averages based on an observed difference between white and black case fatality rates in the ARIC cohort study.8 Annual probabilities of stroke after myocardial infarction15 and the probability of coronary heart disease in stroke patients were based on natural history studies and were assumed to be the same for both race/ethnic groups (Supplementary Text). Deaths, disability, and costs of heart failure due to coronary heart disease were tabulated by the CVD policy model. Deaths attributable to heart failure due to hypertensive heart disease (and not due to coronary heart disease) were tabulated, but chronic costs and nonfatal disability due to hypertensive heart disease were not.
Hypertension treatment model inputs
The 2014 US hypertension guidelines (“JNC 8”) recommended a goal of <140/90mm Hg for diabetes and/or CKD, diastolic BP < 90 if age under 60 years and BP < 150/90 if age > 60 years without diabetes or CKD.16 The number of US non-Hispanic black and white adults eligible for treatment under JNC 8 was estimated using these categories and BP and treatment status information in NHANES 2007–2010.
BP change due to antihypertensive medications was determined by pretreatment BP and the number of standard doses of medications needed to reach the guideline BP goal, according to a trials-based formula.17 BP changes were calculated based on pretreatment BP, age, and sex. We assumed the same BP reduction per standard dose of the main drug classes (Table 1; Supplementary Text).17,18 Quality of life penalties were applied for side effects.17 Treatment costs included hypertension monitoring costs, drug side effects, and the average wholesale cost of drugs. We assumed the 75% adherence observed in the same clinical trials meta-analysis that provided the association between BP reduction and CVD risk reduction.18 In our prior analysis, lower adherence reduced effectiveness and costs proportionally; thus, lower adherence reduced population impact but had little effect on cost-effectiveness.5
Table 1.
Main assumptions and uncertainty ranges used for the comparative effectiveness analysis of US BP treatment guidelines
| Variable | Estimate (range in upper and lower bound estimates if a variation assumed according to age and/or sex) | Sources | ||
|---|---|---|---|---|
| Main | Lower | Upper | ||
| Effectiveness | ||||
| Average RR per 5mm Hg reduction in diastolic BP or 10mm Hg reduction in systolic BP, ages 35–59 yearsa | ||||
| CHD | 0.73 (0.72–0.74) | 0.70 (0.67–0.72) | 0.77 (0.76–0.78) | Law, Morris, and Wald meta-analysis18 |
| Stroke | 0.64 (0.61–0.66) | 0.59 (0.54–0.63) | 0.69 (0.68–0.69) | |
| All-cause mortality | 0.86 (0.83–0.89) | 0.76 | 0.95 | |
| Average RR per 10mm Hg reduction in systolic BP or 5mm Hg reduction in diastolic BP, ages 60–74 yearsa | ||||
| CHD | 0.77 (0.74–0.78) | 0.74 (0.72–0.76) | 0.79 (0.76–0.81) | Law, Morris, and Wald meta-analysis,18 Systolic Hypertension in the Elderly Program (SHEP) all-cause mortality upper bound 95% interval for upper estimate19 |
| Stroke | 0.69 (0.66–0.71) | 0.64 (0.62–0.64) | 0.74 (0.69–0.78) | |
| All-cause mortality | 0.91 (0.91–0.92) | 0.80 | 1.02 | |
| Average systolic BP-lowering effect (mm Hg)b | ||||
| Stage 2 hypertension | ||||
| Age <60 years, pretreatment ≥ 160mm Hg target 140mm Hg, 3–4 standard dose medications | 31.0–34.7 | 26.0–29.4 | 36.0–39.9 | Law, Morris, and Wald meta-analysis18 |
| Age ≥60 years, pretreatment ≥ 160mm Hg target 150mm Hg, 2–3 standard dose medications | 22.1–24.2 | 18.1–18.9 | 27.2–29.2 | |
| Stage 1 hypertension | ||||
| Age <60 years, pretreatment 140–159mm Hg target 140mm Hg, 0.5–2.0 standard dose medications | 7.9–10.9 | 5.9–8.3 | 9.9–13.4 | |
| Age ≥60 years, pretreatment 150–159mm Hg target 150mm Hg, 0.5 standard dose medications | 7.1 | 3.2 | 11.0 | |
| Diastolic BP-lowering effect (mm Hg)b | ||||
| All ages, stage 2 hypertension (≥100mm Hg) | 17.1 | 12.0 | 22.2 | Law, Morris, and Wald meta- analysis18 |
| All ages (target 90mm Hg, 3 standard dose medications | ||||
| All ages, stage 1 hypertension (90–99mm Hg) | 5.3 | 3.7 | 6.9 | |
| All ages (target 90mm Hg, 1 standard dose medication | ||||
| Annual costs per person treated (2010 costs; inflated to 2014 costs in all results) | ||||
| Physician office visit | ||||
| Treatment monitoring visits (number) | ||||
| Stage 2 hypertension | 4 | 3 | 5 | ALLHAT trial (Heidenreich et al.),31 JNC 7 recommendation. Outpatient visit, Medicare Physician Fee Schedule (code 99213, nonfacility limiting charge)20 |
| Stage 1 hypertension | 3 | 2 | 4 | |
| Cost per routine monitoring visit | $71 | Not modeled | Not modeled | |
| Hospitalization | ||||
| Average cost (used for infrequent hospitalized drug-related adverse events) | $12,000 | Not modeled | Not modeled | National Inpatient Sample survey |
| High cost (used for rare hospitalized drug-related adverse events) | $21,000 | |||
| Laboratory test (electrolytes monitoring on treatment) | ||||
| Number of tests | 1 | 1 | 2 | U.S. Joint National Committee 7 recommendation Centers for Medicare and Medicaid laboratory fee schedule |
| Cost per test | $15 | Not modeled | Not modeled | |
| Antihypertensive drug costs (total daily doses)c | ||||
| 0.5 standard dose | $124 | Not modeled | $296 | Average wholesale prices reported by manufacturers (“Red Book”; 2010),21 see Methods text for estimation method |
| 1.0 standard dose | $166 | $363 | ||
| 1.5 standard doses | $215 | $409 | ||
| 2.0 standard doses | $238 | $567 | ||
| 3.0 standard doses | $357 | $850 | ||
| 3.5 standard doses | $430 | $1,311 | ||
| 4.0 standard doses | $496 | $1,374 | ||
| Pharmacy dispensing feesd | $27 | $33 | ||
| Acute and chronic CVD treatment costs | ||||
| Myocardial infarction hospitalization | ||||
| Nonfatal | $33,000 | California Office of Statewide Health Planning and Development (OSHPD) hospital data, 200822 | ||
| Fatal | $46,000 | |||
| Coronary revascularization procedures | ||||
| Percutaneous coronary intervention | $21,000–$23,000 | |||
| Coronary artery bypass graft surgery | $57,000–$59,000 | |||
| Stroke | ||||
| Fatal | $21,000–$26,000 | |||
| Nonfatal | $15,000–$21,000 | |||
| Chronic CHD costs | ||||
| First year | $11,000 | US Medical Expenditure Panel Surveys (MEPS), 1998–2008 | ||
| Subsequent years | $2,000 | |||
| Chronic post-stroke costs | ||||
| First year | $16,000 | |||
| Subsequent years | $5,000 | |||
| Inflation from 2010 to 2014 costs | 9% | 11% | Main = change in general US consumer price index; upper = change in medical component | |
| Serious adverse effects of medications (incidence per 100,000 person-years) | ||||
| Common, outpatient management | ||||
| 3 standard doses | 10,039.20 | 6,950.21 | 12,742.06 | Based on Law 200317 |
| 2 standard doses | 7,572.41 | 5,242.43 | 9,611.13 | |
| 1 standard dose | 5,200.00 | 3,600.06 | 6,600.00 | |
| 0.5 standard dose | 2,600.00 | 1,800.00 | 3,300.00 | |
| Infrequent, hospitalized | ||||
| 3 standard doses | 193.06 | 19.31 | 965.31 | Trials, medication labels, post- marketing reports |
| 2 standard doses | 145.62 | 14.56 | 728.12 | |
| 1 standard dose | 100.00 | 10.00 | 500.00 | |
| 0.5 standard dose | 50.00 | 5.00 | 250.00 | |
| Rare, hospitalized/severe | ||||
| 3 standard doses | 1.93 | 0.0193 | 19.31 | |
| 2 standard doses | 1.46 | 0.0146 | 14.56 | |
| 1 standard dose | 1.00 | 0.0100 | 10.00 | |
| 0.5 standard dose | 0.50 | 0.0050 | 5.00 | |
| Death | ||||
| 3 standard doses | 0.0193 | 0.0002 | 0.1931 | |
| 2 standard doses | 0.0146 | 0.0001 | 0.1456 | |
| 1 standard dose | 0.0100 | 0.0001 | 0.1000 | |
| 0.5 standard dose | 0.0050 | 0.0001 | 0.0500 | |
| Utility (QALY weight penalty, duration) | ||||
| Drug side effects managed as outpatient (1 day) | 0.23 | Montgomery23 | ||
| Drug side effect requiring hospitalization (1 day) | 0.50 | Clinical judgment | ||
| Acute stroke (1 month) | 0.86 | Global Burden of Disease 2010 Study24 | ||
| Chronic stroke survivors (12 months) | 0.85–0.88 | |||
| Acute myocardial infarction (1 month) | 0.91 | |||
| Acute unstable angina (1 month) | 0.95 | |||
| Chronic CHD (12 months) | 0.91–0.98 | |||
| Death | 1.00 | |||
| Disutility due to taking daily medications | 0.00 | 0.01–0.02 | Past cost-effectiveness analyses20,25,26 | |
| Adherence to medications (percent of patients continuing prescribed treatment) | 75% | 25% or 50% lower than observed in trials | Not modeled | Law, Morris, and Wald meta-analysis for main estimate18 |
| Annual discount rate | 3% | Not modeled | Not modeled | Assumed |
Abbreviations: BP = blood pressure; CHD = coronary heart disease; CVD = cardiovascular disease; QALY = quality-adjusted life year.
aRelative risk reductions vary by age and sex category, see Methods and Supplementary Appendix for details.
bChange in BP dependent on age- and sex-specific distribution of baseline BPs within stage 1 or stage 2 category and number of standard dose antihypertensive medications.
cStandard dose medications used to estimate costs: captopril 25mg twice daily, nifedipine 30mg daily, amlodipine 5mg daily, hydrochlorothiazide 25mg daily, and atenolol 50mg daily. Combination medications equivalent to two-half standard doses were amlodipine/benazepril 2.5/10mg daily and captopril/hydrochlorothiazide 25/25mg daily. Combination medications equivalent to 1.5 standard doses were captopril/hydrochlorothiazide 25/25mg once daily, benazepril/amlodipine 10/2.5mg daily, or hydrochlorothiazide/propranolol 25/80mg daily. Combination medications equivalent to 2 standard doses were atenolol/chlorthalidone 50/25mg daily and captopril/hydrochlorothiazide 50/25mg daily. Of the medications listed, only captopril (angiotensin converting enzyme inhibitor) taken divided doses (25mg twice daily).
dVariation dependent on 90 or 100 pill packaging.
We assumed CVD risk reduction is due to BP reduction,18 and that BP is lowered to a similar extent across classes when comparing per-class standard doses.17,27 We started with observational Prospective Studies Collaboration age-specific relative risks and 95% confidence intervals for coronary heart disease and stroke per 10mm Hg change in systolic BP or 5mm Hg diastolic BP (Table 1).28 Age-specific relative risk inputs were calibrated to be within ≤0.02 of these estimates and overall relative risks within 95% confidence interval bounds of the summary estimate from a large meta-analysis of randomized clinical trials of hypertension treatment (Supplementary Tables 2 and 3).18 The stroke relative risk estimate was found to be close to the pooled estimate from the East Asian trials included in that analysis (0.59 (0.49–0.71); Supplementary Text). The resulting relative risk assumptions were validated for treatment of systolic BP in ages 60–74 years by simulating the treatment and placebo groups of the Systolic Hypertension in the Elderly Program (SHEP) trial and comparing simulated relative rate ratios with those observed in the trial (Supplementary Text; Supplementary Table 4).
Main analysis
A status quo simulation projected CVD events, CVD deaths, and heart failure deaths, costs, and QALYs for non-Hispanic black and white adults aged 35–74 years with untreated hypertension from 2014 to 2024. We then simulated CVD outcomes treating to JNC 8 BP targets in non-Hispanic blacks and whites with subgroup analysis stratified by age groups (under and over 60 years) and status of CVD, CKD, or diabetes. Incremental cost-effectiveness ratios (ICERs) were calculated as change in costs divided by incremental change in QALYs. ICERs < $50,000/QALY gained were considered cost-effective, ≥$50,000 and <$150,000 of intermediate value, and ≥$150,000 of low value.29 All analyses were approached from a payer’s perspective. Future costs and QALYs were discounted at 3% per year. ICERs compared costs and costs savings associated with treating patients to JNC 8 guidelines vs. no treatment within age groups and by CVD, diabetes, and CKD status.
In an exploratory analysis, we simulated alternative scenarios applying nonpharmacologic diet and exercise (lifestyle) interventions alone to lower BP in stage 1 hypertension without diabetes or CKD based on the upper and lower 95% confidence interval bounds of a summary effectiveness estimate from lifestyle intervention trials.30 Reduction in total cardiovascular events due to lifestyle interventions was estimated, but cost analysis for lifestyle intervention was not done due to poor and inconsistent data available on the cost of these interventions.
Multivariate, probabilistic sensitivity analysis
We did not perform 1-way sensitivity analyses since uncertainties surrounding each model input were explored extensively in our prior analysis.5 Uncertainty distributions of the main inputs, including clinical effectiveness of BP lowering, relative risk of CVD associated with BP change, quality of life decrements due to side effects, and costs related to side effects, medications, and monitoring, were randomly sampled 1,000 times in probabilistic simulations. From these results, we calculated 95% uncertainty intervals (UIs) for all model outputs.
RESULTS
About 130,000 non-Hispanic blacks and 570,000 non-Hispanic whites with CVD and hypertension but not currently treated with medications would be eligible for secondary prevention every year during the 2014–2024. Treating these patients was estimated to prevent about 2,000 and 10,000 CVD events in black and white populations, respectively (Table 2). Additionally, 1.7 million non-Hispanic blacks and 5.4 million non-Hispanic whites aged 35–74 years with hypertension were eligible for primary prevention treatment. Treating these patients to 2014 guideline targets was projected to prevent about 6,000 and 25,000 CVD events in non-Hispanic black and non-Hispanic white populations, respectively (Table 2).
Table 2.
Annual population treated and cost-effectiveness of implementing JNC 8 hypertension treatment guidelines in previously untreated non-Hispanic black (NHB) and non-Hispanic white (NHW) adults with hypertension aged 35–74 years. Average annual results from a years 2014–2024 simulation, the CVD policy model.
| Strategy | Annual incremental results | ||||
|---|---|---|---|---|---|
| Number of people newly treated | CVD events averted compared with status quo | CVD costs compared with status quo ($US, thousands) | ICER compared with status quo ($US per QALY gained) | ICER compared with prior strategy ($US per QALY gained) | |
| NHB patients | |||||
| NHB women ages 35–74 years | |||||
| Treat CVD population | 35,000 | −1,000 (1,000; 1,000) | −48,000 (−70,000; −31,000) | Cost savinga | Cost savinga |
| Treat stage 2 along with CVD | 484,000 | −2,000 (2,000; 3,000) | −103,000 (−142,000; −73,000) | Cost savinga | Cost savinga |
| Treat stage 1 with diabetes or CKD, along with CVD and stage 2 | 703,000 | −3,000 (2,000; 3,000) | −112,000 (−157,000; −76,000) | Cost savinga | Cost savinga |
| Treat all hypertension | 855,000 | −3,000 (3,000; 4,000) | −115,000 (−164,000; −76,000) | Cost savinga | Cost saving (cost saving; 4,000) |
| NHB men ages 35–74 years | |||||
| Treat CVD population | 96,000 | −1,000 (1,000; 2,000) | −100,000 (−148,000; −67,000) | Cost savinga | Cost savinga |
| Treat stage 2 along with CVD | 592,000 | −4,000 (3,000; 5,000) | −258,000 (−336,000; −193,000) | Cost savinga | Cost savinga |
| Treat stage 1 with diabetes or CKD, along with CVD and stage 2 | 698,000 | −4,000 (4,000; 6,000) | −294,000 (−386,000; −219,000) | Cost savinga | Cost savinga |
| Treat all hypertension | 940,000 | −5,000 (4,000; 7,000) | −337,000 (−446,000; −244,000) | Cost savinga | Cost savinga |
| NHW patients | |||||
| NHW women ages 35–74 years | |||||
| Treat CVD population | 86,000 | −4,000 (3,000; 5,000) | −163,000 (−240,000; −105,000) | Cost savinga | Cost savinga |
| Treat stage 2 along with CVD | 785,000 | −10,000 (8,000; 12,000) | −286,000 (−431,000; −160,000) | Cost savinga | Cost savinga |
| Treat stage 1 with diabetes or CKD, along with CVD and stage 2 | 1,527,000 | −11,000 (9,000; 13,000) | −273,000 (−440,000; −130,000) | Cost savinga | 6,000 (−4,000; 18,000) |
| Treat all hypertension | 2,559,000 | −13,000 (10,000; 15,000) | −203,000 (−399,000; −43,000) | Cost savinga | 19,000 (9,000; 31,000) |
| NHW men ages 35–74 years | |||||
| Treat CVD population | 482,000 | −6,000 (5,000; 8,000) | −455,000 (−702,000; −292,000) | Cost savinga | Cost savinga |
| Treat stage 2 along with CVD | 1,368,000 | −16,000 (12,000; 20,000) | −884,000 (−1,269,000; −597,000) | Cost savinga | Cost savinga |
| Treat stage 1 with diabetes or CKD, along with CVD and stage 2 | 1,961,000 | −19,000 (14,000; 24,000) | −964,000 (−1,399,000; −632,000) | Cost savinga | Cost savinga |
| Treat all hypertension | 3,436,000 | −22,000 (18,000; 28,000) | −971,000 (−1,473,000; −581,000) | Cost savinga | Cost savinga |
Abbreviations: CKD = chronic kidney disease; CVD = cardiovascular disease; ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life year.
aUpper and lower uncertainty interval bounds of ICERs were cost saving.
Hypertension treatment was cost saving overall in both non-Hispanic blacks and non-Hispanic whites compared with no treatment (Table 2). Incremental addition of treatment of stage 1 hypertension for primary prevention to treatment of CVD and stage 2 patients was also cost-effective in both groups overall.
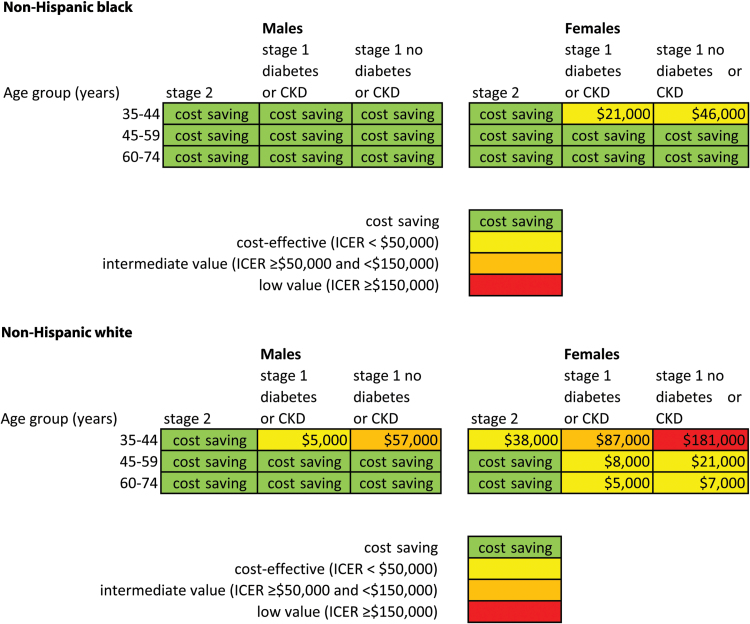
When treatment was assessed in progressively less cost-effective strategies, cost savings were observed in a greater proportion of subgroups in the non-Hispanic black population compared with the non-Hispanic white adults. In non-Hispanic blacks, treating hypertension for primary prevention in males of all ages and BP stages was cost saving, regardless of diabetes or CKD status (Figure 1). Treatment was also cost saving in all non-Hispanic black women with stage 2 hypertension and in those aged 45–74 years with stage 1 hypertension. Treating non-Hispanic black women aged 35–44 years with stage 1 hypertension was cost-effective both in those with diabetes or CKD ($21,000/QALY; 95% UI $3,000–$43,000) and in those without diabetes or CKD ($46,000/QALY; 95% UI $17,000–$76,000).
Figure 1.
Incremental cost-effectiveness of implementing JNC 8 hypertension treatment guidelines in previously untreated non-Hispanic black (NHB) and non-Hispanic white (NHW) adults with hypertension aged 35–74 years, by age, sex, hypertension stage, and status of diabetes or chronic kidney disease (CKD). Within age and sex row, each group is compared with the higher risk group to its left; stage 2 hypertension is compared with patients living with cardiovascular disease (CVD) and hypertension. Average annual results from a years 2014–2024 simulation, the CVD policy model. Abbreviation: ICER = incremental cost-effectiveness ratio.
Treating hypertension was cost saving in all non-Hispanic white men with stage 2 hypertension and those aged 45–74 years with stage 1 hypertension. Treatment was cost-effective in non-Hispanic white males aged 35–44 years with stage 1 hypertension and diabetes or CKD with an ICER of about $5,000/QALY gained (95% UI from cost saving to $30,000), but of only intermediate value in those with stage 1 hypertension but without diabetes or CKD (ICER of about $57,000/QALY; 95% UI $15,000–$100,000). In non-Hispanic white females, hypertension treatment was only cost saving in those 45–74 years old and with stage 2 hypertension. Treating non-Hispanic white women aged 35–44 years with stage 2 hypertension or those aged 45–74 years with stage 1 hypertension was cost-effective (Figure 1). Treating hypertension in white women aged 35–44 years with stage 1 hypertension was of intermediate or low value (ICERS ≥ $50,000/QALY; 95% UI $46,000–$121,000 with diabetes or CKD, $111,000–$235,000 without diabetes or CKD).
For non-Hispanic whites with stage 1 hypertension but without diabetes or CKD, lifestyle interventions alone would result in about 1,000–3,000 CVD events avoided per year, less than the approximately 5,000 events prevented by pharmacologic treatment alone in the same patients according to JNC 8. For non-Hispanic blacks, lifestyle intervention alone in stage 1 patients without diabetes or CKD would prevent <1,000 CVD events (about 300–700 fewer), less than the approximately 1,000 events that would be prevented by pharmacologic treatment alone.
DISCUSSION
Implementation of 2014 hypertension treatment guideline goals would reduce CVD morbidity and mortality while saving costs overall in both Non-Hispanic whites and blacks with untreated hypertension. Compared with non-Hispanic whites, cost savings and cost-effectiveness extended to younger ages in non-Hispanic black patients with stage 1 hypertension. In the short term, implementing guideline goals would be cost-effective in 35- to 44-year-old non-Hispanic black females with stage 1 hypertension, but appeared to be of intermediate or low value in non-Hispanic white females of the same age and hypertension stage.
Over 41% of non-Hispanic blacks aged ≥18 years have hypertension, more than 10 percentage points higher than other US race/ethnic groups.32 Awareness and treatment have improved in all US adults in the past 2 decades,1 but even now only about a half of non-Hispanic whites have controlled hypertension (50.3%), and the proportion controlled is almost 9 percentage points lower in non-Hispanic blacks (41.4%).33 Uncontrolled hypertension is a leading cause of death and disability in the non-Hispanic black population, and non-Hispanic black men have the highest rates of hypertension-related death (hypertensive heart disease, other heart diseases, and stroke) of any race/ethnic group.3 Hypertension treatment and control in non-Hispanic black adults is therefore an urgent national public health priority. Our results suggest that implementing hypertension treatment guidelines in younger non-Hispanic black adults is a high-value strategy for health system payers, yielding cost savings in older and higher risk patients, and cost-effectiveness in younger patients.
Clinical practice-based studies have demonstrated that intensive focus on hypertension treatment can lead to remarkable improvements in BP control.34 Investment in a team-based approach—supplementing usual care with intensive interactions between patient and a nonphysician health care provider—may achieve substantial gains.35 Non-Hispanic black men aged 21–54 years old in inner-city Baltimore who received educational-behavioral-pharmacologic intervention from a team including a nurse practitioner and community health worker had a >10% higher proportion of controlled hypertension after a 36-month intervention, compared with those receiving usual care.36 A systematic clinic-based quality improvement intervention in the 15 Veteran’s Administration medical centers achieved a >30% point improvement in hypertension control over 10 years, with similar improvements in non-Hispanic black and white patients.37 A multicomponent intervention combining patient education, home BP monitoring, and lifestyle counseling in low-income non-Hispanic black patients (72% female) did not improve hypertension control compared with usual care—in part perhaps because the complexity of the intervention led to <50% adherence to component interventions.38 The results of the SPRINT trial add to decades of evidence demonstrating that BP-lowering therapies save lives.18,39 Further research is needed on the most effective ways to deliver this highly effective and cost-effective therapy to all patients seen in clinical practice, including non-Hispanic black patients. Lifestyle interventions alone in stage 1 patients without diabetes or CKD would prevent a more modest number of CVD events compared with pharmacologic therapy alone; however, lifestyle measures are likely to augment prevention benefits when combined with pharmacologic treatment or serve as an alternative for patients who decline to take medications.
Due in part to documented barriers to accessing and accepting treatment, a significant proportion of non-Hispanic black patients do not regularly seek hypertension and other preventive care in medical clinics.3 Recent initiatives have tested the strategy of diagnosing and initiating treatment in non-Hispanic black adults outside of the clinic and in other community settings, including barbershops, community centers, and faith-based organizations.3 In the intervention arm of a trial in Dallas, barbers in 17 black-owned barbershops were trained to offer BP checks, deliver health education, and refer hypertensive patients to medical care.40 After 10 months, hypertension control was 8.8 percentage points higher in the intervention, compared with the usual care arm. The Georgia Stroke and Heart Attack Prevention Program (SHAPP) is a statewide initiative focused on hypertension screening and control in 16 predominantly rural public health districts that are about 56% non-Hispanic black. Through provider training, affordable medications, adherence support, and community partnerships,41 SHAPP achieved approximately 60% hypertension control and was projected to save costs compared with no treatment.42 Our analysis provides further evidence supporting the value of investing in both clinic- and community-based interventions to improve hypertension control in non-Hispanic blacks and other high-risk groups with high hypertension prevalence.
The methods and reporting of this study conform to Consolidated Health Economic Evaluation Reporting Standards (CHEERS; see checklist in the Supplementary Text),43 and Quality of Health Economic Studies Instrument standards recommended for cost-effectiveness analyses of US CVD risk factor guidelines.29 Effectiveness assumptions were grounded in a large meta-analysis of randomized antihypertensive medication treatment trials and inputs for non-Hispanic whites and blacks were estimated from national data. However, like all computer-simulation analyses, this analysis was limited by reliance on multiple assumptions and inputs derived from multiple data sources and study types. Because of our conservative approach, we did not fully capture disability and health care costs attributed to hypertensive heart disease or end-stage renal disease—both of which are more common in non-Hispanic blacks—so that we may have underestimated cost savings and QALY gains with hypertension treatment compared with no treatment and in between-group comparisons. Additionally, while the 2014 national guidelines recommended different first-line agents for hypertension control in non-Hispanic blacks (diuretics or calcium channel blockers) and whites (the same agents or renin-angiotensin system blockers),16 the BP response to different agents is similar in most patients,44 and we chose not to model differential use of antihypertensive medication classes in order not to bias cost-of-treatment inputs. Our projections of future CVD incorporated demographic trends but did not account for any secular or clinical practice changes in the next decade. Our analysis is further limited by its exclusion of other race/ethnic groups, including Hispanic Americans and Asian Americans due to the lack of high-quality data on stroke and coronary heart disease incidence in these groups. Further research is needed to elucidate hypertension treatment cost-effectiveness in these groups as higher quality data becomes available.
Hypertension treatment cost savings and cost-effectiveness extend to younger ages among non-Hispanic black, compared with non-Hispanic white patients. Given their the higher hypertension prevalence and heavier burden of hypertension-related disease compared with any other US race/ethnic group, investment in effective clinic- and community-based hypertension control programs in non-Hispanic black Americans is a particularly high-value investment in population health.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from NHLBI (R01 HL107475-01), NINDS (5-U54NS081760-03), the American Heart Association Founder’s Affiliate (10CRP4140089), and HRSA (T32HP10260). This manuscript was prepared using Framingham Cohort and Framingham Offspring Research Materials obtained from the US National Heart, Lung and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the Framingham Cohort, Framingham Offspring, or the NHLBI. Kirsten Bibbins-Domingo is a member of the US Preventive Services Task Force (USPSTF) and current co-Vice Chair. This work does not necessarily represent the views and policies of the USPSTF. Eshan Vasudeva and Andrew Moran had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Moran; Acquisition of data: Vasudeva, Coxson, and Moran; Analysis and interpretation of data: Vasudeva and Moran; Drafting of the manuscript: Vasudeva, Moran, and Moise; Critical revision of manuscript for important intellectual content: Moran, Coxson, Bibbins-Domingo, Goldman, and Moise; Statistical analysis: Vasudeva, Huang, and Moran; Obtained funding: Moran; Study supervision: Moran.
REFERENCES
- 1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. 2010; 303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 2. Xu J, Kochanek KD. National vital statistics reports. Natl Vital Stat Rep 2009; 58:1–52. [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. A Closer Look at African American Men and High Blood Pressure Control: A Review of Psychosocial Factors and Systems-Level Interventions. Atlanta, GA: US Department of Health and Human Services, 2010. [Google Scholar]
- 4. Fuchs FD. Why do black Americans have higher prevalence of hypertension?: an enigma still unsolved. Hypertension 2011; 57:379–380. [DOI] [PubMed] [Google Scholar]
- 5. Moran AE, Odden MC, Thanataveerat A, Tzong KY, Rasmussen PW, Guzman D, Williams L, Bibbins-Domingo K, Coxson PG, Goldman L. Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med 2015; 372:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med 2013; 173:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999; 30:736–743. [DOI] [PubMed] [Google Scholar]
- 9. Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 1998; 147:259–268. [DOI] [PubMed] [Google Scholar]
- 10. Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, Woo D, Szaflarski J, Gebel J, Moomaw C, Pancioli A, Jauch E, Shukla R, Broderick J. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke 2004; 35:426–431. [DOI] [PubMed] [Google Scholar]
- 11. Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Broderick JP, Kissela BM. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke 2010; 41:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kittner SJ, White LR, Losonczy KG, Wolf PA, Hebel JR. Black-white differences in stroke incidence in a national sample. The contribution of hypertension and diabetes mellitus. JAMA 1990; 264:1267–1270. [PubMed] [Google Scholar]
- 13. Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011; 69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wellenius GA, Mittleman MA. Disparities in myocardial infarction case fatality rates among the elderly: the 20-year Medicare experience. Am Heart J 2008; 156:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Witt BJ, Brown RD, Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792. [DOI] [PubMed] [Google Scholar]
- 16. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 17. Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003; 326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265:3255–3264. [PubMed] [Google Scholar]
- 20. Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation 2011; 124:146–153. [DOI] [PubMed] [Google Scholar]
- 21. Red Book Drug References. 2014; http://redbook.com/redbook/awp/ Accessed 2 May 2014.
- 22. Auw Yang KG, Raijmakers NJ, van Arkel ER, Caron JJ, Rijk PC, Willems WJ, Zijl JA, Verbout AJ, Dhert WJ, Saris DB. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthr Cartilage 2008; 16:498–505. [DOI] [PubMed] [Google Scholar]
- 23. Montgomery AA, Harding J, Fahey T. Shared decision making in hypertension: the impact of patient preferences on treatment choice. Fam Pract 2001; 18:309–313. [DOI] [PubMed] [Google Scholar]
- 24. Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N, Shah R, Karyana M, Kosen S, Farje MR, Moncada G, Dutta A, Sazawal S, Dyer A, Seiler J, Aboyans V, Baker L, Baxter A, Benjamin EJ, Bhalla K, Bin Abdulhak A, Blyth F, Bourne R, Braithwaite T, Brooks P, Brugha TS, Bryan-Hancock C, Buchbinder R, Burney P, Calabria B, Chen H, Chugh SS, Cooley R, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, Davis A, Degenhardt L, Díaz-Torné C, Dorsey ER, Driscoll T, Edmond K, Elbaz A, Ezzati M, Feigin V, Ferri CP, Flaxman AD, Flood L, Fransen M, Fuse K, Gabbe BJ, Gillum RF, Haagsma J, Harrison JE, Havmoeller R, Hay RJ, Hel-Baqui A, Hoek HW, Hoffman H, Hogeland E, Hoy D, Jarvis D, Karthikeyan G, Knowlton LM, Lathlean T, Leasher JL, Lim SS, Lipshultz SE, Lopez AD, Lozano R, Lyons R, Malekzadeh R, Marcenes W, March L, Margolis DJ, McGill N, McGrath J, Mensah GA, Meyer AC, Michaud C, Moran A, Mori R, Murdoch ME, Naldi L, Newton CR, Norman R, Omer SB, Osborne R, Pearce N, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Pourmalek F, Prince M, Rehm JT, Remuzzi G, Richardson K, Room R, Saha S, Sampson U, Sanchez-Riera L, Segui-Gomez M, Shahraz S, Shibuya K, Singh D, Sliwa K, Smith E, Soerjomataram I, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Taylor HR, Tleyjeh IM, van der Werf MJ, Watson WL, Weatherall DJ, Weintraub R, Weisskopf MG, Whiteford H, Wilkinson JD, Woolf AD, Zheng ZJ, Murray CJ, Jonas JB. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 2012; 380:2129–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choudhry NK, Patrick AR, Glynn RJ, Avorn J. The cost-effectiveness of C-reactive protein testing and rosuvastatin treatment for patients with normal cholesterol levels. J Am Coll Cardiol 2011; 57:784–791. [DOI] [PubMed] [Google Scholar]
- 26. Greving JP, Visseren FL, de Wit GA, Algra A. Statin treatment for primary prevention of vascular disease: whom to treat? Cost-effectiveness analysis. BMJ 2011; 342:d1672. [DOI] [PubMed] [Google Scholar]
- 27. Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003; 362:1527–1535. [DOI] [PubMed] [Google Scholar]
- 28. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 29. Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED, Shaw LJ. ACC/AHA Statement on Cost/Value Methodology in Clinical Practice Guidelines and Performance Measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63. [DOI] [PubMed] [Google Scholar]
- 30. Lin JS, O’Connor E, Whitlock EP, Beil TL. Behavioral counseling to promote physical activity and a healthful diet to prevent cardiovascular disease in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2010; 153:736–750. [DOI] [PubMed] [Google Scholar]
- 31.Heidenreich PA, Davis BR, Cutler JA, Furberg CD, Lairson DR, Shlipak MG, Pressel SL, Nwachuku C, Goldman L. Cost‐effectiveness of chlorthalidone, amlodipine, and lisinopril as first‐step treatment for patients with hypertension: an analysis of the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Gen Intern Med May 2008; 23:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gillespie CD, Hurvitz KA; Centers for Disease Control and Prevention Prevalence of hypertension and controlled hypertension - United States, 2007–2010. MMWR Surveill Summ 2013; 62(Suppl 3): 144–148. [PubMed] [Google Scholar]
- 33. Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation 2012; 126:2105–2114. [DOI] [PubMed] [Google Scholar]
- 34. Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev 2010(3):CD005182. [DOI] [PubMed] [Google Scholar]
- 35. Carter BL, Ardery G, Dawson JD, James PA, Bergus GR, Doucette WR, Chrischilles EA, Franciscus CL, Xu Y. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med 2009; 169:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hill MN, Han HR, Dennison CR, Kim MT, Roary MC, Blumenthal RS, Bone LR, Levine DM, Post WS. Hypertension care and control in underserved urban African American men: behavioral and physiologic outcomes at 36 months. Am J Hypertens 2003; 16:906–913. [DOI] [PubMed] [Google Scholar]
- 37. Fletcher RD, Amdur RL, Kolodner R, McManus C, Jones R, Faselis C, Kokkinos P, Singh S, Papademetriou V. Blood pressure control among US veterans: a large multiyear analysis of blood pressure data from the Veterans Administration health data repository. Circulation 2012; 125:2462–2468. [DOI] [PubMed] [Google Scholar]
- 38. Ogedegbe G, Tobin JN, Fernandez S, Cassells A, Diaz-Gloster M, Khalida C, Pickering T, Schwartz JE. Counseling African Americans to Control Hypertension: cluster-randomized clinical trial main effects. Circulation 2014; 129:2044–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Group SR, Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Victor RG, Ravenell JE, Freeman A, Leonard D, Bhat DG, Shafiq M, Knowles P, Storm JS, Adhikari E, Bibbins-Domingo K. Effectiveness of a barber-based intervention for improving hypertension control in black men: the BARBER-1 study: a cluster randomized trial. Arch Intern Med 2011; 171:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Constantine R, Brownstein JN, Hoover S, Wordlaw-Stinson L, Orenstein D, Jones P, Farris R. Strategies for controlling blood pressure among low-income populations in Georgia. Prev Chronic Dis 2008; 5:A52. [PMC free article] [PubMed] [Google Scholar]
- 42. Rein DB, Constantine RT, Orenstein D, Chen H, Jones P, Brownstein JN, Farris R. A cost evaluation of the Georgia Stroke and Heart Attack Prevention Program. Prev Chronic Dis 2006; 3:A12. [PMC free article] [PubMed] [Google Scholar]
- 43. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E, Force CT. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013; 346:f1049. [DOI] [PubMed] [Google Scholar]
- 44. Johnson JA. Ethnic differences in cardiovascular drug response: potential contribution of pharmacogenetics. Circulation 2008; 118:1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.