Abstract
Background
Investigations of the association of combination antiretroviral therapy (ART) with pregnancy outcomes often rely on routinely collected clinical data, which are prone to missing data and measurement error. Measurement error in gestational age may bias the relationship between combination ART and gestational age-based outcomes.
Methods
We demonstrate the use of multiple overimputation to address missing data and measurement error in gestational age. Using routinely collected clinical data from public health facilities in Lusaka, Zambia, we multiply imputed missing data and multiply overimputed observed values of gestational age. Poisson models with robust variance estimators were used to estimate risk ratios (RRs) for the associations of duration of combination ART with small for gestational age (SGA) and preterm birth. We compared results from a complete-case analysis, using multiple imputation to address missing data only, and using multiple overimputation to address missing data and measurement error.
Results
In the complete-case analysis, there was no evidence of an association between duration of combination ART and SGA or preterm birth. When we performed multiple overimputation, RRs for SGA moved past the null, but remained imprecise. For preterm birth, RRs for 9-32 weeks of combination ART moved away from the null as the variance due to measurement error increased.
Conclusion
When we used multiple overimputation to account for measurement error and missing data, we observed an increased risk of preterm birth with longer duration of combination ART. Future analyses examining associations between combination ART and pregnancy outcomes should consider using multiple overimputation to address measurement error in gestational age.
Introduction
In 2013 the WHO recommended lifetime combination antiretroviral therapy (ART) for all HIV-infected pregnant women in countries with a generalized HIV epidemic.(1) When taken consistently during pregnancy and breastfeeding, combination ART reduces the risk of mother-to-child HIV transmission to <5%.(2) However, the effect of combination ART on fetal growth and length of gestation remains controversial; its use during pregnancy has been associated with preterm birth (3, 4) and low infant birthweight (LBW(5) in Europe and the United States.(6) In resource-limited settings, combination ART use during pregnancy has also been associated with an increased risk of preterm birth (7), LBW (8) and small for gestational age (SGA).(9)
The impact of combination ART on pregnancy outcomes is of great concern in sub-Saharan Africa, where HIV prevalence remains high among women of reproductive age and lifetime combination ART initiation during pregnancy is rapidly being scaled up.(10) With nearly 13 million people on combination ART globally, the need to understand combination ART's association with pregnancy outcomes is critical.(11) In resource-limited settings, investigations of combination ART's association with pregnancy outcomes often rely on routinely collected clinical data.(9) Clinical data provides an important source of information for monitoring pregnancy outcomes of HIV-infected women, but are typically not collected for research purposes. Routinely collected clinical data are consequently often plagued by missing data and measurement error.(12)
Missing data and measurement error in gestational age may introduce bias when evaluating the relationship between combination ART and gestational age-based outcomes like preterm birth and SGA. Gestation age dating based on last menstrual period (LMP) may include error due to natural variation in menstrual cycle length (and thus timing of ovulation, relative to LMP), errors in recall or missing LMP dates.(13) Inaccurate LMP dates may be more common among women in the developing world, where malnutrition, high fertility rates and longer breastfeeding duration may result in women not resuming regular menstrual cycles before becoming pregnant again.(14) Menstrual abnormalities are common in HIV-infected women, which may further limit the reliability of LMP dating (15) and have implications for our understanding of combination ART's association with preterm birth and SGA.
To assess the impact of bias from missing data and measurement error in GA on the associations of duration of combination ART with SGA and preterm birth, we demonstrate the use of multiple overimputation. We describe multiple overimputation and illustrate its use as applied to routinely collected clinical data from public healthcare facilities in Lusaka, Zambia. We end with a discussion of our results and an evaluation of whether MOs assumptions are likely met in our data.
Methods
Multiple overimputation
Multiple overimputation is a convenient approach to address missing data and measurement error simultaneously. Practical and technical details for multiple overimputation has been described elsewhere.(19-23) Our goal is to illustrate the use of multiple overimputation to address missing data and measurement error in gestational age. Briefly, multiple overimputation addresses missing data in the same way as multiple imputation: missing values are multiply imputed based on observed covariates. Observed, but mismeasured, values are handled slightly differently. Mismeasured values are overimputed (replaced) with multiply imputed values based on observed covariates, with an additional step: observed mismeasured values are used to create observation-level Bayesian priors for the imputation model.(19) The goal of specifying observation-level Bayesian priors is to incorporate prior knowledge (in the form of the observed mismeasured value), as well as appropriate uncertainty, about an observed variable's true value into the imputation model.(19) Specifying observation-level Bayesian priors involves two parts: specifying the mean of the prior distribution and the variance due to measurement error. Additional details about specifying observation-level priors, multiple overimputation, and its assumptions are available in eAppendix 1.
Data Analysis
We provide an illustrative example of multiple overimputation to address measurement error in gestational age when examining the association of duration of combination ART with SGA and preterm birth. Duration of combination ART during pregnancy may affect length of gestational or fetal growth, (26) leading to an increased risk of preterm birth or SGA.(7, 9) In order to better understand the relationship between duration of combination ART with SGA and preterm birth, accurately measuring gestational age is critical. Data for the present retrospective cohort analysis come from the Zambia Electronic Perinatal Record System (ZEPRS), which has collected routine maternity and HIV clinical information in 24 public facilities in Lusaka, Zambia since 2007. Clinics in ZEPRS see a high volume of patients and errors in in remembering or recording LMP dates are common. Therefore measurement error within each week of gestational age was considered likely to be normally distributed. The goal of our illustrative example was to assess whether associations for duration of combination ART with SGA and preterm birth changed under differing assumptions about measurement error in gestational age.
Study population
Women who presented for antenatal care and delivered between January 1, 2009 and September 2, 2013 were included in the present analysis if they had a CD4 count of ≤350 cells/uL, were not on combination ART at entry into antenatal care and delivered a singleton pregnancy at a public healthcare facility at ≥28 weeks gestation (fetal viability cut-off in Zambia). Women with chronic conditions such as known heart disease, hypertension, and diabetes were excluded since they have poorer pregnancy outcomes (27, 28) and may be more likely to seek antenatal care earlier due to their preexisting conditions. A related analysis used a similar study population, but evaluated LBW as an outcome(29). Ethical approval for the analysis of routinely collected clinical data was obtained from the University of Zambia Biomedical Research Ethics Committee (Lusaka, Zambia) and the University of North Carolina, Chapel Hill (Chapel Hill, NC).
Definitions
For the present analysis, SGA was considered the primary outcome in order to evaluate fetal growth adjusted for length of gestation. SGA was defined as birthweight below the 10th percentile for each week of gestational age at birth (weeks 28-41). The reference curve used to define SGA was based on fetal weight and adjusted to account for lower overall birthweights in a Zambian population.(30) This method has previously been shown to reliably classify SGA infants, in comparison to more complex customized reference curves.(30) Preterm birth (delivery at <37 weeks gestation) was considered as a secondary outcome. The exposure of interest was duration of combination ART before delivery, measured in completed weeks and assessed at 32 weeks gestation. Duration of combination ART during pregnancy is intrinsically linked to length of gestation. Consequently, longer duration of treatment may appear protective against preterm birth simply by being a marker of longer gestation. To mitigate the dependency between duration of treatment and duration of gestation, and allow the majority of women to complete their exposure duration before delivery, we assessed combination ART duration at 32 weeks. Duration of combination ART was categorized into four groups: never initiated or did not initiate by 32 weeks gestation (referent), 1-8 weeks, or 9-32 weeks. Category cut-points were based on the functional form of the relationship between the exposure and outcome, as well as clinical considerations to approximately correspond with early and mid-to-late pregnancy.
We identified likely confounders using directed acyclic graphs (31, 32) and included age, baseline body mass index (BMI), baseline CD4 count, baseline hemoglobin, education, intermittent presumptive therapy (IPT) for malaria, parity, reported previous preterm birth (<37 weeks gestation), syphilis screening/treatment, self-reported tuberculosis status and number of antenatal care visits. Number of antenatal care visits and education were used as proxies for health seeking behavior.(33) The functional form of the relationship between continuous confounders and outcome was assessed and confounders were modeled using restricted quadratic splines.(34) All confounders were included in multivariable models. Information on viral load, WHO clinical stage, antiretroviral adherence and drug regimen was not available.
Assessment of Measurement Error in Gestational Age
As is common practice in many resource-limited settings, (35) gestational age was calculated by last menstrual period (LMP) for pregnancies less than 20 weeks at time of enrollment into antenatal care. For those ≥20 weeks at enrollment, both LMP and symphysis-fundal height were used. If these two methods yielded gestational ages within 3 weeks of each other, the date based on the LMP was used. If not, the fundal height–derived gestational age was used.
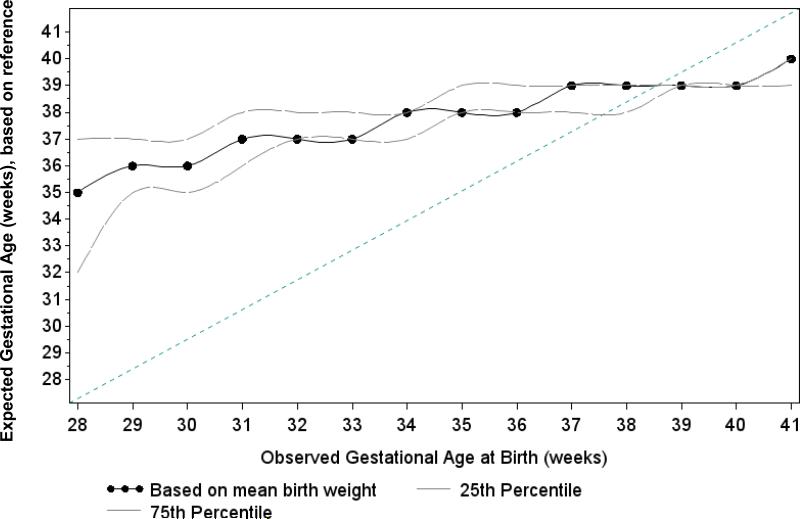
We assessed the presence and direction of measurement error in observed gestational age values by comparing the mean birthweight for each week of gestational age at birth to a reference curve adjusted to a Zambian population.(30) For each observed week of gestational age at birth, we determined the expected gestational age value by finding the smallest absolute difference in mean birthweight among reference curve values. For example, if the mean birthweight for infants born at observed gestational age 35 weeks was 2,900 grams, based on reference value mean birthweights the gestational age was more likely to be 38 weeks. We repeated the same process for the 25th and 75th percentiles of weight to assess if the pattern of observed versus expected values was consistent across the birthweight distribution (Figure 1). The difference between observed and expected values of gestational age was used to account for the bias (ai) (or non-random nature) of the measurement error in gestational age.
Figure 1.
Observed versus expected gestational age of 9,529 HIV-infected women in Lusaka, Zambia 2009-2013. The X-axis indicates the value of gestational age (weeks) observed in the data. To calculate expected values of gestational age (Y-axis), we compared each week of gestational age at birth to a reference curve adjusted to a Zambian population and found the smallest absolute difference in birthweight among reference curve values. The dotted line indicates expected gestational age based on mean birthweight and the dashed lines indicate expected values based on the 25th and 75th percentiles of birthweight. The diagonal line indicates perfect agreement between observed and expected values. The divergence of the dotted and dashed lines from the line of agreement at gestational ages <37 weeks) suggests that the measurement error in at these gestational ages is non-random in the direction of gestational age being incorrectly specified as too early. At gestational ages ≥37 weeks, measurement error appeared to be more randomly distributed around the line of agreement.
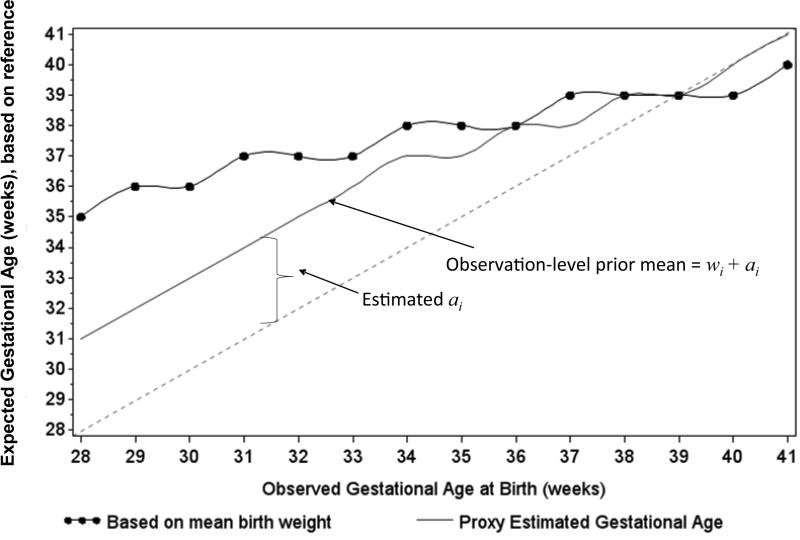
To investigate the possibility of digit preference in measured birthweight values (which could affect expected gestational age values), we inspected histograms of birthweight for each week of gestational age (eAppendix 2). We attempted to account for digit preference in gestational age values by choosing estimates of bias (ai) between observed and expected gestational ages. Values of ai ranging from 4 to 0 weeks were used to account for both random and non-random measurement error across the distribution of gestational age (Figure 2).
Figure 2.
Estimated bias (ai) and observation-level prior mean values (wi + ai) for 9,529 HIV-infected women in Lusaka, Zambia 2009-2013. The solid line with dots indicates expected gestational age values, based on reference curve mean birthweights. The dashed line indicates perfect agreement between observed and expected gestational age values. The divergence of expected values from the line of agreement indicates non-random measurement error. To correct this non-random measurement error, an estimate of the bias was calculated (ai) and added to each observed value of gestational age (wi) to set the mean value of the prior for each week of gestational age (solid line). Due to concerns over digit preference at earlier gestational ages, we selected values for ai such that observation-level prior mean values were between expected values and observed values. To accommodate the apparent shift from non-random and to random measurement error across the distribution of gestational ages, we selected offset values ranging from 4 to 0 weeks.
Statistical Analyses
We used Poisson models with robust variance estimators to estimate risk ratios (RR) and 95% confidence intervals (CIs),(36) due to lack of convergence of log-binomial models, for the associations of duration of combination ART with SGA and preterm birth in three separate analyses. First, we conducted a complete-case analysis using only the observed data (naïve-analysis). Second, we used multiple imputation to impute missing data for all confounders, the exposure and the GA as a continuous measure (used to define both outcomes). The imputation model included predictors of any missing data (not only missing GA), all confounders, the exposure and the outcome.(20) Third, we used multiple overimputation to impute missing confounder and exposure information and to overimpute GA. Due to the fact that the data arise from routine clinical care and not all women are under observation from a uniform time point in pregnancy, we note that reported effect estimates are interpretable as associations and not causal effects.
We conducted four sensitivity analyses. First, we conducted an analysis assuming only normal random measurement error in gestational age. Second, we included only women who delivered after 32 weeks of gestation. Third, to account for the possibility of measurement error in birthweight, we overimputed both gestational age and birthweight. Finally, we altered the specification of the imputation model and assessed whether results meaningfully changed.
Observation-level priors were specified for all measured values of gestational age. To account for non-random measurement error in the distribution of gestational age, we set each observation-level prior mean to the observed value of gestational age (wi) plus an estimate of the bias (ai) (eAppendix Table 1) in gestational age. Conditional on each observation-level prior mean and observed covariates, measurement error was assumed to be random. Information on the degree of measurement error in gestational age in our data was not available. To be conservative we specified a range of variances due to measurement error, assuming that measurement error accounted for between 15% and 60% of the variance in gestational age, and was most likely 30%. Multiple overimputation was performed using the Amelia II package in R; all other statistical analyses were performed using SAS version 9.2 (R Development Core Team Vienna, Austria; SAS Institute Inc. Cary, NC).(21)
Results
Among 50,765 HIV-infected pregnant women, 9,529 women met inclusion criteria for our study population. Of the 9,529 women included, 583 (8%) delivered SGA infants, 3,656 (45%) delivered preterm infants and 108 (1%) delivered infants that were both preterm and SGA. Most women did not initiate combination ART by 32 weeks gestation (n=6,925, 77%; of these, 778 initiated combination ART at or after 32 weeks), 1415 (16%) women had between 1 and 8 weeks of combination ART by 32 weeks gestation and 611 (8%) had between 9 and 32 weeks of combination ART by 32 weeks gestation.
Missing data were common. Of the 9,529 women included, 1,399 (15%) were missing gestational age at birth. A SGA value could not be calculated for additional 733 women who had recorded gestational ages >41 weeks (SGA defined for weeks 28-41 only) or who were missing birthweight information (total missing SGA: n=2,132 (22%)). Only 5% (n=542) of women were missing exposure information due to not having a gestational age value at first antenatal care recorded. Overall, 6,625 (70%) of women were missing exposure, outcome or confounder information (Table 1).
Table.
Sociodemographic and obstetric characteristics of 9,529 HIV-infected women eligible for cART initiation during pregnancy in Lusaka, Zambia 2009-2013.
| Characteristic | Small for gestational age a N(%) or Median (IQR) |
Normal for gestational age a N(%) or Median (IQR) |
Missing Data N (%) |
|---|---|---|---|
| N=583 (8) | N=6,814 (92) | N=9,529 | |
| Age | 27 (23, 31) | 27 (23, 31) | 17 (0) |
| Education | 1,201 (13) | ||
| Primary or None | 252 (50) | 2,720 (46) | |
| Secondary or higher | 255 (50) | 3,223 (54) | |
| Parity | 903 (10) | ||
| 0 | 145 (27) | 1,150 (19) | |
| 1 | 140 (26) | 1,571 (24) | |
| 2 | 108 (20) | 1,517 (25) | |
| 2+ | 146 (27) | 1,912 (31) | |
| BMI | 23 (21, 25) | 23 (22, 25) | 3,130(33) |
| CD4 count | 230 (156, 286) | 239 (168, 297) | 0 (0) |
| Hemoglobin | 11 (10, 12) | 11 (10, 12) | 759 (8) |
| Syphilis screening | 0 (0) | ||
| Non-reactive | 372 (64) | 4,246 (62) | |
| Reactive | 28 (5) | 331 (5) | |
| Not tested | 183 (31) | 2,237 (33) | |
| Tuberculosis | 2,590 (27) | ||
| No | 410 (97) | 4,380 (97) | |
| Yes | 14 (3) | 137 (3) | |
| IPT b for Malaria | 0 (0) | ||
| None | 154 (26) | 1,701 (25) | |
| 1 dose SP | 301 (52) | 3,543 (52) | |
| 2 doses SP | 82 (14) | 1,086 (16) | |
| 3 doses SP | 46 (8) | 478 (7) | |
| Number of antenatal care visits | 0 (0) | ||
| 1 | 298 (51) | 3,607 (54) | |
| 2 | 141 (24) | 1,734 (26) | |
| 3 | 97 (17) | 978 (14) | |
| ≥4 | 47 (8) | 432 (6) | |
| Previous preterm birth | 0 (0) | ||
| No | 564 (96.7) | 6,602 (96.9) | |
| Yes | 19 (3.3) | 212 (3.1) | |
Missing outcomes: SGA 2,132 (22.4%), preterm birth 1,399 (14.7%).
cART : combination antiretroviral therapy
BMI = body mass index; IPT: intermittent presumptive therapy; SP: sulfadoxine-pyrmethamine
Comparisons of birthweights for each week of gestational age with reference curve values suggested appreciable measurement error in gestational age. Among infants born preterm, observed gestational age was lower than expected, suggesting it was incorrectly specified as too early, perhaps due to the fact that women in this population may not be menstruating regularly. Among infants born at term, measurement error appeared to be more random (i.e. expected values randomly distributed around observed values) (Figure 1). Inspection of histograms of birthweight overall and by gestational age week revealed some digit preference at birthweight 3,000 grams.
Small for gestational age
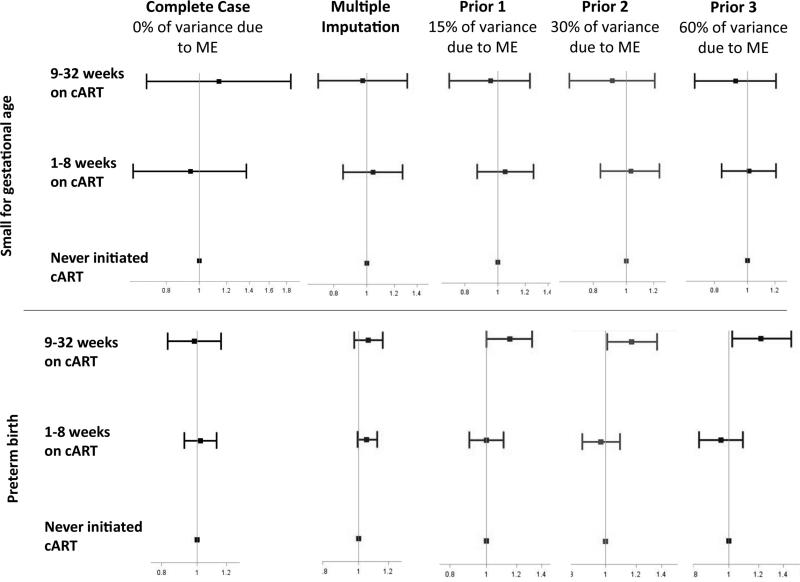
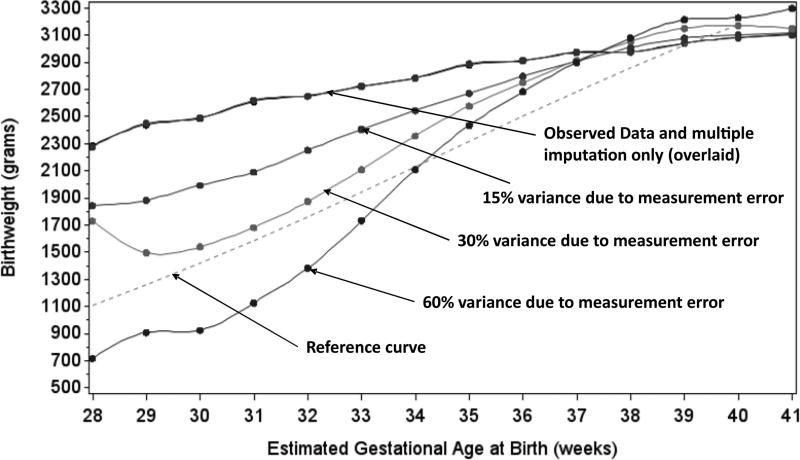
There was no evidence of an association between combination ART and SGA in the naïve analysis: RR1.1 (95% CI 0.7, 1.9) for 9-32 weeks combination ART and RR 0.9 (95% CI 0.6, 1.4) for =<8 weeks combination ART, by 32 weeks gestation (Figure 3). In the multiple overimputation analysis, when 30% of variance due to measurement error was assumed, receiving 9-32 weeks of combination ART was associated with RR 0.9 (95% CI 0.7, 1.21) and ≤8 weeks of combination ART was associated with RR 1.03 (95% CI 0.8, 1.3). As the amount of variance in gestational age due to measurement error increased, the joint distribution of gestational age and mean birthweight approached that of the reference curve values, suggesting that values for gestational age from the multiple overimputation analysis were closer to what might be expected, based on reference curve values (Figure 4).
Figure 3.
Associations between duration of combination ART before delivery with SGA and preterm birth (RRs and 95% CIs). All models adjusted for: number of antenatal care visits, age, BMI, CD4 count, education, hemoglobin, intermittent presumptive therapy, parity, syphilis screening/treatment, tuberculosis status and prior preterm birth. All models are estimated using Poisson regression with robust variance estimators.
Figure 4.
Gestational age at birth by mean birthweight for 9,529 HIV-infected women in Lusaka, Zambia 2009-2013 in the observed, multiply imputed and multiply overimputed data. The dashed line indicates reference curve values for gestational age by mean birthweight.
Preterm birth
Similarly, there was no evidence of an association between duration of combination ART and preterm birth in the naïve-analysis: RR 1.0 (95% CI 0.8, 1.2) for 9-32 weeks of combination ART and RR 1.0 (95%CI 0.9, 1.1) for ≤8 weeks of combination ART, by 32 weeks. When 30% of variance due to measurement error was assumed, the risk of preterm birth increased with longer duration of combination ART: RR 1.2 (95% CI 1.0, 1.4) for 9-32 weeks of combination ART and RR 1.0 (95% CI 0.9, 1.1) for ≤8 weeks of combination ART(Figure 3). The proportion of preterm births decreased from 45% in the naïve analysis to 30%, when 60% of the variance in gestational age was considered due to measurement error. For both SGA and preterm birth, performing multiple imputation alone dramatically improved precision, compared with the naïve-analysis. Additionally performing multiple overimputation appropriately propagated uncertainty about the true value of gestational age through to final confidence intervals, slightly decreasing precision from the multiple imputation analysis. For both preterm birth and SGA, results were similar across sensitivity analyses.
Discussion
The goal of our analysis was to demonstrate the use of multiple overimputation and to assess whether associations between duration of combination ART and pregnancy outcomes were sensitive to assumptions about measurement error in gestational age. We found no evidence of an increased risk of SGA across a range of assumptions about measurement error in gestational age. This finding aligns with some previous evidence that combination ART use during pregnancy does not appear to increase the risk of fetal growth restriction.(37) More recent work from animal models suggest that protease inhibitor (PI)-based combination ART may reduce progesterone levels during pregnancy, which could affect fetal growth.(26) In Zambia, PI-based combination ART is reserved for second line therapy. All women in our analysis were on first line therapy, which may explain why we did not observe an association between duration of combination ART and SGA.
Associations between duration of combination ART and preterm birth in our analysis were sensitive to assumptions about measurement error in gestational age. In the naive and multiple imputation analyses, there was no evidence that duration of combination ART increased the risk of preterm birth. However, when measurement error was additionally considered, the risk of preterm birth for 9-32 weeks of combination ART increased as the amount of measurement error assumed increased. An association between combination ART use early during pregnancy (consistent with longer duration of therapy) and preterm birth has been reported in observational studies. (38, 39) In a recent randomized controlled trial, PI-based combination ART was found to increase the risk of preterm birth.(40)
In our analysis, a priori we assumed that the measurement error in gestational age was likely to account for approximately 30% of its variance. However, point estimates were slightly closer to those observed in other studies (38, 39) when 60% variance due to measurement error was assumed. This suggests that the proportion of the variance due to measurement error in gestational age may have been closer to 60%. Interestingly, our results did not change meaningfully when only random measurement error was assumed (despite clear evidence of non-random measurement error). However, point estimates when only random measurement error was assumed were close to those seen in the multiple imputation analysis, suggesting that changes in effect estimates were largely driven by the substantial amount of missing data.
Assumptions and Limitations
Multiple overimputation rests on several assumptions, of which we examine the plausibility in our data. It assumes that measurement error and missing data depend only on observed data.(22) In our data, error in gestational age was largely considered to be due to patient's memory or recording errors by staff. Such errors in memory or recording are unlikely to depend on unmeasured factors. Nevertheless, there may be important predictors of measurement error in gestational age that were not measured, such as whether a woman experienced irregular menstrual cycles. However, in our population of HIV infected women where breastfeeding is routine, irregular menstrual cycles may be the norm.
Multiple overimputation also assumes that measurement error is random, conditional on observed covariates and the observation-level prior (wi + ai). In our population, measurement error in gestational age was primarily due to errors in recording or remembering LMP dates. Such measurement error is likely to be normal, conditional on observed gestational age. Some groups of women, such as those with irregular menstrual cycles, may be more likely to have a mismeasured value of gestational age. However, it is unlikely that women with irregular menstrual cycles are more likely to deliver at a particular gestational age (e.g. conditional on observed gestational age, measurement error is random).
Additionally, multiple overimputation assumes that observation-level priors and the imputation model are correctly specified.(22) We evaluated the robustness of our results to this assumption in several ways: 1) by varying the proportion of variance due to measurement error assumed, 2) in sensitivity analyses assuming only random measurement error and 3) by varying the specification of imputation model, all with similar results.
Finally, our analysis assumed that a reference curve based on a HIV-uninfected population correctly defined SGA in a population of HIV-infected women. Such an approach assumes that differences between observed and expected gestational age are due to measurement error only, and not HIV status. While reference curves for HIV-infected women are not available, similar patterns of measurement error in gestational age were observed in our data when both HIV-infected and uninfected women were considered.
Measurement error is rarely addressed in epidemiologic studies, yet the ability to measure variables correctly is fundamental to making inference.(41) In our analysis of duration of combination ART before delivery and its associations with SGA and preterm birth we developed a multiple overimputation model to address missing data and measurement error in gestational age, using LMP and fundal height measurements. An association between longer duration of combination ART and preterm birth was observed only after accounting for missing data and measurement error. Our findings suggest that the measurement error in gestational age may have implications for associations between duration of combination ART and pregnancy outcomes, particularly preterm birth. As lifelong combination ART is scaled up to all HIV-infected pregnant women, routinely collected clinical data will likely continue to play an important role in monitoring the relationship between combination ART use and pregnancy outcomes. Future analyses examining these associations should consider the use of multiple overimputation to address measurement error in gestational age.
Supplementary Material
Acknowledgements
Traineeship support was provided by the National Institutes of Health (T32 AI007001).
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Presentations at conferences: A version of this work was presented at the Society for Epidemiologic Research's 47th annual meeting in Seattle, WA, June 25-27, 2014.
References
- 1.WHO . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization; Geneva, Switzerland: 2013. [PubMed] [Google Scholar]
- 2.Jamieson DJ, Chasela CS, Hudgens MG, King CC, Kourtis AP, Kayira D, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet. 2012 Jun 30;379(9835):2449–58. doi: 10.1016/S0140-6736(12)60321-3. PubMed PMID: 22541418. Pubmed Central PMCID: Pmc3661206. Epub 2012/05/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorenzi P, Spicher VM, Laubereau B, Hirschel B, Kind C, Rudin C, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS (London, England) 1998;12(18):F241–7. doi: 10.1097/00002030-199818000-00002. [DOI] [PubMed] [Google Scholar]
- 4.European Collaborative Study, Swiss Mother Child HIV Cohort Study Combination antiretroviral therapy and duration of pregnancy. AIDS (London, England) 2000;14(18):2913–20. doi: 10.1097/00002030-200012220-00013. [DOI] [PubMed] [Google Scholar]
- 5.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. Aids. 2007 May 11;21(8):1019–26. doi: 10.1097/QAD.0b013e328133884b. PubMed PMID: 17457096. Epub 2007/04/26. eng. [DOI] [PubMed] [Google Scholar]
- 6.Cotter AM, Garcia AG, Duthely ML, Luke B, O'Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? The Journal of infectious diseases. 2006;193(9):1195–201. doi: 10.1086/503045. [DOI] [PubMed] [Google Scholar]
- 7.Powis KM, Kitch D, Ogwu A, Hughes MD, Lockman S, Leidner J, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. The Journal of infectious diseases. 2011;204(4):506–14. doi: 10.1093/infdis/jir307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekouevi DK, Coffie PA, Becquet R, Tonwe-Gold B, Horo A, Thiebaut R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d'Ivoire. AIDS (London, England) 2008;22(14):1815–20. doi: 10.1097/QAD.0b013e32830b8ab9. [DOI] [PubMed] [Google Scholar]
- 9.Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012 Dec 1;206(11):1695–705. doi: 10.1093/infdis/jis553. PubMed PMID: 23066160. Pubmed Central PMCID: 3488194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Lettow M, Bedell R, Mayuni I, Mateyu G, Landes M, Chan AK, et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+). J Int AIDS Soc. 2014;17:18994. doi: 10.7448/IAS.17.1.18994. PubMed PMID: 25079437. Pubmed Central PMCID: Pmc4116618. Epub 2014/08/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UNAIDS . Gap Report. UNAIDS; Geneva, Switzerland: 2014. [Google Scholar]
- 12.Lanzola G, Parimbelli E, Micieli G, Cavallini A, Quaglini S. Data quality and completeness in a web stroke registry as the basis for data and process mining. Journal of healthcare engineering. 2014;5(2):163–84. doi: 10.1260/2040-2295.5.2.163. PubMed PMID: 24918182. Epub 2014/06/12. eng. [DOI] [PubMed] [Google Scholar]
- 13.Howards PP, Hertz-Picciotto I, Weinberg CR, Poole C. Misclassification of gestational age in the study of spontaneous abortion. Am J Epidemiol. 2006 Dec 1;164(11):1126–36. doi: 10.1093/aje/kwj327. PubMed PMID: 16985078. Epub 2006/09/21. eng. [DOI] [PubMed] [Google Scholar]
- 14.Egbuonu I, Ezechukwu CC, Chukwuka JO, Ikechebelu JI. Breast-feeding, return of menses, sexual activity and contraceptive practices among mothers in the first six months of lactation in Onitsha, South Eastern Nigeria. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2005 Jul;25(5):500–3. doi: 10.1080/01443610500171250. PubMed PMID: 16183590. Epub 2005/09/27. eng. [DOI] [PubMed] [Google Scholar]
- 15.Ezechi OC, Jogo A, Gab-Okafor C, Onwujekwe DI, Ezeobi PM, Gbajabiamila T, et al. Effect of HIV-1 infection and increasing immunosuppression on menstrual function. The journal of obstetrics and gynaecology research. 2010 Oct;36(5):1053–8. doi: 10.1111/j.1447-0756.2010.01253.x. PubMed PMID: 21058440. Epub 2010/11/09. eng. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, Robins JM. Large-sample theory for parametric multiple imputation procedures. Biometrika. 1998;85(4):935–48. [Google Scholar]
- 17.Cole SR, Chu H, Greenland S. Multiple-imputation for measurement-error correction. Int J Epidemiol. 2006 Aug;35(4):1074–81. doi: 10.1093/ije/dyl097. PubMed PMID: 16709616. Epub 2006/05/20. eng. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong BG. The effects of measurement errors on relative risk regressions. Am J Epidemiol. 1990 Dec;132(6):1176–84. doi: 10.1093/oxfordjournals.aje.a115761. PubMed PMID: 2260550. Epub 1990/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 19.Blackwell MH, James and King, Gary A Unified Approach to Measurement Error and Missing Data: Overview and Applications. Sociological Methods and Research. 2015:1–39. Epub June 30, 2015. [Google Scholar]
- 20.Honaker J, King G. What to do about missing values in time-series cross-section data. American Journal of Political Science. 2010;54(2):561–81. [Google Scholar]
- 21.Honaker J, King G, Blackwell M. Amelia II: A program for missing data. Journal of Statistical Software. 2011;45(7):1–47. [Google Scholar]
- 22.Blackwell M, Honaker J, King G. A Unified Approach to Measurement Error and Missing Data: Details and Extensions. Sociological Methods and Research. 2015:1–28. Epub June 22, 2015. [Google Scholar]
- 23.Schomaker M, Hogger S, Johnson LF, Hoffmann CJ, Barnighausen T, Heumann C. Simultaneous Treatment of Missing Data and Measurement Error in HIV Research Using Multiple Overimputation. Epidemiology. 2015 Sep;26(5):628–36. doi: 10.1097/EDE.0000000000000334. PubMed PMID: 26214336. Epub 2015/07/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoover DR, Graham NM, Chen B, Taylor JM, Phair J, Zhou SY, et al. Effect of CD4+ cell count measurement variability on staging HIV-1 infection. Journal of acquired immune deficiency syndromes. 1992;5(8):794–802. [PubMed] [Google Scholar]
- 25.Rubin DB. Multiple imputation for nonresponse in surveys. Wiley; New York, New York: 1987. [Google Scholar]
- 26.Papp E, Mohammadi H, Loutfy MR, Yudin MH, Murphy KE, Walmsley SL, et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels; a potential mechanism contributing to fetal growth restriction. J Infect Dis. 2014 Jul 16; doi: 10.1093/infdis/jiu393. PubMed PMID: 25030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livingston JC, Maxwell BD, Sibai BM. Chronic hypertension in pregnancy. Minerva ginecologica. 2003 Feb;55(1):1–13. PubMed PMID: 12598838. Epub 2003/02/25. eng. [PubMed] [Google Scholar]
- 28.Svare JA, Hansen BB, Molsted-Pedersen L. Perinatal complications in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2001 Oct;80(10):899–904. doi: 10.1034/j.1600-0412.2001.801006.x. PubMed PMID: 11580734. Epub 2001/10/03. eng. [DOI] [PubMed] [Google Scholar]
- 29.Bengtson AM, Chibwesha CJ, Westreich D, Mubiana-Mbewe M, Vwalika B, Miller WC, et al. Duration of cART before delivery and low infant birthweight among HIV-infected women in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2015 Nov 30; doi: 10.1097/QAI.0000000000000909. PubMed PMID: 26627103. Epub 2015/12/03. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gulmezoglu AM, et al. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377(9780):1855–61. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 31.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass) 1999;10(1):37–48. [PubMed] [Google Scholar]
- 32.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. American Journal of Epidemiology. 2002;155(2):176–84. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 33.Jain T, Garg S, Singh MM, Kaushik A, Batra S, Gupta VK, et al. Antepartum morbidities and health seeking behaviour among women in an urban slum of Delhi. Journal of the Indian Medical Association. 2011;109(5):315–7. [PubMed] [Google Scholar]
- 34.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr. Splines for trend analysis and continuous confounder control. Epidemiology (Cambridge, Mass) 2011;22(6):874–5. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geerts L, Poggenpoel E, Theron G. A comparison of pregnancy dating methods commonly used in South Africa: a prospective study. S Afr Med J. 2013 Aug;103(8):552–6. doi: 10.7196/samj.6751. PubMed PMID: 23885738. Epub 2013/07/28. eng. [DOI] [PubMed] [Google Scholar]
- 36.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004 Apr 1;159(7):702–6. doi: 10.1093/aje/kwh090. PubMed PMID: 15033648. Epub 2004/03/23. eng. [DOI] [PubMed] [Google Scholar]
- 37.Briand N, Mandelbrot L, Le Chenadec J, Tubiana R, Teglas JP, Faye A, et al. No relation between in-utero exposure to HAART and intrauterine growth retardation. Aids. 2009 Jun 19;23(10):1235–43. doi: 10.1097/QAD.0b013e32832be0df. PubMed PMID: 19424054. Epub 2009/05/09. eng. [DOI] [PubMed] [Google Scholar]
- 38.Watts DH, Williams PL, Kacanek D, Griner R, Rich K, Hazra R, et al. Combination antiretroviral use and preterm birth. The Journal of infectious diseases. 2013;207(4):612–21. doi: 10.1093/infdis/jis728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Merwe K, Hoffman R, Black V, Chersich M, Coovadia A, Rees H. Birth outcomes in South African women receiving highly active antiretroviral therapy: a retrospective observational study. J Int AIDS Soc. 2011;14:42. doi: 10.1186/1758-2652-14-42. PubMed PMID: 21843356. Pubmed Central PMCID: 3163172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fowler M, Qin M, Shapiro D, Fiscus S, Currier JS, Makanani B, et al. PROMISE: Efficacy and Safety of 2 Strategies to Prevent Perinatal HIV Transmission.. CROI; Seattle, Washington. February 23-26, 2015.2015. [Google Scholar]
- 41.Edwards JK, Cole SR, Westreich D. All your data are always missing: incorporating bias due to measurement error into the potential outcomes framework. International journal of epidemiology. 2014 doi: 10.1093/ije/dyu272. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.