ABSTRACT
The cyanobacterium Synechococcus sp. strain PCC 7002 is a cobalamin auxotroph and utilizes this coenzyme solely for the synthesis of l-methionine by methionine synthase (MetH). Synechococcus sp. strain PCC 7002 is unable to synthesize cobalamin de novo, and because of the large size of this tetrapyrrole, an active-transport system must exist for cobalamin uptake. Surprisingly, no cobalamin transport system was identified in the initial annotation of the genome of this organism. With more sophisticated in silico prediction tools, a btuB-cpdA-btuC-btuF operon encoding components putatively required for a B12 uptake (btu) system was identified. The expression of these genes was predicted to be controlled by a cobalamin riboswitch. Global transcriptional profiling by high-throughput RNA sequencing of a cobalamin-independent form of Synechococcus sp. strain PCC 7002 grown in the absence or presence of cobalamin confirmed regulation of the btu operon by cobalamin. Pérez et al. (A. A. Pérez, Z. Liu, D. A. Rodionov, Z. Li, and D. A. Bryant, J Bacteriol 198:2743–2752, 2016, http://dx.doi.org/10.1128/JB.00475-16) developed a cobalamin-dependent yellow fluorescent protein reporter system in a Synechococcus sp. strain PCC 7002 variant that had been genetically modified to allow cobalamin-independent growth. This reporter system was exploited to validate components of the btu uptake system by assessing the ability of targeted mutants to transport cobalamin. The btuB promoter and a variant counterpart mutated in an essential element of the predicted cobalamin riboswitch were fused to a yfp reporter. The combined data indicate that the btuB-cpdA-btuF-btuC operon in this cyanobacterium is transcriptionally regulated by a cobalamin riboswitch.
IMPORTANCE With a cobalamin-regulated reporter system for expression of yellow fluorescent protein, genes previously misidentified as encoding subunits of a siderophore transporter were shown to encode components of cobalamin uptake in the cyanobacterium Synechococcus sp. strain PCC 7002. This study demonstrates the importance of experimental validation of in silico predictions and provides a general scheme for in vivo verification of similar cobalamin transport systems. A putative cobalamin riboswitch was identified in Synechococcus sp. strain PCC 7002. This riboswitch acts as a potential transcriptional attenuator of the btu operon that encodes the components of the cobalamin active-transport system.
INTRODUCTION
Synechococcus sp. strain PCC 7002 is a euryhaline, unicellular cyanobacterium that tolerates high light intensities and a wide range of NaCl concentrations (1, 2). This organism has one of the highest growth rates among cyanobacteria (1, 3) and is naturally transformable (4). Furthermore, the genome of Synechococcus sp. strain PCC 7002 has been sequenced (http://www.ncbi.nlm.nih.gov/), and a versatile system for genetic complementation and overexpression exists for this organism (5). Although it is generally considered to be a photoautotroph, Synechococcus sp. strain PCC 7002 actually has an obligate requirement for exogenous vitamin B12 (cobalamin) (6). The average reported concentration of cobalamin in seawater is around 3 ng liter−1 (or 0.003 μg liter−1) (7) but exhibits variable vertical distribution (8). As a marine organism incapable of synthesizing cobalamin de novo (9, 10), Synechococcus sp. strain PCC 7002 thus needs a specific and effective method to transport this essential compound from its surrounding medium.
Cobalamin is a large tetrapyrrole molecule that cannot traverse the cellular membrane passively (10, 11). The uptake of scarce nutrients in Gram-negative bacteria requires a series of transporters that can actively transport nutrients across the outer and inner membranes. Components in the periplasmic space are also sometimes required for the transport of these essential nutrients into the cytosol (11). A vitamin B twelve uptake system, or btu, occurs in Gram-negative bacteria. It consists of BtuB, an outer membrane TonB-dependent transporter (TBDT) (12) and an inner membrane transporter (BtuCDF) of the ATP-binding cassette (ABC) family (13). BtuB is coupled to an inner membrane complex consisting of TonB, ExbB, and ExbD and shares the canonical 22-strand, β-barrel architecture of other TBDTs together with an N-terminal, globular periplasmic domain that occludes a channel through the transporter and thus prevents passive diffusion into the periplasm (12). The inner membrane ABC transporter consists of a high-affinity cobalamin-binding periplasmic protein (BtuF) that binds cobalamin in the periplasmic space and delivers it to an inner membrane ABC transporter (BtuCD). This complex consists of a pair of membrane-spanning permease subunits (BtuC) and a pair of ATP-binding and hydrolysis subunits (BtuD) (14). The inner membrane ABC transporter system utilizes ATP hydrolysis-driven conformational changes for translocation of cobalamin into the cytoplasm, but BtuB relies on TonB, ExbB, and ExbD to couple proton motive force to cobalamin uptake (12, 14). Although Synechococcus sp. strain PCC 7002 is a cobalamin auxotroph, the initial annotation of the genome of this cyanobacterium failed to identify the expected genes for cobalamin uptake.
Like that of many of its eukaryotic microalgal and marine cyanobacterial counterparts, methionine biosynthesis is seemingly responsible for the cobalamin auxotrophy of Synechococcus sp. strain PCC 7002 (15, 16). Cobalamin is an essential coenzyme for cobalamin-dependent methionine synthase, MetH, which utilizes the adenosylated vitamer of cobalamin to catalyze the transfer of a methyl group from N5-methyl-5,6,7,8-tetrahydrofolate to l-homocysteine (17). In contrast, an unrelated isozyme, the cobalamin-independent methionine synthase (MetE), exists in many organisms and catalyzes the transfer of the methyl group from N5-methyl-5,6,7,8-tetrahydrofolate to l-homocysteine directly without the use of cobalamin as an intermediary carrier (18). Some marine algae possess genes for both MetH and MetE and thus are able to acclimate to fluctuations in the availability of exogenous cobalamin (19); however, MetH is usually the preferred catalyst for methionine biosynthesis because of its higher turnover rate (20). Studies indicate that cobalamin auxotrophy in these organisms is correlated with the loss of the metE gene (15).
In this study, in silico prediction tools (21–25) indicated that genes SYNPCC7002_A0634 to SYNPCC7002_A0637, previously annotated as encoding an ABC-type siderophore transporter, instead might encode the canonical ABC transporter for cobalamin (BtuCDF) and the associated TBDT outer membrane transporter BtuB. On the basis of cobalamin-dependent regulation of the yfp gene, encoding enhanced yellow fluorescent protein (YFP), an assay system was developed that successfully confirmed the roles of the predicted btuB, btuC, and btuF components in cobalamin uptake. Surprisingly, the btuD gene could not be identified in this study. Global transcription profiling by high-throughput RNA sequencing (RNA-seq) and characterization of the btu promoter variants with yfp reporter fusions support the presence of a predicted cobalamin riboswitch in the untranslated leader sequence upstream of this newly validated btu operon.
MATERIALS AND METHODS
Strains, culture conditions, and transformation procedure.
Synechococcus sp. strain PCC 7002 was obtained from the Pasteur Culture Collection (Institut Pasteur, Paris, France) (26). Pérez et al. (16) have described a cobalamin-independent Synechococcus sp. strain PCC 7002, designated strain AAP005, that expresses the metE gene of Synechococcus sp. strain PCC 73109 from pAQ1Ex under the control of the Synechocystis sp. strain PCC 6803 PpsbA2 promoter and harbors a cobalamin-sensing YFP reporter system. Strain AAP005 was utilized as the background strain for all of the mutants used in this study. Strain AAP005 and mutants derived from it (described below) were grown in medium A supplemented with 1 mg of NaNO3 ml–1 (designated medium A+), which additionally contains 4 μg of cyanocobalamin liter−1 (4), also designated A+ B12+, or in medium A+ with cyanocobalamin omitted, designated A+ B12–. All Synechococcus sp. strain PCC 7002 mutants were grown photoautotrophically in 20-mm culture tubes containing 10 ml of liquid medium. Cultures were grown at 38°C with continuous illumination from cool white fluorescent lights at 250 μmol of photons m–2 s–1 and sparging with 1% (vol/vol) CO2 in air, defined as “standard conditions” (1). Antibiotics at the following concentrations were supplied when required: 50 μg of spectinomycin ml–1, 100 μg of kanamycin ml–1, 20 μg of erythromycin ml–1, and 20 μg of gentamicin ml–1. Transformation of Synechococcus sp. strain PCC 7002 was performed as described previously (5).
PCR amplification, digestions, and ligations.
The sequences of the primers utilized for PCR in this study are provided in Tables S1 and S2 in the supplemental material. PCR amplifications in this study were performed with Phusion High-Fidelity DNA polymerase (catalog number M0530S; New England BioLabs Inc.). Purification of PCR amplicons prior to digestion with restriction enzymes was achieved by an isopropanol-and-ethanol purification method as described previously (5). Digested fragments were electrophoretically resolved on 0.8% (wt/vol) agarose gels and purified with the EZ-10 Spin Column DNA Extraction kit from Bio Basic Inc. (catalog number BS353). Ligations were carried out for 8 h at 16°C with T4 DNA ligase (New England BioLabs Inc.) in equimolar reaction mixtures (volume, 20 μl).
Prediction of a putative B12 uptake (btu) operon in Synechococcus sp. strain PCC 7002.
Previously reconstructed B12 riboswitch regulons in the cyanobacterial genomes were extracted from the RegPrecise database (http://regprecise.lbl.gov/) (21). Comparative genomic analysis of the btuBFC genes in Synechococcus genomes was performed with the SEED platform (http://pubseed.theseed.org/), which supports the identification and projection of metabolic subsystems across prokaryotic genomes (22). Additionally, identification of orthologs in closely related genomes and genome neighborhood analysis were performed with the MicrobesOnline tool (http://www.microbesonline.org) (23). Computational identification of B12 riboswitches in untranslated upstream leader regions was performed with the RibEx Riboswitch Explorer tool (27). The RNA secondary structures of B12 riboswitches, including potential antiterminators and antisequestors, were predicted with Zuker's algorithm of free-energy minimization (24), as implemented in the Mfold program (http://www.bioinfo.rpi.edu/applications/mfold/). Multiple-sequence alignments of DNA regions upstream of btuB genes were constructed with ClustalX (25).
Deletion of genes for predicted components of cobalamin transport.
The ermC erythromycin resistance cassette from pRL409 (29) was amplified with primers ermC_F and erm_R (see Table S1 in the supplemental material). Multiple primers (see Table S2) were designed to obtain upstream and downstream flanking regions of the genes to be deleted (Fig. 1). A ligation reaction mixture containing the digested flanking fragments and the ermC erythromycin resistance cartridge was prepared to obtain ΔbtuX::ermC (X represents different btu gene loci) constructs. The ligation reaction for each ΔbtuX::ermC construct was used as the template for a nested PCR with appropriate flanking primers (see Table S2 and Fig. S1 in the supplemental material). Each putative btu gene was deleted by transforming the ΔbtuX::ermC construct into strain AAP005 (Fig. 2A) as described by Pérez et al. (16). Segregation of the various btuX and ΔbtuX::ermC alleles was performed on plates prepared with A+ B12– medium, which also contained erythromycin, spectinomycin, and kanamycin. Full segregation of alleles was confirmed by colony PCR performed with screening primers (see Table S2).
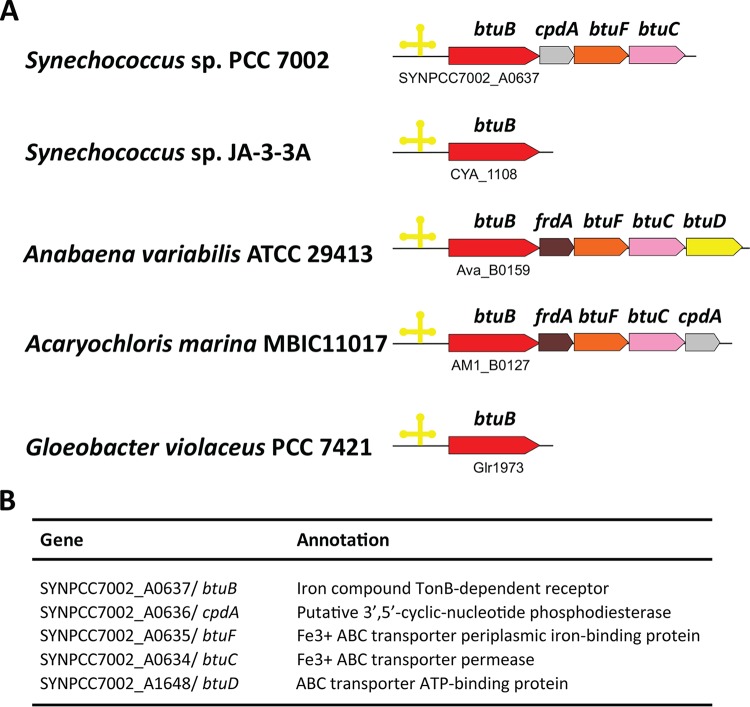
FIG 1.
(A) Organization of btuBCDF orthologs and cobalamin riboswitches in various cyanobacterial genomes. The yellow cloverleaf structures show the locations of predicted cobalamin riboswitches. (B) The btu operon in Synechococcus sp. strain PCC 7002 containing btuB, btuF, btuC, and a putative 3′,5′-cyclic-nucleotide phosphodiesterase gene (cpdA). The predicted btuD gene (SYNPCC7002_A1648), which might encode the ATPase component of the inner membrane ABC transporter, is not located in the btu operon. btuB encodes the TonB-dependent outer membrane transporter, btuF encodes the periplasmic cobalamin-binding protein of the inner membrane ABC transporter, and btuC encodes the permease subunit of the inner membrane cobalamin ABC transporter. The annotation column shows the original descriptions, not those determined functionally in this study, which are reflected by the btu gene designations.
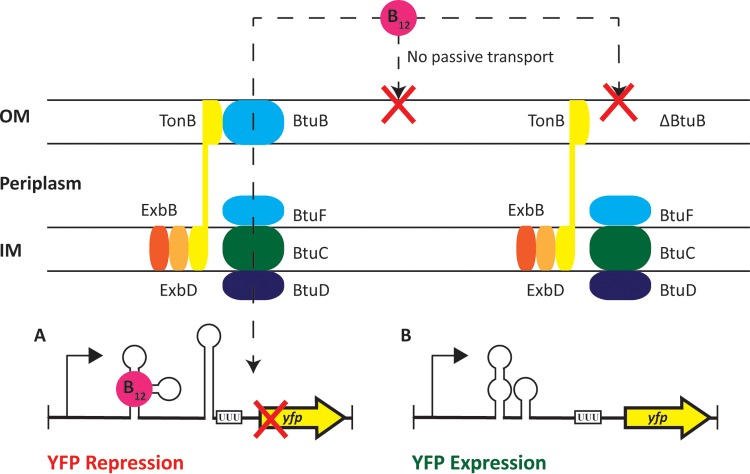
FIG 2.
In vivo fluorescence reporter system for verification of genes essential for cobalamin uptake. (A) Strain AAP005, a cobalamin-independent Synechococcus sp. strain PCC 7002 variant, the result of MetE complementation, was used as a background strain for this assay. In this strain, the yfp gene is under the control of a fused cpcBA/metE promoter construct that can sense cobalamin via a cobalamin riboswitch (16). Uptake of cobalamin into the cell will lead to repression of yfp expression in the presence of exogenous cobalamin. (B) Cobalamin is a large tetrapyrrole molecule that is only capable of traversing the cell membrane via active transport. If a knockout mutation eliminates an essential component of this uptake system, cobalamin will not traverse the inner and outer cell membranes (IM and OM, respectively) and YFP levels will be independent of exogenous cobalamin.
In vivo evaluation of putative btu components in Synechococcus sp. strain PCC 7002.
A schematic explaining the in vivo assay for evaluating essential cobalamin uptake genes is presented in Fig. 2. AAP005 ΔbtuX::ermC strains (16) were grown in liquid medium A+ B12– supplemented with erythromycin, spectinomycin, and kanamycin under standard growth conditions (1). Starter cultures were diluted to an optical density at 730 nm (OD730) of 0.05 in A+ or A+ B12– medium with the appropriate antibiotics. Cultures were grown under standard growth conditions to an OD730 of 1.5. Each AAP005 ΔbtuX::ermC strain was grown in the presence or absence of cobalamin to allow fluorometric comparison of the YFP contents under the two conditions. The YFP fluorescence amplitude at 527 nm per OD730 unit was determined with an SLM-Aminco 8100C fluorometer modernized for computer data acquisition by On-Line Instrument Systems (Bogart, GA) (5). Cell suspensions were adjusted to an OD730 of 1.0 for data acquisition. The excitation wavelength was 488 nm.
Global transcriptional profiling.
Starter cultures of Synechococcus sp. strain PCC 7002 were grown under standard conditions with the appropriate antibiotics at an OD730 of 0.05 and diluted to produce triplicate cultures. The triplicate cultures were harvested in exponential phase at an OD730 of 0.7 for RNA extraction for global transcriptional profiling experiments as described previously (1). Construction of cDNA libraries and SOLiD sequencing were performed in the Genomics Core Facility at The Pennsylvania State University (University Park, PA). Data analysis was performed as described previously (1). Transcript levels of a cobalamin-independent Synechococcus sp. strain PCC 7002 variant with the native metH gene deleted (strain AAP002) (16) were compared when the strain was grown in the presence or absence of cobalamin. For the complete data, see Table S3 in the supplemental material.
In vivo fluorescence assay shows that the putative btuB leader sequence contains a predicted cobalamin riboswitch.
A 564-bp region upstream of SYNPCC7002_A0637 (btuB) was amplified with primers 120 and 121 (see Table S2 in the supplemental material); this PbtuB promoter, which is predicted to contain a cobalamin riboswitch, was transcriptionally fused to the yfp gene in pAQ3Ex as described previously (16). A double cytosine-to-thymine transition variant (CC to TT or CC to UU in RNA) of the putative btuB cobalamin riboswitch at the P1 helix-B12 box interface was synthesized (GenScript, Piscataway, NJ) to generate PbtuB-1. This variant promoter was also transcriptionally fused to the yfp gene in pAQ3Ex. Both constructs were transformed into strain AAP002 (16). In vivo fluorescence of YFP controlled by PbtuB and PbtuB-1 was measured as described above (5).
Deletion of the native metH gene from Synechococcus sp. strain PCC 7002 in btu mutant strains.
The aacC1 gentamicin resistance cassette from pMS255 (30) was amplified from a pAQ1Ex construct containing this gene with primers aacC1_F and aacC1_R (see Table S1 in the supplemental material). This gene was used to replace the ermC erythromycin resistance cassette previously used to delete the native metH gene from strain AAP002 (16). The ΔmetH::ermC construct was amplified by colony PCR from strain AAP002 (16) with primers Nest_dmetH_F and Nest_dmetH_R (see Table S1). Digestion of the ΔmetH::ermC amplicon with BamHI and EcoRI was followed by purification of the digested flanking sites as described above. The BamHI- and EcoRI-digested aacC1 amplicon and the digested upstream and downstream metH fragments were mixed and ligated, and the product was used as a template for the amplification of ΔmetH::aacC1 with primers Nest_dmetH_F and Nest_dmetH_R (see Fig. S2 in the supplemental material). This ΔmetH::aacC1 amplicon was used to transform the AAP005 strains harboring the individual ΔbtuX::ermC mutations that also carried the cobalamin-dependent YFP fluorescence reporter system. Transformants were streaked on A+ B12– medium plates supplemented with gentamicin, spectinomycin, kanamycin, and erythromycin. Full segregation of the ΔmetH::aacC1 and ΔmetH::ermC alleles was confirmed with the Nest_dmetH_F and Nest_dmetH_R primers (see Table S1) for colony PCR. Evaluation of the individual btu components was assayed by measuring YFP fluorescence in vivo as described above.
Accession number.
The cDNA sequence data have been submitted to the NCBI Sequence Read Archive under accession number SRP076516.
RESULTS
The btu operon of Synechococcus sp. strain PCC 7002.
Candidate genes encoding the vitamin B12 uptake transporter (btuBCD) were identified as predicted members of vitamin B12 riboswitch regulons in cyanobacteria (Fig. 1). The Synechococcus sp. strain PCC 7002 btu operon resembles that of Acaryochloris marina MBIC11017 but lacks the frdA gene that encodes a subunit of fumarate reductase (Fig. 1A). In Synechococcus sp. strain PCC 7002, this putative vitamin B12 transporter is encoded by open reading frames SYNPCC7002_A0637 to SYNPCC7002_A0634. However, the putative btuD gene, SYNPCC7002_A1648, which is predicted to encode an ATPase subunit of an ABC transporter, is not included in this operon (Fig. 1A). These genes had previously been annotated as encoding components of a siderophore transporter (Fig. 1B).
The btu cobalamin uptake genes are not essential in a cobalamin-independent strain of Synechococcus sp. strain PCC 7002.
The predicted btu genes (Fig. 1) were deleted from strain AAP005 (16). Deletion of each btu gene (see Fig. S1 in the supplemental material) was achieved by individually replacing it with the ermC erythromycin resistance cassette from pRL409 (29). The facile deletion of these genes from cobalamin-independent strain AAP005 (see Fig. S3 in the supplemental material) demonstrates that these genes are not essential in this genetic background. These strains grow well in the absence of cobalamin but require more frequent transfers to maintain viability. This probably denotes a loss of fitness of the cells as a result of the extensive genetic alterations.
Identification of the btuB, btuC, and btuF genes.
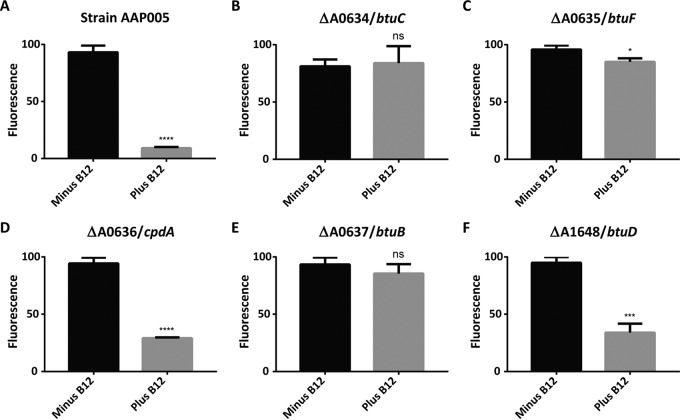
The AAP005 ΔbtuX::ermC strains (Fig. 2 and 3) were grown in the presence or absence of cobalamin to determine if the deleted genes encode proteins required for cobalamin uptake. If a gene product functions in cobalamin uptake, the YFP fluorescence amplitude levels measured in vivo should be the same in the presence or absence of exogenous cobalamin because no cobalamin will enter the cell and bind to the riboswitch to relieve transcription attenuation (Fig. 2B). Deletion of the individual predicted btu genes should be sufficient to inactivate cobalamin transport (12, 13) and thus derepress the expression of yfp through the metE riboswitch of the reporter system. Deletion of SYNPCC7002_A0634 (btuC), which is predicted to encode the permease subunit of the ABC transporter for cobalamin, produced a strain in which yfp expression was insensitive to exogenous cobalamin. Similar YFP fluorescence amplitudes were measured in the presence or absence of exogenous cobalamin (Fig. 3B). This result shows that BtuC plays an essential role in cobalamin uptake. A strain lacking SYNPCC7002_A0635 (btuF), which is predicted to encode the periplasmic cobalamin-binding protein of the ABC transporter, also showed similar (and elevated) YFP fluorescence amplitudes in the presence or absence of exogenous cobalamin (Fig. 3C). The deletion of SYNPCC7002_A0637 (btuB), predicted to encode the TonB-dependent outer membrane transporter of cobalamin, likewise confirmed that it is essential for cobalamin uptake (Fig. 3E).
FIG 3.
Validation of cobalamin uptake genes. (A) Strain AAP005, in which addition of cobalamin to growth medium causes repression of YFP fluorescence. All btu deletion strains were generated with this strain. (B) AAP005 btuC (SYNPCC7002_A0634) mutant. (C) AAP005 btuF (SYNPCC7002_A0635) mutant. (D) AAP005 cpdA (SYNPCC7002_A0636) mutant. (E) AAP005 btuB (SYNPCC7002_A0637) mutant. (F) AAP005 putative btuD (SYNPCC7002_A1648) mutant. Each bar represents the average emission amplitude of YFP at 527 nm with excitation at 488 nm of three biological replicates after normalization to set the highest fluorescence value as 100%. Cell densities were normalized to an OD730 of 1.0. The error bars represent the standard deviations of these measurements. The asterisks represent statistical significance, with P values as follows: none, P > 0.05; *, P ≤ 0.05; ***, P ≤ 0.001; ****, P ≤ 0.0001; ns, not significant.
Bioinformatic analyses suggested that SYNPCC7002_A1648 was the most likely candidate gene to encode BtuD, the ATPase subunit of the inner membrane cobalamin ABC transporter. However, deletion of SYNPCC7002_A1648 produced a strain in which the yfp reporter gene was repressed in the presence of exogenous cobalamin (Fig. 3F). This indicates that cobalamin transport was still functional in the mutant with SYNPCC7002_A1648 deleted. Similarly, a deletion mutant lacking SYNPCC7002_A0636 also showed repression of yfp in the presence of cobalamin (Fig. 3D). SYNPCC7002_A0636 is annotated as a putative 3′,5′-cyclic-nucleotide phosphodiesterase (CpdA) that is not predicted to play a role in cobalamin uptake but is nevertheless a part of the btu operon, along with btuB, btuC, and btuF (Fig. 1). It should be noted that the patterns of yfp expression were the same as those in Fig. 3 when the metH gene was also deleted from the btu deletion strains (data not shown; Fig. S2 in the supplemental material shows confirmation of the deletion of metH in each mutant). However, the deletion mutants that also lacked metH grew more slowly and did not propagate as well on plates.
Global transcriptional profiling of a cobalamin-independent Synechococcus sp. strain PCC 7002 variant shows that the btu operon is regulated by cobalamin.
Strain AAP002 (16) was grown in the presence or absence of cobalamin to evaluate cobalamin-dependent regulation of genes by global transcriptional profiling (1). The relative transcript abundances of the btu operon genes were much higher in the absence of exogenous cobalamin (Table 1; Fig. 4), and only the four genes of the btu operon changed transcript abundance levels to an appreciable extent. For example, the btuB gene (SYNPCC7002_A0637), which is immediately downstream from the promoter region for the btu operon, showed relative transcript levels that were 249-fold lower in the presence of exogenous cobalamin. The relative transcript levels of the other genes in the operon decreased more moderately in the presence of cobalamin and were only about 20-fold lower (Table 1). The obvious correlation between transcript levels and exogenous cobalamin suggests that cobalamin-dependent transcriptional attenuation of the btu operon occurs. Analysis of the promoter region of the Synechococcus sp. strain PCC 7002 btu operon revealed the presence of a B12 box, an essential and conserved region of cobalamin riboswitches (31), as well as elements suggesting control by transcriptional attenuation, such as a putative terminator and a poly-U tract (Fig. 5A; see Fig. S4 in the supplemental material) (28).
TABLE 1.
Relative transcript abundances of genes of the btu operon in cells of strain AAP002
| Gene | Relative transcript abundancea |
Fold decrease with cobalamin | P value | |
|---|---|---|---|---|
| Without cobalamin | With cobalamin | |||
| SYNPCC7002_A0634 (btuC) | 2.79E−04 | 1.29E−05 | 21.6 | <0.001 |
| SYNPCC7002_A0635 (btuF) | 6.35E−04 | 3.51E−05 | 18.1 | <0.001 |
| SYNPCC7002_A0636 (cpdA)b | 7.78E−04 | 3.98E−05 | 19.5 | <0.001 |
| SYNPCC7002_A0637 (btuB) | 1.46E−03 | 5.85E−06 | 249.5 | <0.001 |
Cultures were grown with or without exogenous cobalamin.
Encodes a putative 3′,5′-cyclic-nucleotide phosphodiesterase.
FIG 4.
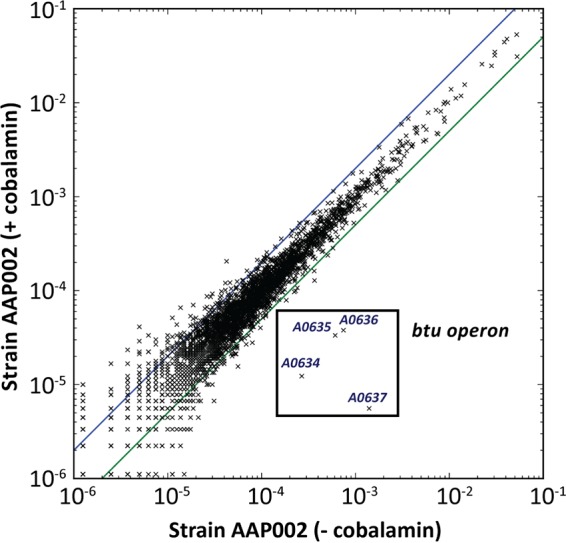
Changes in the relative transcript abundances of the btu operon genes. RNA was isolated from triplicate cultures of strain AAP002. Cells were grown in medium A+ with or without cobalamin under standard conditions. The blue and green lines indicate a 2-fold increase and a 50% decrease in relative transcript abundance, respectively.
FIG 5.
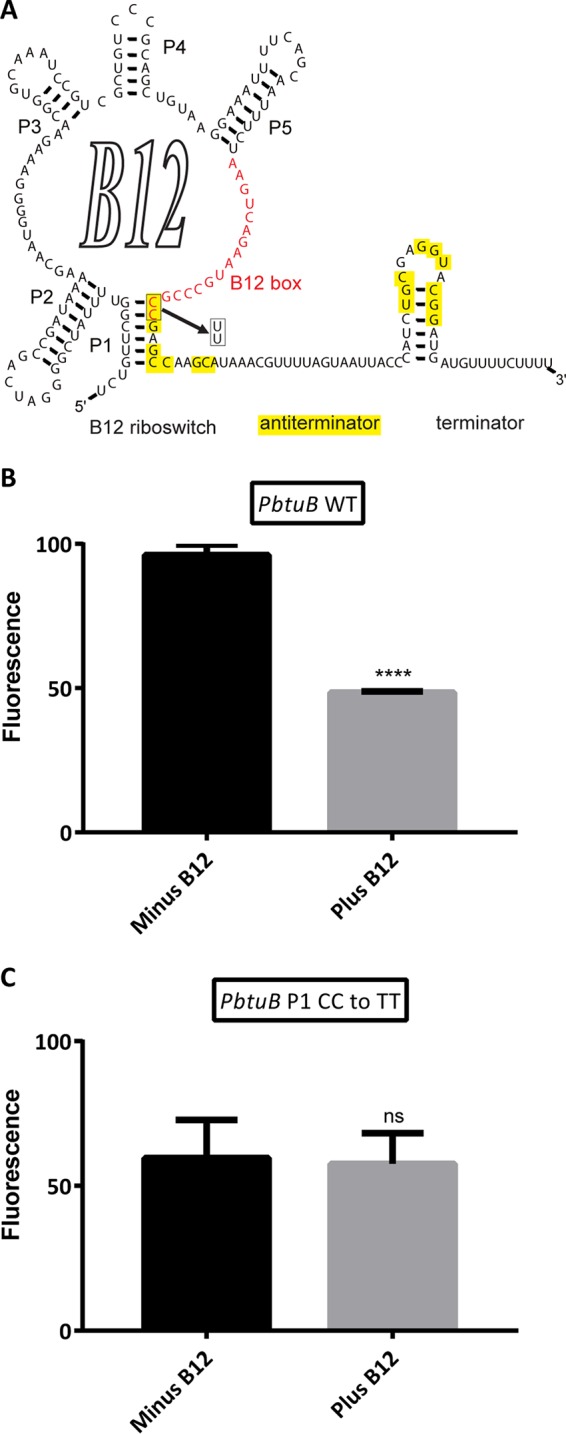
Predicted secondary structure of the cobalamin riboswitch in the btuB leader region of Synechococcus sp. strain PCC 7002. (A) The boxed bases represent the B12 box-P1 helix interface, where a CC-to-TT (UU in the RNA structure) transition mutation was introduced to generate promoter variant PbtuB-1. Comparison of YFP expression in the presence or absence of cobalamin for the PbtuB (B) and PbtuB-1 (C) promoter variants. Each bar represents the average emission amplitude of YFP at 527 nm with excitation at 488 nm of three biological replicates after normalization by using the highest fluorescence value as 100%. Cell densities were normalized to an OD730 of 1.0. The error bars represent the standard deviations of these measurements. The asterisks represent statistical significance with P values as follows: none, P > 0.05; ****, P ≤ 0.0001; ns, not significant. WT, wild type.
A double cytosine-to-thymine transition mutation in the conserved 3′ site of the B12 box causes loss of cobalamin regulation.
To demonstrate that cobalamin regulation by PbtuB is the result of a cobalamin riboswitch, mutations in conserved sequence regions such as the B12 box are frequently employed (31). A transition mutation of the conserved CC motif at the 3′ end of the B12 box-P1 helix interface to TT (UU in RNA) was introduced (Fig. 5A). In comparison with the wild-type promoter PbtuB (Fig. 5B), Fig. 5C demonstrates that cobalamin-dependent regulation is eliminated when yfp expression is controlled by the variant promoter PbtuB-1.
DISCUSSION
The marine cyanobacterium Synechococcus sp. strain PCC 73109, a close relative of Synechococcus sp. strain PCC 7002 (32), contains genes for both cobalamin-dependent methionine synthase (MetH) and cobalamin-independent methionine synthase (MetE), which are isozymes catalyzing the conversion of l-homocysteine to l-methionine (16). Heterologous expression of metE from Synechococcus sp. strain PCC 73109 is sufficient to complement the cobalamin auxotrophy of Synechococcus sp. strain PCC 7002, which solely possesses the cobalamin-dependent isozyme MetH and is incapable of de novo cobalamin biosynthesis (16). The use of MetH for methionine synthesis must therefore be complemented by a high-affinity uptake system to transport the large cobalamin molecule into cells from the environment (16). Initial annotation of the Synechococcus sp. strain PCC 7002 genome failed to identify candidate genes for cobalamin transport. With more sophisticated bioinformatic tools and comparative genomics, candidate genes, previously annotated as siderophore uptake genes (Fig. 1), were predicted to encode components of the cobalamin transport system (BtuBCDF) (12, 13). A yfp reporter system controlled by a cobalamin riboswitch was used to assess predicted btu genes by in vivo fluorometric assays of deletion mutants for the predicted genes (Fig. 2). These studies confirmed the assignments of btuB, btuC, and btuF in cobalamin transport but failed to confirm the predicted btuD gene (12, 13).
The btuB, btuF, and btuC genes are included in an operon (Fig. 1) together with a gene (cpdA; SYNPCC7002_A0636) for 3′,5′-cyclic-nucleotide phosphodiesterase, which apparently has no role in cobalamin uptake (Fig. 3D). This is expected, because cpdA exhibits no amino acid sequence similarity or conserved domains related to ABC transporter subunits (data not shown). It is possible that the slight derepression observed in Fig. 3D compared to the control in Fig. 3A is due to downstream effects on essential components in the btu operon such as btuF and btuC. The putative btuD gene encoding the ATPase subunit of the inner membrane ABC transporter complex was predicted to occur elsewhere in the genome. The location of this gene, downstream of a ferric uptake regulator (fur; SYNPCC7002_A1649), might indicate that the ATPase subunit produced by the gene plays a role in siderophore uptake (33). The facile deletion and full allele segregation of all of the btu genes in the cobalamin-independent Synechococcus sp. strain PCC 7002 variant indicate that these components are no longer essential for viability in this genetic background (see Fig. S3 in the supplemental material). It was observed that complete deletion of the SYNPCC7002_A0635 (btuF) gene is possible in wild-type Synechococcus sp. strain PCC 7002, but this mutation caused an obvious decrease in the growth rate; this observation suggests that some cobalamin may enter cells without the participation of BtuF (results not shown). In Salmonella enterica serovar Typhimurium, deletion of individual btu components still allowed that bacterium to grow at high concentrations of exogenous cobalamin, whereas deletion of multiple btu genes significantly impaired cobalamin uptake even at high concentrations of exogenous cobalamin (34). In Synechococcus sp. strain PCC 7002, it is possible that cobalamin can accumulate in the periplasmic space in the absence of BtuF and support some transport through the BtuC-BtuD complex (35). This is also demonstrated in Fig. 3C, which shows that despite deletion of btuF, some repression of YFP might occur in comparison to btuC (Fig. 2B) and btuB (Fig. 3E).
There are multiple possibilities for why it was not possible to identify BtuD in this study. One is that the ATPase subunit of the cobalamin transporter has diverged substantially from other subunits of ABC transporters for this compound. While this may be the case, an alternative is that a btuD mutant can be rescued by ATPase subunits of ABC transporters for other compounds. It is also possible that ABC transporters in cyanobacteria share ATPase subunits. Support for this idea comes from the observation that operons for ABC transporters frequently lack an associated gene for the ATPase subunit. ABC transporters in prokaryotes and eukaryotes are known to experience functional redundancy among members of this large protein superfamily (36). The BtuB transporter obviously has binding specificity for cobalamin, which could not be easily replaced with other outer membrane importers for structurally unique siderophores (34, 37), and the BtuC permease must form a cavity that is large enough to accommodate cobalamin (35). In contrast, the BtuD components of ABC transporters are often quite highly conserved among members of the ABC transporter superfamily (38). Further comparative genomic analyses, as well as biochemical studies of the ABC transporter for cobalamin, could help to identify the missing subunit(s).
Global transcriptional profiling (1) was used to investigate whether relative transcript abundances were correlated with the presence or absence of cobalamin in a cobalamin-independent variant of Synechococcus sp. strain PCC 7002. This approach provided strong evidence for a cobalamin-responsive element in the regulation of the btuB-cpdA-btuC-btuF operon (Fig. 4). The relative transcript abundances for genes in this operon decreased at least 20-fold or more, with btuB experiencing a 250-fold decrease, in the presence of exogenous cobalamin (Table 1). Coupled with the prediction of a cobalamin riboswitch within the btu operon promoter region (see Fig. S1 in the supplemental material), these data suggested the presence of a potential cobalamin riboswitch that is responsible for the transcriptional regulation of the genes for cobalamin uptake in Synechococcus sp. strain PCC 7002. Additional experimentation is required to ascertain the precise mode of regulation of the btuB cobalamin riboswitch; however, the large differences in relative transcript levels observed in the RNA-seq data (Fig. 4), as well as the predicted structure of the riboswitch itself (Fig. 5A), suggest that this riboswitch functions to attenuate transcription. No other cobalamin riboswitches were predicted to occur in the genome of Synechococcus sp. strain PCC 7002 (D. A. Rodionov, results not shown), a result that is supported by the global transcription profiling data. No btuD candidate was identified in the RNA-seq analyses, again suggesting that it is possible that no single ABC transporter ATPase subunit is responsible for cobalamin uptake.
Despite the possibility of homologous recombination with the chromosomal btuB promoter, the btuB leader region controlling the btuB-cpdA-btuF-btuC operon was capable of regulating YFP via repression in the presence of exogenous cobalamin (Fig. 5B). Mutagenesis of essential nucleotides at the B12 box–P1 helix-loop interface eliminated the response to cobalamin of the btuB promoter, confirming the presence of a cobalamin riboswitch in its leader region (Fig. 5C). Interestingly, expression levels observed for the mutant construct were lower than those observed for the wild-type counterpart. This phenomenon was first observed by Warner et al. (31), and though it is unknown what leads to this change in expression, it is likely that the nucleotide changes in the mutant cobalamin riboswitch could generate an alternative secondary-structure change that affects the processivity of RNA polymerase in the btuB operator/promoter.
Wilhelm and Trick (6) provided the first evidence of proteins/genes regulated by exogenous cobalamin in Synechococcus sp. strain PCC 7002. The results of this study show that those proteins were probably the products of the btu operon, which is regulated by a cobalamin riboswitch. As a marine organism that is incapable of synthesizing its own cobalamin de novo, Synechococcus sp. strain PCC 7002 requires a highly specific btu transport system to take up cobalamin produced by other marine bacteria (9, 10) for use as a coenzyme for methionine biosynthesis (16). It is possible that Synechococcus sp. strain PCC 7002 once had a metE gene like closely related strain Synechococcus sp. strain PCC 73109, but because of the availability of cobalamin in its environment, it may have lost the metE gene at some point in its evolutionary history, a situation similar to that documented in some eukaryotic algae (19). This could explain the presence of a cobalamin riboswitch for regulation of the btu operon, which could be a vestige of a system that also enabled Synechococcus sp. strain PCC 7002 to switch between the two methionine synthase isozymes. The presence of metE on a plasmid that can be highly variable in sequence and gene content in Synechococcus sp. strain PCC 73109 is consistent with this possibility.
This study shows that the cobalamin-independent Synechococcus sp. strain PCC 7002 reporter variant can potentially be utilized as a platform to test cobalamin riboswitches from other organisms for which axenic cultures do not exist or genetic systems are unavailable. For example, microbial mat communities possess a metabolic exchange network that sustains all of the organisms present (39, 40), and vitamin exchange, including vitamin B12 exchange, seems to be an important component of this network (41) (D. A. Rodionov, unpublished data). Metagenomics provide an indispensable tool in studying such communities, and this study describes one tool that can be used to confirm genomic inferences experimentally.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Craig Praul and Candace Price for advice and oversight of cDNA sequencing in the Genomics Core Facility, Huck Institutes for the Life Sciences, Penn State University, University Park, PA.
This work was supported by NSF grant MCB-1021725 and DOE contract DE-FG-02-05-ER46222 to D.A.B. Both D.A.B. and D.A.R. were supported by the Genomic Science Program (GSP), Office of Biological and Environmental Research (OBER), U.S. Department of Energy (DOE). We also acknowledge the NSF GK-12 CarbonEARTH grant (award number 0947962) for providing full tuition and stipend support to A.A.P.
Footnotes
This is a contribution of the Pacific Northwest National Laboratory (PNNL) Foundational Scientific Focus Area.
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00476-16.
For a companion article on this topic, see doi:10.1128/JB.00475-16.
REFERENCES
- 1.Ludwig M, Bryant DA. 2011. Transcription profiling of the model cyanobacterium Synechococcus sp. strain PCC 7002 by Next-Gen (SOLiD™) sequencing of cDNA. Front Microbiol 2:41. doi: 10.3389/fmicb.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakamoto T, Bryant DA. 2002. Synergistic effect of high-light and low temperature on cell growth of the Delta12 fatty acid desaturase mutant in Synechococcus sp. PCC 7002. Photosynth Res 72:231–242. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein HC, Konopka A, Melnicki MR, Hill EA, Kucek LA, Zhang S, Shen G, Bryant DA, Beliaev AS. 2014. Effect of mono- and dichromatic light quality on growth rates and photosynthetic performance of Synechococcus sp. PCC 7002. Front Microbiol 5:488. doi: 10.3389/fmicb.2014.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens SE, Porter RD. 1980. Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci U S A 77:6052–6056. doi: 10.1073/pnas.77.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Alvey RM, Byrne PO, Graham JE, Shen G, Bryant DA. 2011. Expression of genes in cyanobacteria: adaptation of endogenous plasmids as platforms for high-level gene expression in Synechococcus sp. PCC 7002. Methods Mol Biol 684:273–293. doi: 10.1007/978-1-60761-925-3_21. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm SW, Trick CG. 1995. Effects of vitamin B12 concentration on chemostat cultured Synechococcus sp. strain PCC 7002. Can J Microbiol 41:145–151. doi: 10.1139/m95-019. [DOI] [Google Scholar]
- 7.Carlucci AF. 1970. The ecology of the plankton off La Jolla, California, in the period April through September, 1967, edited by J. D. H. Strickland. Part II. Vitamin B12, thiamine, and biotin. Bull Scripps Inst Oceanogr 17:23–31. http://escholarship.org/uc/item/4q01m9gk. [Google Scholar]
- 8.Daisley KW, Fisher LR. 1958. Vertical distribution of vitamin B12 in the sea. J Mar Biol Assn UK 37:683–686. [Google Scholar]
- 9.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. 2003. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J Biol Chem 278:41148–41159. doi: 10.1074/jbc.M305837200. [DOI] [PubMed] [Google Scholar]
- 10.Roth JR, Lawrence JG, Bobik TA. 1996. Cobalamin (coenzyme B12): synthesis and biological significance. Annu Rev Microbiol 50:137–181. doi: 10.1146/annurev.micro.50.1.137. [DOI] [PubMed] [Google Scholar]
- 11.Wiener MC. 2005. TonB-dependent outer membrane transport: going for Baroque? Curr Opin Struct Biol 15:394–400. doi: 10.1016/j.sbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Shultis DD, Purdy MD, Banchs CN, Wiener MC. 2006. Outer membrane active transport: structure of the BtuB: TonB complex. Science 312:1396–1399. doi: 10.1126/science.1127694. [DOI] [PubMed] [Google Scholar]
- 13.Borths EL, Poolman B, Hvorup RN, Locher KP, Rees DC. 2005. In vitro functional characterization of BtuCD-F, the Escherichia coli ABC transporter for vitamin B12 uptake. Biochemistry 44:16301–16309. doi: 10.1021/bi0513103. [DOI] [PubMed] [Google Scholar]
- 14.Locher KP. 2004. Structure and mechanism of ABC transporters. Curr Opin Struct Biol 14:426–431. doi: 10.1016/j.sbi.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Croft MT, Warren MJ, Smith AG. 2006. Algae need their vitamins. Eukaryot Cell 5:1175–1183. doi: 10.1128/EC.00097-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez AA, Liu Z, Rodionov DA, Li Z, Bryant DA. 2016. Complementation of cobalamin auxotrophy in Synechococcus sp. strain PCC 7002 and validation of a putative cobalamin riboswitch in vivo. J Bacteriol 198:2743–2752. doi: 10.1128/JB.00475-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee RV, Matthews RG. 1990. Cobalamin-dependent methionine synthase. FASEB J 4:1450–1459. [DOI] [PubMed] [Google Scholar]
- 18.Pejchal R, Ludwig ML. 2005. Cobalamin-independent methionine synthase (MetE): a face-to-face double barrel that evolved by gene duplication. PLoS Biol 3:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helliwell KE, Wheeler GL, Leptos KC, Goldstein RE, Smith AG. 2011. Insights into the evolution of vitamin B12 auxotrophy from sequenced algal genomes research. Mol Biol Evol 28:2921–2933. doi: 10.1093/molbev/msr124. [DOI] [PubMed] [Google Scholar]
- 20.González JC, Banerjee RV, Huang S, Sumner JS, Matthews RG. 1992. Comparison of cobalamin-independent and cobalamin-dependent methionine synthases from Escherichia coli: two solutions to the same chemical problem. Biochemistry 31:6045–6056. doi: 10.1021/bi00141a013. [DOI] [PubMed] [Google Scholar]
- 21.Novichkov PS, Kazakov AE, Ravcheev DA, Leyn SA, Kovaleva GY, Sutormin RA, Kazanov MD, Riehl W, Arkin AP, Dubchak I, Rodionov DA. 2013. RegPrecise 3.0—a resource for genome-scale exploration of transcriptional regulation in bacteria. BMC Genomics 14:745. doi: 10.1186/1471-2164-14-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dehal PS, Joachimiak MP, Price MN, Bates JT, Baumohl JK, Chivian D, Friedland GD, Huang KH, Keller K, Novichkov PS, Dubchak IL, Alm EJ, Arkin AP. 2010. MicrobesOnline: an integrated portal for comparative and functional genomics. Nucleic Acids Res 38:D396–D400. doi: 10.1093/nar/gkp919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyngsø RB, Zuker M, Pedersen CN. 1999. Fast evaluation of internal loops in RNA secondary structure prediction. Bioinformatics 15:440–445. doi: 10.1093/bioinformatics/15.6.440. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 278:1–61. [Google Scholar]
- 27.Abreu-Goodger C, Merino E. 2005. RibEx: a web server for locating riboswitches and other conserved bacterial regulatory elements. Nucleic Acids Res 33:W690–W692. doi: 10.1093/nar/gki445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. 2004. Comparative genomics of the methionine metabolism in Gram-positive bacteria: a variety of regulatory systems. Nucleic Acids Res 32:3340–3353. doi: 10.1093/nar/gkh659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frigaard NU, Bryant DA. 2001. Chromosomal gene inactivation in the green sulfur bacterium Chlorobium tepidum by natural transformation. Appl Environ Microbiol 67:2538–2544. doi: 10.1128/AEM.67.6.2538-2544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frigaard NU, Sakuragi Y, Bryant DA. 2004. Gene inactivation in the cyanobacterium Synechococcus sp. PCC 7002 and the green sulfur bacterium Chlorobium tepidum using in vitro-made DNA constructs and natural transformation. Methods Mol Biol 274:325–340. [DOI] [PubMed] [Google Scholar]
- 31.Warner DF, Savvi S, Mizrahi V, Dawes SS. 2007. A riboswitch regulates expression of the coenzyme B12-independent methionine synthase in Mycobacterium tuberculosis: implications for differential methionine synthase function in strains H37Rv and CDC1551. J Bacteriol 189:3655–3659. doi: 10.1128/JB.00040-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson BR, Tezuka N, Watanabe MM. 2001. Phylogenetic analyses of Synechococcus strains (cyanobacteria) using sequences of 16S rDNA and part of the phycocyanin operon reveal multiple evolutionary lines and reflect phycobilin content. Int J Syst Evol Microbiol 51:861–871. doi: 10.1099/00207713-51-3-861. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig M, Chua TT, Chew CY, Bryant DA. 2015. Fur-type transcriptional repressors and metal homeostasis in the cyanobacterium Synechococcus sp. PCC 7002. Front Microbiol 6:1217. doi: 10.3389/fmicb.2015.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Bibber M, Bradbeer C, Clark N, Roth JR. 1999. A new class of cobalamin transport mutants (btuF) provides genetic evidence for a periplasmic binding protein in Salmonella typhimurium. J Bacteriol 181:5539–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locher KP, Borths E. 2004. ABC transporter architecture and mechanism: implications from the crystal structures of BtuCD and BtuF. FEBS Lett 564:264–268. doi: 10.1016/S0014-5793(04)00289-3. [DOI] [PubMed] [Google Scholar]
- 36.Zwiers LH, Stergiopoulos I, Gielkens MMC, Goodall SD, De Waard M. 2003. ABC transporters of the wheat pathogen Mycosphaerella graminicola function as protectants against biotic and xenobiotic toxic compounds. Mol Genet Genomics 269:499–507. doi: 10.1007/s00438-003-0855-x. [DOI] [PubMed] [Google Scholar]
- 37.Chimento DP, Kadner RJ, Wiener MC. 2003. The Escherichia coli outer membrane cobalamin transporter BtuB: structural analysis of calcium and substrate binding, and identification of orthologous transporters by sequence/structure conservation. J Mol Biol 332:999–1014. doi: 10.1016/j.jmb.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Hung LW, Wang IX, Nikaido K, Liu PQ, Ames GF, Kim SH. 1998. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature 396:703–707. doi: 10.1038/25393. [DOI] [PubMed] [Google Scholar]
- 39.Ward DM, Bateson MM, Ferris MJ, Kühl M, Wieland A, Koeppel A, Cohan FM. 2006. Cyanobacterial ecotypes in the microbial mat community of Mushroom Spring (Yellowstone National Park, Wyoming) as species-like units linking microbial community composition, structure and function. Philos Trans R Soc Lond B Biol Sci 361:1997–2008. doi: 10.1098/rstb.2006.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klatt CG, Wood JM, Rusch DB, Bateson MM, Hamamura N, Heidelberg JF, Grossman AR, Bhaya D, Cohan FM, Kühl M, Bryant DA, Ward DM. 2011. Community ecology of hot spring cyanobacterial mats: predominant populations and their functional potential. ISME J 5:1262–1278. doi: 10.1038/ismej.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z, Klatt CG, Ludwig M, Rusch DB, Jensen SI, Kuhl M, Ward DM, Bryant DA. 2012. ‘Candidatus Thermochlorobacter aerophilum’: an aerobic chlorophotoheterotrophic member of the phylum Chlorobi defined by metagenomics and metatranscriptomics. ISME J 6:1869–1882. doi: 10.1038/ismej.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.