Abstract
Sorting internalized proteins and lipids back to the cell surface controls the supply of molecules throughout the cell and regulates integral membrane protein activity at the surface. One central process in mammalian cells is the transit of cargo from endosomes back to the plasma membrane directly, along a route that bypasses retrograde movement to the Golgi. Despite recognition of this pathway for decades we are only beginning to understand the machinery controlling this overall process. Saccharomyces cerevisiae, a stalwart genetic system that has routinely helped identify key proteins and mechanisms for other fundamental trafficking steps, presents several obstacles that obscure the study of recycling, particularly through early endosomes. Here we briefly discuss how recycling could be a more prevalent process in yeast than is widely appreciated and how tools might be built to better study it.
Endocytic recycling
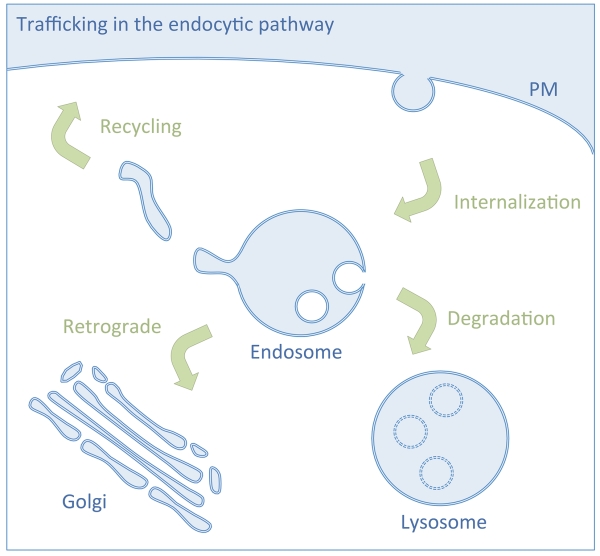
The process of endocytosis involves packaging of cell surface material into vesicles that are internalized and incorporated into the endosomal system, a complex network that intersects various intracellular trafficking pathways. In addition to sorting lipids and soluble molecules, endosomes parse internalized integral membrane proteins into different pathways to either effect their lysosomal degradation or their return to the plasma membrane (PM), trans-Golgi network (TGN), or other compartments within the endomembrane system (Figure 1). Early work following the internalization kinetics of receptors revealed that membrane turnover at the surface was constantly in flow from endosomes and that receptors used this process to repopulate the surface following ligand-uncoupling [1, 2]. Studies from cultured mammalian cells revealed a general itinerary for proteins that travel through the endocytic pathway [3]. Here, proteins internalized by a variety of routes converge in early sorting endosomes, defined by a mildly acidic pH and markers such as Rab5 and EEA1. Proteins returning to the PM can transit via a direct, fast recycling route that remains poorly defined at the mechanistic level. Alternatively, proteins travel to recycling endosomes marked by Rab11 [4], and then return to the PM via tubulovesicular structures controlled by a number of known proteins such as Rab11, its effectors, and Eps15 homology domain (EHD) proteins. Even from recycling endosomes, cargo may be segregated into different pathways; despite this plethora of sub-pathways, the general ability of proteins to recycle back to the PM is thought to reflect a ‘default’ pathway, since proteins without any known trafficking signals can be conveyed back to the PM after internalizing [5]. Additionally, specialized signals have been described that allow efficient transit along early endosome-to-PM recycling routes [6].
Figure 1. Endosomal trafficking pathways.
Internalized cell surface membrane proteins move to a common, early endosome. Cargo proteins localized to endosomes follow several distinct trafficking pathways: including the ubiquitin-mediated degradation pathway to the lysosome; the retrograde pathway to the Golgi; and the recycling pathway back to the plasma membrane. Trafficking within these broad directional classes of transit can be further subcategorized and there is significant overlap between each pathway.
Aside from recycling, other pathways from early sorting endosomes are also available. One is the multivesicular body (MVB) pathway, which packages protein cargo into intralumenal vesicles that form from the limiting membrane of endosomes and that are ultimately delivered to the lysosome for degradation or to the PM for secretion as exosomes. The canonical signal for entry into the MVB pathway is ubiquitin (Ub), which can be attached directly to cargo as a sorting signal [7] or attached to a protein associated with cargo to allow sorting in trans [8-10]. Alternatively, cargo can follow a ‘retrograde’ route, taking them back to the TGN where they re-join the secretory pathway [11, 12]. Such retrograde pathways include retromer-mediated sorting, which harnesses actin to form tubules from endocytic compartments to carry proteins back to the TGN [13]. Sorting signals for a variety of retrograde pathways have now been described, as has some of the sorting machinery that recognizes them, including retromer, AP1, GGA and SNX sorting nexin proteins [14]. The features (and even existence) of these pathways are in many ways defined by the cargo proteins that travel along them. Proteins such as the Transferrin receptor (TfR1) spend the majority of their time passing through the fast recycling pathway or through Rab11-positive recycling endosomes. Thus, cargo proteins such as TfR1 have been invaluable for revealing recycling pathways. However, it should be noted that TfR1 also follows a retrograde pathway back to the TGN [15] and a Ub-dependent pathway to the lysosome [16]. In yeast, direct recycling pathways that convey proteins from early endosomes to the PM have been more difficult to define owing to the lack of proteins that exclusively follow them.
Deubiquitnation as a trigger for recycling
Covalent attachment of ubiquitin (Ub) to integral membrane proteins is a conserved signal for entry into the multivesicular body / lysosomal degradation pathway, which is controlled by the Endosomal Sorting Complexes Required for Transport (ESCRT) apparatus [17]. The role of Ub in lysosomal degradation is extensive; therefore it is conceptually attractive to consider deubiqutintating peptidases (DUbs) as potential and permissive facilitators of recycling simply for their ability to intentionally antagonize the signals for lysosomal degradation (Figure 2). This has been documented for the Epidermal Growth Factor Receptor (EGFR), where deubiquitnating activity of AMSH, USP8, and Cezanne-1 serve to attenuate receptor turnover [18-21] allowing EGFR to repopulate the cell surface [22]. Yet, the effects of perturbing these DUbs can be explained by the possibility they regulate the sorting machinery itself rather than deubiquitinating cargo per se. Moreover, the search for other DUbs that might directly promote recycling of endocytosed proteins back to the PM of mammalian cells has revealed a paucity of candidates [17]. In yeast, however, evidence is stronger; supporting a model in which deubiquitination liberates membrane proteins from their degradative fate allowing them to utilize other sorting signals that can take them back to the PM. For instance, the Ftr1-Fet3 cell surface iron transporter complex is ubiquitinated and targeted for degradation along the MVB pathway in the presence of excess iron [23]. Yet, when the stimulus for that ubiquitination is removed, the complex is able to access a retromer-dependent route through a sorting motif that binds the retomer Snx3/Grd19 subunit [24]. Similarly, the sugar transporter Jen1 can transit back to the PM from endosomes once the signal that drives its Rod1-Rsp5 mediated ubiquitination is attenuated [25]. In addition, disruption of the large polymers of ESCRT-III that might trap ubiquitinated proteins on endosomes [26] or deletion of a newly described yeast family of cargo carrier proteins that sequester ubiquitinated proteins on endosomes [8], increases levels of cell surface proteins that are otherwise efficiently sent to the MVB pathway. In agreement with these findings, engineering yeast to deubiquitinate cargo once it contacts the ESCRT sorting machinery leads to high steady state levels of a variety of cell surface proteins, supporting the idea that deubiquitination can reveal a ‘default’ pathway for many cell surface proteins - not just cargo with specific sorting signals for retromer - to regain access to the PM after release from the clutches of the Ub-mediated degradation pathway [27]. Recently, we provided formal evidence that deubiquitination of cargo can mediate its transfer from endosomes back the cell surface. Here, the yeast methionine permease (Mup1), was sent to late endosomes by inducing its Art1-Rsp5-dependent ubiquitination, and was then subjected to chemically induced deubiquitnation using the FKBP-FRB system to attach a deubiquitinating enzyme directly to the cargo. While other ubiquitinated cargo remained in endosomes, the Mup1~DUb complex was transported back to the PM where it remained stable [8]. Importantly, Mup1 is not known to have specialized signals for entry into the known endosomal retrieval pathways. Thus, while such sorting signals may eventually be found, an alternative is that a wide variety of cell surface proteins have the capacity to recycle from endosomes in the absence of ubiquitination via a ‘default’ bulk transport mechanism.
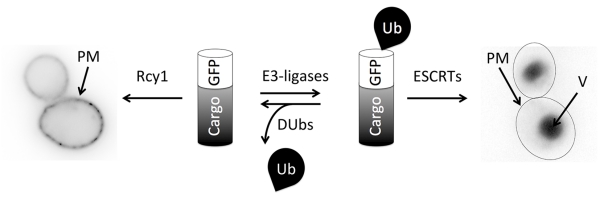
Figure 2. Ubiquitination status of cargo as an endosomal trafficking determinant.
Schematic representation of the counteracting activity between Ub-ligases and deubiquitinating enzymes (DUbs), the balance of which dictates directional trafficking through the endolysosomal system. The R-SNARE protein Snc1 labelled with GFP serves as a useful example, as it exhibits steady state localization at the plasma membrane, cycling between early endosomes and the cell surface in an Rcy1 dependent manner (left). However, Snc1 can also undergo ubiquitination and sort to the vacuole, which occurs very efficiently when cells are cultured beyond late log phase (right).
Recycling from early endosomes & the yeast system
The best studied pathways in yeast that convey proteins from endosomes back to the cell surface operate in late endosomal/post Golgi compartments and take back cargo to the secretory pathway along a ‘retrograde’ route [28]. In this way they differ from the ‘recycling’ pathway as understood from the itinerary of TfR1, which delivers cargo to the PM while largely avoiding intersecting the secretory pathway and TGN. Despite a lack of reporters equivalent to TfR1, yeast cells do possess functionally and morphologically distinct early and late endosomal compartments [29, 30]. Observing trafficking along a recycling route between early endosomes and the cell surface in yeast has been difficult, but is best defined by two cargoes proposed to follow it, and protein machinery required for it to operate. One cargo is the yeast R-SNARE, Snc1, which catalyses fusion of secretory vesicles to the PM, in concert with the cognate Sso1/Sec9 complex [31]. Snc1 also forms complexes with endosomal SNAREs and is required for maintaining morphology of the endosomal system, indicating it functions and transits within multiple compartments [32-34]. GFP labelled Snc1 has been experimentally valuable to report on recycling/retrograde pathways because it mainly travels among early endosomes, the TGN and the PM, and not late endosomes or the vacuole. Another cargo is the styrl dye FM4-64, which binds the PM and is internalized via the same actin-based machinery that internalizes cell surface proteins [35, 36]. While a portion of FM4-64 eventually travels to the limiting membrane of the vacuole, a greater proportion is externalized back into the medium [37]. Although retrograde trafficking of GFP-Snc1 to the Golgi can occur after internalization, it is not clear that the bulk of FM4-64 does. After internalization, co-localization of FM4-64 with the Golgi is marginal, with alignment in compartments juxtaposed rather than coincident with the Golgi marker Sec7 [38]. Blocking exit from the Golgi enhances this labelling, but the effect is not as profound as with GFP-Snc1. A retrograde route for FM4-64 predicts that a block in the fusion of late secretory vesicles would trap FM4-64 within vesicle clusters polarized for delivery to the daughter cell. However, sec1-temperature sensitive mutants at the non-permissive temperature accumulate FM4-64 diffusely in the cytosol likely reflecting other findings showing that prolonged blocks in the late secretory pathway disrupt endocytosis and internalization [35, 36]. COP1 has been implicated in moving materials from early endosomes to the Golgi, and while this perturbs GFP-Snc1 trafficking [38, 39], it does not appear to cause FM4-64 to remain in early endosomal structures and prevent its residual delivery to late endosomal compartments [40]. Thus, FM4-64 may follow a non-retrograde recycling route from endosomes back to the cell surface and partake in such a route far more than the GFP-Snc1 cargo protein. Better cartography of FM4-64 pathways using quantitative time-lapse fluorescence microscopy combined with various inducible trafficking blocks might provide a stringent test for a hypothetical direct endosome to PM route.
The seminal machinery that defines the trafficking pathway(s) taken by these two cargoes on their way from endosomes back to the cell surface is Rcy1, loss of which causes accumulation of both FM4-64 and GFP-tagged Snc1 within punctate endosomal structures [37, 41]. Rcy1 is an F-box containing protein that was originally implicated in recycling because cells lacking it mislocalized the fluid endocytic tracer Lucifer Yellow [42]. Rcy1 has a C-terminal CAAX box predicting that it is isoprenylated and which is important for its function and localization [41]. Rcy1 shows sequence homology to Sec10, the exocyst component involved in targeting vesicles. Rcy1 also has an N-terminal F-box motif, which is found in a large family of proteins that complex with Skp1 and Cullin to form SCF ubiquitin ligases. Although Rcy1 forms a complex with Skp1, it has not been found to form SCF complexes in vivo and its F-box domain does not fulfil the known structural requirements to allow it to bind Cullin [41, 43, 44]. It remains unclear whether Rcy1 ligase activity operates in vivo and how that might control its function in trafficking through early endosomes. Interestingly, Snc1 undergoes ubiquitination itself and this has been proposed to promote its trafficking back to the PM [45]. However, why this signal would rein over a typical ubiquitin-dependent and ESCRT-driven delivery to the vacuole is not clear.
Two additional proteins have been implicated in regulating Rcy1: firstly, the Rab11-related GTPases, Ypt31 and Ypt32, which control the levels of Rcy1 and are required for its localization [46]. Secondly, the P-type ATPase / flippase Drs2, in complex with the non-catalytic subunit Cdc50, is thought to drive the lipid organization and membrane deformation required for recycling vesicle biogenesis [47, 48]. Ypt31/32 and Drs2/Cdc50 all physically interact with Rcy1, which in turn binds Snc1 [46, 47, 49]. The fact all these components appear to be required for recycling and the knowledge that Snc1 SNARE complexes drive fusion at the cell surface [34], presents the possibility that this core complex constitutes the basic machinery for cell surface recycling. Thus, Snc1 is as much part of the multipurpose trafficking machine as it is a cargo, a notion that undermines the usefulness of Snc1 as a simple reporter for recycling. The discovery of passive cargoes that exclusively transit this pathway may allow better delineation of the pathways leading from early endosomes and help reveal other cargoes and regulators.
Conclusion
To what extent does the early endosome to PM pathway in yeast mirror that of animal cells, and is there one distinct from the retrograde pathway? For Snc1, there is clear evidence that it can travel along a retrograde route from endosomes back to the secretory pathway, and it can be trapped in secretory pathway mutants [34]. One approach to reveal a direct recycling route analogous to TfR1 in animal cells might be to diminish retrograde traffic. Good candidates for this include the Arf Gap Gcs1 and the COPI coatomer, loss of which traps GFP-Snc1 almost exclusively in early endosomes and the PM [39]. An alternative to engineering a crippled yeast strain would be to create tools to report on distinct trafficking routes. As discussed above, one of the problems in studying the trafficking of most cell surface proteins is that they ultimately become modified by Ub and are sent into the MVB pathway. This problem even plagues GFP-Snc1, which is sent to the vacuole when cells are simply grown past log phase, [8] and Figure 2. Recently, we developed a tool that negates the effects of ubiquitination on cargoes: one such example is a chimeric protein based on the recycling cargo Ste3 fused to catalytic domain of a DUb. This reporter, which is resistant to ubiquitination, is unaffected by mutations in late endosomal ESCRT machinery but maintains reliance on Rcy1 for efficient sorting to the PM [50]. Such synthetic reporters, excluded from the degradation pathway and dedicated to early endosome recycling, might be useful to study this trafficking in the yeast system.
Acknowledgments
This work was funded by the American Heart Association [CM, 15POST-22980010] and the National Institutes of Health [RCP, NIHRO1-GM058202].
Footnotes
No conflict of interest is declared.
References
- 1.Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annual review of cell biology. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A, Schwartz AL, Lodish HF. Sorting and recycling of cell surface receptors and endocytosed ligands: the asialoglycoprotein and transferrin receptors. Journal of cellular biochemistry. 1983;23:107–130. doi: 10.1002/jcb.240230111. [DOI] [PubMed] [Google Scholar]
- 3.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 6.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 7.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald C, Payne JA, Aboian M, Smith W, Katzmann DJ, Piper RC. A family of tetraspans organizes cargo for sorting into multivesicular bodies. Dev Cell. 2015;33:328–342. doi: 10.1016/j.devcel.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald C, Stamnes MA, Katzmann DJ, Piper RC. Tetraspan cargo adaptors usher GPI-anchored proteins into multivesicular bodies. Cell cycle. 2015 doi: 10.1080/15384101.2015.1100773. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDonald C, Stringer DK, Piper RC. Sna3 Is an Rsp5 Adaptor Protein that Relies on Ubiquitination for Its MVB Sorting. Traffic. 2012 doi: 10.1111/j.1600-0854.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chia PZ, Gunn P, Gleeson PA. Cargo trafficking between endosomes and the trans-Golgi network. Histochemistry and cell biology. 2013;140:307–315. doi: 10.1007/s00418-013-1125-6. [DOI] [PubMed] [Google Scholar]
- 12.Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–1187. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol. 2012;14:29–37. doi: 10.1038/ncb2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burd C, Cullen PJ. Retromer: a master conductor of endosome sorting. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snider MD, Rogers OC. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J Cell Biol. 1985;100:826–834. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tachiyama R, Ishikawa D, Matsumoto M, Nakayama KI, Yoshimori T, Yokota S, Himeno M, Tanaka Y, Fujita H. Proteome of ubiquitin/MVB pathway: possible involvement of iron-induced ubiquitylation of transferrin receptor in lysosomal degradation. Genes to cells : devoted to molecular & cellular mechanisms. 2011;16:448–466. doi: 10.1111/j.1365-2443.2011.01499.x. [DOI] [PubMed] [Google Scholar]
- 17.Piper RC, Dikic I, Lukacs GL. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullough J, Clague MJ, Urbe S. AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol. 2004;166:487–492. doi: 10.1083/jcb.200401141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuno E, Kobayashi K, Yamamoto A, Kitamura N, Komada M. A deubiquitinating enzyme UBPY regulates the level of protein ubiquitination on endosomes. Traffic. 2006;7:1017–1031. doi: 10.1111/j.1600-0854.2006.00452.x. [DOI] [PubMed] [Google Scholar]
- 20.Row PE, Prior IA, McCullough J, Clague MJ, Urbe S. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J Biol Chem. 2006;281:12618–12624. doi: 10.1074/jbc.M512615200. [DOI] [PubMed] [Google Scholar]
- 21.Pareja F, Ferraro DA, Rubin C, Cohen-Dvashi H, Zhang F, Aulmann S, Ben-Chetrit N, Pines G, Navon R, Crosetto N, Kostler W, Carvalho S, Lavi S, Schmitt F, Dikic I, Yakhini Z, Sinn P, Mills GB, Yarden Y. Deubiquitination of EGFR by Cezanne-1 contributes to cancer progression. Oncogene. 2012;31:4599–4608. doi: 10.1038/onc.2011.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirisaengtaksin N, Gireud M, Yan Q, Kubota Y, Meza D, Waymire JC, Zage PE, Bean AJ. UBE4B protein couples ubiquitination and sorting machineries to enable epidermal growth factor receptor (EGFR) degradation. J Biol Chem. 2014;289:3026–3039. doi: 10.1074/jbc.M113.495671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felice MR, De Domenico I, Li L, Ward DM, Bartok B, Musci G, Kaplan J. Post-transcriptional regulation of the yeast high affinity iron transport system. J Biol Chem. 2005;280:22181–22190. doi: 10.1074/jbc.M414663200. [DOI] [PubMed] [Google Scholar]
- 24.Strochlic TI, Setty TG, Sitaram A, Burd CG. Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J Cell Biol. 2007;177:115–125. doi: 10.1083/jcb.200609161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becuwe M, Vieira N, Lara D, Gomes-Rezende J, Soares-Cunha C, Casal M, Haguenauer-Tsapis R, Vincent O, Paiva S, Leon S. A molecular switch on an arrestin-like protein relays glucose signaling to transporter endocytosis. J Cell Biol. 2012;196:247–259. doi: 10.1083/jcb.201109113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teis D, Saksena S, Emr SD. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev Cell. 2008;15:578–589. doi: 10.1016/j.devcel.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald C, Buchkovich NJ, Stringer DK, Emr SD, Piper RC. Cargo ubiquitination is essential for multivesicular body intralumenal vesicle formation. EMBO Rep. 2012;13:331–338. doi: 10.1038/embor.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burd CG. Physiology and pathology of endosome-to-Golgi retrograde sorting. Traffic. 2011;12:948–955. doi: 10.1111/j.1600-0854.2011.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hicke L, Zanolari B, Pypaert M, Rohrer J, Riezman H. Transport through the yeast endocytic pathway occurs through morphologically distinct compartments and requires an active secretory pathway and Sec18p/N-ethylmaleimide-sensitive fusion protein. Mol Biol Cell. 1997;8:13–31. doi: 10.1091/mbc.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prescianotto-Baschong C, Riezman H. Ordering of compartments in the yeast endocytic pathway. Traffic. 2002;3:37–49. doi: 10.1034/j.1600-0854.2002.30106.x. [DOI] [PubMed] [Google Scholar]
- 31.Rossi G, Salminen A, Rice LM, Brunger AT, Brennwald P. Analysis of a yeast SNARE complex reveals remarkable similarity to the neuronal SNARE complex and a novel function for the C terminus of the SNAP-25 homolog, Sec9. J Biol Chem. 1997;272:16610–16617. doi: 10.1074/jbc.272.26.16610. [DOI] [PubMed] [Google Scholar]
- 32.Lewis MJ, Pelham HR. A new yeast endosomal SNARE related to mammalian syntaxin 8. Traffic. 2002;3:922–929. doi: 10.1034/j.1600-0854.2002.31207.x. [DOI] [PubMed] [Google Scholar]
- 33.Holthuis JC, Nichols BJ, Dhruvakumar S, Pelham HR. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 1998;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grote E, Vlacich G, Pypaert M, Novick PJ. A snc1 endocytosis mutant: phenotypic analysis and suppression by overproduction of dihydrosphingosine phosphate lyase. Mol Biol Cell. 2000;11:4051–4065. doi: 10.1091/mbc.11.12.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll SY, Stimpson HE, Weinberg J, Toret CP, Sun Y, Drubin DG. Analysis of yeast endocytic site formation and maturation through a regulatory transition point. Mol Biol Cell. 2012;23:657–668. doi: 10.1091/mbc.E11-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiederkehr A, Avaro S, Prescianotto-Baschong C, Haguenauer-Tsapis R, Riezman H. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J Cell Biol. 2000;149:397–410. doi: 10.1083/jcb.149.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis MJ, Nichols BJ, Prescianotto-Baschong C, Riezman H, Pelham HR. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol Biol Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson M, Poon PP, Schindler C, Murray LE, Kama R, Gabriely G, Singer RA, Spang A, Johnston GC, Gerst JE. The Gcs1 Arf-GAP mediates Snc1,2 v-SNARE retrieval to the Golgi in yeast. Mol Biol Cell. 2006;17:1845–1858. doi: 10.1091/mbc.E05-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabriely G, Kama R, Gerst JE. Involvement of specific COPI subunits in protein sorting from the late endosome to the vacuole in yeast. Mol Cell Biol. 2007;27:526–540. doi: 10.1128/MCB.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galan JM, Wiederkehr A, Seol JH, Haguenauer-Tsapis R, Deshaies RJ, Riezman H, Peter M. Skp1p and the F-box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Mol Cell Biol. 2001;21:3105–3117. doi: 10.1128/MCB.21.9.3105-3117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiederkehr A, Meier KD, Riezman H. Identification and characterization of Saccharomyces cerevisiae mutants defective in fluid-phase endocytosis. Yeast. 2001;18:759–773. doi: 10.1002/yea.726. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt MW, McQuary PR, Wee S, Hofmann K, Wolf DA. F-box-directed CRL complex assembly and regulation by the CSN and CAND1. Mol Cell. 2009;35:586–597. doi: 10.1016/j.molcel.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kus BM, Caldon CE, Andorn-Broza R, Edwards AM. Functional interaction of 13 yeast SCF complexes with a set of yeast E2 enzymes in vitro. Proteins. 2004;54:455–467. doi: 10.1002/prot.10620. [DOI] [PubMed] [Google Scholar]
- 45.Chen SH, Shah AH, Segev N. Ypt31/32 GTPases and their F-Box effector Rcy1 regulate ubiquitination of recycling proteins. Cellular logistics. 2011;1:21–31. doi: 10.4161/cl.1.1.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen SH, Chen S, Tokarev AA, Liu F, Jedd G, Segev N. Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol Biol Cell. 2005;16:178–192. doi: 10.1091/mbc.E04-03-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furuta N, Fujimura-Kamada K, Saito K, Yamamoto T, Tanaka K. Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31p/32p-Rcy1p pathway. Mol Biol Cell. 2007;18:295–312. doi: 10.1091/mbc.E06-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hua Z, Fatheddin P, Graham TR. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol Biol Cell. 2002;13:3162–3177. doi: 10.1091/mbc.E02-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanamatsu H, Fujimura-Kamada K, Yamamoto T, Furuta N, Tanaka K. Interaction of the phospholipid flippase Drs2p with the F-box protein Rcy1p plays an important role in early endosome to trans-Golgi network vesicle transport in yeast. Journal of biochemistry. 2014;155:51–62. doi: 10.1093/jb/mvt094. [DOI] [PubMed] [Google Scholar]
- 50.Stringer DK, Piper RC. A single ubiquitin is sufficient for cargo protein entry into MVBs in the absence of ESCRT ubiquitination. J Cell Biol. 2011;192:229–242. doi: 10.1083/jcb.201008121. [DOI] [PMC free article] [PubMed] [Google Scholar]