Abstract
Objectives
To estimate FTD prevalence, identify FTD-related mutations, correlate FTD phenotype with mutations in a Southern Italian population.
Methods
Study population consisting of subjects ≥50 years of age residing in the Community of Biv. on January 1, 2004. Door-to-door two-phase design. Genetic and biochemical analyses were done on samples collected from 32 patients.
Results
Prevalence rates were 0.6 for AD, 0.4 for VD, 3.5 for FTD, 0.2 for Parkinson Dementia and 1.2 for unspecified dementia. Three GRN (one known and two novel) mutations with reduced plasma protein levels were found associated to three distinct phenotypes (behavioural, affective and delirious type).
Conclusions
We report an unusually high FTD prevalence in the investigated population, but a low prevalence of AD. We confirm the heterogeneity of FTD phenotype associated with different GRN mutations.
Keywords: door-to-door study frontotemporal dementia, gene, progranulin, mutation, prevalence studies
1. Introduction
Frontotemporal dementia (FTD) is the second most common form of dementia after Alzheimer’s Disease (AD) (Rosso et al., 2003), characterized by predominant and gradual behavioural changes associated with language and affective disorders. Epidemiologic studies of FTD have been rare and conducted through clinical survey, (Borroni et al., 2010; Harvey et al., 2003; Ikeda et al., 2004; Rosso et al., 2003; Ratnavalli et al., 2002) while door-to-door studies have not been reported. Up to 50% of FTD patients have positive family history of dementia, mainly with autosomal dominant inheritance (Rosso et al., 2003). FTD is a genetically complex disorder with several genes associated with disease etiology (http://www.molgen.ua.ac.be/ADMutations), including the MAPT (Poorkaj et al., 1998) and GRN (Baker et al., 2006; Cruts et al., 2006) genes responsible for the disease in FTD families linked to chromosome 17q21.The most common mutation reported to date is an expanded hexanucleotide repeat in a non-coding region of C9orf72, which is the cause of chromosome 9p21-linked FTD and Amyotrophic lateral sclerosis (ALS) with TDP-43/p62 associated brain pathology (DeJesus-Hernandez et al., 2011; Renton et al., 2011).
Known GRN mutations have different pathogenic potential depending on the type of mutation (i.e. deletion, nonsense, frameshift, splice-site and some missense substitutions), causing FTD with different degrees of severity (Baker et al., 2006; Cruts et al., 2006; Shankaran et al., 2008; Wang et al., 2010). Recently, it was demonstrated that plasma progranulin protein levels could predict the presence of GRN mutations in FTD patients (Ghidoni et al., 2012) and that it may be a valuable tool in predicting the pathogenic significance of GRN mutations (Finch et al., 2009). However, different clinical characteristics are observed in carriers of the same GRN mutation, even within the same family. Some of this clinical variability could be explained by specific variants of a modifying gene. Common variations in the GRN gene (rs9897526, rs5848) (Fenoglio et al., 2009) and the Transmembrane protein 106B gene (TMEM106B) (rs1990622, rs1020004) (Finch et al., 2011) were reported to influence age of onset. Since 1990 we have been studying a large FTD family encompassing 37 affected subjects over four generations segregating the c.1145insA mutation of the GRN gene (Bruni et al., 2007). However some patients, who present with a clear phenotype of FTD and belong to the pedigree, do not carry the c.1145insA mutation thus suggesting a different genetic cause responsible for the disease in these patients (Bruni et al., 2007). The Southern Italian population in which this family is nested, peculiar for its historical and geographic isolation over the centuries, was very cooperative in participating in a door-to-door population study. The principal aims of the study were: (i) to calculate the prevalence of FTD, (ii) to identify other FTD-related mutations, (iii) to analyze genotypes of modifier genes reported to influence age at onset, and (iv) to characterize the FTD phenotype with specific reference to genetic and biochemical parameters.
2. Methods
2.1. Subjects
The study population targeted all subjects who were ≥ 50 years of age residing in the Community of Biv. (Province of Reggio Calabria, Italy) on January 1, 2004. Biv. is a small town in a mountainous area (25 km2) of Southern Italy. Eligible subjects were identified from data maintained by the municipality’s registrar office. Both household and institutionalized individuals were included; all subjects who were official residents of Biv. on January 1, 2004, but who were living in another town temporarily were interviewed whenever possible.
2.2. General study design
An informed written consent was signed by all subjects or by their legal representative. This study was performed according to the Declaration of Helsinki and was supported by the Italian Health Ministry (DGRST n° 4/2760-P/I.9.ab, 2007; RFPS-2006-7-334858, 2006) with appropriate Ethics committee approval.
We used a door-to-door two-phase design (see figure e-1, Supplemental data Appendix E-1 for further details).
2.3. Genetic and biochemical analyses
Genomic DNA and total RNA were obtained from peripheral blood using QIAGEN kits. The GRN, MAPT, FUS TARDBP and C9orf72 genes were analyzed for all FTD patients. To assess the presence of large (>30kb) copy number variations (CNV) affecting known FTD genes the DNA samples were genotyped on an Illumina HumanHap 650Y platform and the intensity array data was analysed as described previously (Ghani et al., 2012). All DNA samples were genotyped for SNPs in the GRN (rs5848, rs9897526) and TMEM106B genes (rs1990622, rs1020004) using a direct sequencing approach (PCR conditions are available upon request). The SNP in the PRNP gene (M129V) was genotyped using a restriction assay by digesting the PCR product with BsaAI enzyme (New England Biolabs Inc.). APOE polymorphisms and the 238 bp insertion/deletion in intron 9 of the MAPT gene defining the H1 and H2 haplotypes were genotyped (appendix e-1).
The GRN A266P and C126W variations were assessed in 100 controls (mean age 65.2±6.7, MMSE 28±3.7) and in 140 FTD patients (mean age 65.1±9.4) belonging to the Southern Italian population, using DHPLC (Transgenomic Inc., Omaha, USA). RNA of two patients, one carrying either the GRN c.1145insA or the A266P mutation, were analysed by semi-quantitative RT-PCR, as described in the Supplemental data. Plasma levels of progranulin protein were measured in carriers of the c.1145insA, A266P, C126W mutations and in 15 controls (mean age 64±18.3, MMSE 28±3.7) in three independent experiments in duplicate using an ELISA kit (Human Progranulin ELISA kit, Adipogen Inc., Seoul, Korea) (Finch et al., 2009; Ghidoni et al., 2012).
2.4. Statistical analysis
The prevalence ratio was calculated for dementia in general and for specific types of dementia; age and sex specific ratios were also obtained. Quantitative data between subgroups were compared using the Student two-tailed independent t-test. Difference in clinical symptoms and neurological signs between affected GRN mutation carriers (GRN_positive) and non-GRN cases (GRN_negative) was assessed using Cross-tabulation tables by χ2 test. Descriptive statistics and all analyses were performed using SPSS 11.5 software. For all analyses, statistical significance was considered as p-values<0.05.
3. Results
3.1. Epidemiological results
Of the total 1568 registered residents of Biv. 702 subjects were ≥50 years. In all, 509 individuals (72%, mean age 71.6±11.1; 55.4% of females) agreed to participate, 6% were deceased, 10% lived outside the country, and 12% refused to take part. Figure e-2 shows the demographic and clinical characteristics. Figure e-3 presents the cardio-cerebrovascular risk factors of the population by age.
Of the group of 92 individuals (18.1%) evaluated in Phase II, 30 subjects were diagnosed with dementia (age 77.6±5.6;12M/17F) with a prevalence (number of cases per 100 individuals) of 5.9 among those ≥50 years of age and of 8.1 for those ≥65 years of age.
The distribution of patients with dementia (including specific types of dementia), and corresponding prevalence, considering age and sex, are summarized in Table 1. The prevalence (number of cases per 100 individuals over age 49) was as follows: AD (0.6), VD (0.4), FTD (3.5), Parkinson Dementia (0.2) and unspecified dementia (1.2).
Table 1.
Age- and sex-specific prevalence ratios (cases per 100 population) of dementia and specific dementing disorders in Biv on January 1, 2004
| Age (yrs) (N°) | Men |
Women |
Total |
|||
|---|---|---|---|---|---|---|
| N° | Pr % | N° | PR % | N° | PR % | |
| Dementia | ||||||
| 50-59 (97) | 0 | - | 0 | - | 0 | - |
| 60-69 (106) | 0 | - | 1 | 1.6 | 1 | 0.9 |
| 70-79 (172) | 8 | 10.0 | 10 | 10.9 | 18 | 10.5 |
| 80-89 (108) | 4 | 8.3 | 6 | 10.0 | 10 | 9.3 |
| 90-99 (26) | 0 | - | 1 | 5 | 1 | 3.8 |
| Total (509) | 12 | 5.3 | 18 | 6.4 | 30 | 5.9 |
| Frontotemporal dementia | ||||||
| 50-59 (97) | 0 | - | 0 | - | 0 | - |
| 60-69 (106) | 1 | 2.3 | 1 | 1.6 | 1 | 0.9 |
| 70-79 (172) | 2 | 2.5 | 5 | 5.4 | 7 | 4.1 |
| 80-89 (108) | 3 | 6.3 | 5 | 8.3 | 8 | 7.4 |
| 90-99 (26) | 0 | - | 1 | 5 | 1 | 3.8 |
| Total (509) | 6 | 2.6 | 12 | 4.3 | 18 | 3.5 |
| Alzheimer | ||||||
| Total 50+ | 3 | 0.6 | ||||
| Vascular dementia | ||||||
| Total 50+ | 2 | 0.4 | ||||
| Unspecified dementia | ||||||
| Total 50+ | 6 | 1.2 | ||||
| Parkinson dementia | ||||||
| Total 50+ | 1 | 0.2 | ||||
PR prevalence ratio (per 100 population)
The clinical characteristics of the 18 FTD patients identified through the door-to-door study are reported in Table 2. Mean age at onset was 75.9±9.0 years (66.7% women). 50% (9 subjects) had 4 or fewer years of education and 27.8% (5 subjects) were illiterate. A first degree relative affected by dementia of any type was found in 38.9% of the FTD patients (7 subjects). Cardio-cerebrovascular risk factors were commonly distributed (16/18 FTD patients).
Table 2.
Clinical features of FTD subjects by population study
| FTD patients |
Gender | Education (yrs) |
Age | Onset | Duration at 1st examination |
Family history |
CT/MRI/SPECT | Loss of insight |
Memory impairment |
Disinhibition | Reduction of verbal output |
Irritability | Perseveration | Apathy | Pyramidal signs |
Extrapyramidal signs |
cardio-cerebrovascular risk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 0 | 86 | 86 | 0 | - | - | + | - | + | - | - | + | - | - | + | + |
| 2 | F | 4 | 89 | 87 | 2 | + | - | + | + | + | - | + | - | + | - | - | + |
| 3 | F | 4 | 71 | 70 | 1 | + | + | + | + | + | + | - | - | + | - | + | + |
| 4 | M | 5 | 73 | 64 | 9 | + | + | + | + | - | + | - | - | + | + | + | + |
| 5 | M | 5 | 72 | 70 | 2 | - | + | + | + | - | + | + | - | - | - | + | + |
| 6 | F | 3 | 78 | 76 | 2 | - | + | - | + | + | - | + | - | - | - | - | - |
| 7 | F | 0 | 80 | 72 | 8 | + | + | + | + | + | + | + | - | - | - | - | + |
| 8 | F | 0 | 73 | 63 | 10 | - | - | + | - | + | - | - | + | - | + | - | + |
| 9 | M | 5 | 86 | 84 | 2 | + | - | - | - | - | - | - | - | + | - | - | + |
| 10 | M | 0 | 86 | 84 | 2 | - | + | + | + | + | + | - | - | + | - | - | - |
| 11 | F | 2 | 83 | 82 | 1 | - | + | - | + | + | - | - | - | - | - | + | + |
| 12 | F | 4 | 82 | 82 | 0 | + | - | + | + | + | - | + | - | - | - | - | + |
| 13 | F | 5 | 64 | 62 | 2 | - | - | + | + | + | + | - | - | - | + | + | + |
| 14 | F | 3 | 83 | 81 | 2 | + | + | + | + | - | - | + | - | + | - | - | + |
| 15 | F | 4 | 93 | 90 | 3 | - | - | - | + | + | - | - | - | - | + | - | + |
| 16 | F | 0 | 72 | 72 | 0 | - | - | + | - | + | - | + | + | - | - | - | + |
| 17 | M | 4 | 78 | 65 | 13 | - | - | + | + | - | - | + | + | - | - | - | + |
| 18 | F | 4 | 77 | 77 | 0 | - | - | + | + | - | - | + | + | + | - | + | + |
| Total | 12F/6M | 2.9 | 79.2 | 75.9 | 3.3 | 7/18 | 8/18 | 14/18 | 14/18 | 12/18 | 6/18 | 9/18 | 5/18 | 7/18 | 4/18 | 7/18 | 16/18 |
3.2. Genetic and biochemical results
Mutation analysis was done on 32 FTD patients, including the 18 subjects diagnosed during the door-to-door study (a_group) and 15 previously reported subjects (Bruni et al., 2007) (b_group; 13 affected, 2 at risk who subsequently developed the disease). One subject was present in both the a_ and b_groups. FTD subjects tested negatively for mutations in MAPT, FUS TARDBP and C9orf72 (number of repeats was within a normal range: 2-23). We identified several variations in the GRN gene. One known frameshift mutation (c.1145insA) and two novel heterozygous missense variations, A266P and C126W in the GRN gene were identified in FTD patients (12 carriers of the c.1145insA, 3 carriers of the A266P, belonging and segregating in two different branches of the same large kindred (Bruni et al., 2007) and two apparently sporadic patients bearing C126W both descending from a common founder identified in the 6th generation). In the remaining 15 patients no GRN mutation was found. The A266P is a transversion in exon 8 (g.11002 G>C), while the C126W is a transversion in exon 5 (g.10134 C>G) (Genebank accession number NG_007886.1). The A266P and C126W variations were absent in 100 controls and 140 FTD patients, so we hypothesize that the variations are not common polymorphisms.
Densitometric semi-quantitative analysis of the GRN transcript demonstrated that the amount of mRNA of the c.1145insA mutation carrier corresponds to 33% of the amount of the control mRNA. Sequencing and transcript analysis of the cDNA of the A266P mutation carrier revealed that the amount of mRNA is not different from the control.
None of the analysed variations of potential modifying dementia genes (APOE, MAPT, or common variations in the GRN, TMEM106B and C9orf72) could account for the differences in age of onset of subjects carrying the same GRN mutation. Furthermore, in the FTD patients (GRN_positive and GRN_negative) we did not detect any large CNVs (≥30 Kb) affecting GRN or any other known FTD genes listed at www.molgen.ua.ac.be/ADMutations/.
3.3. Biochemical results
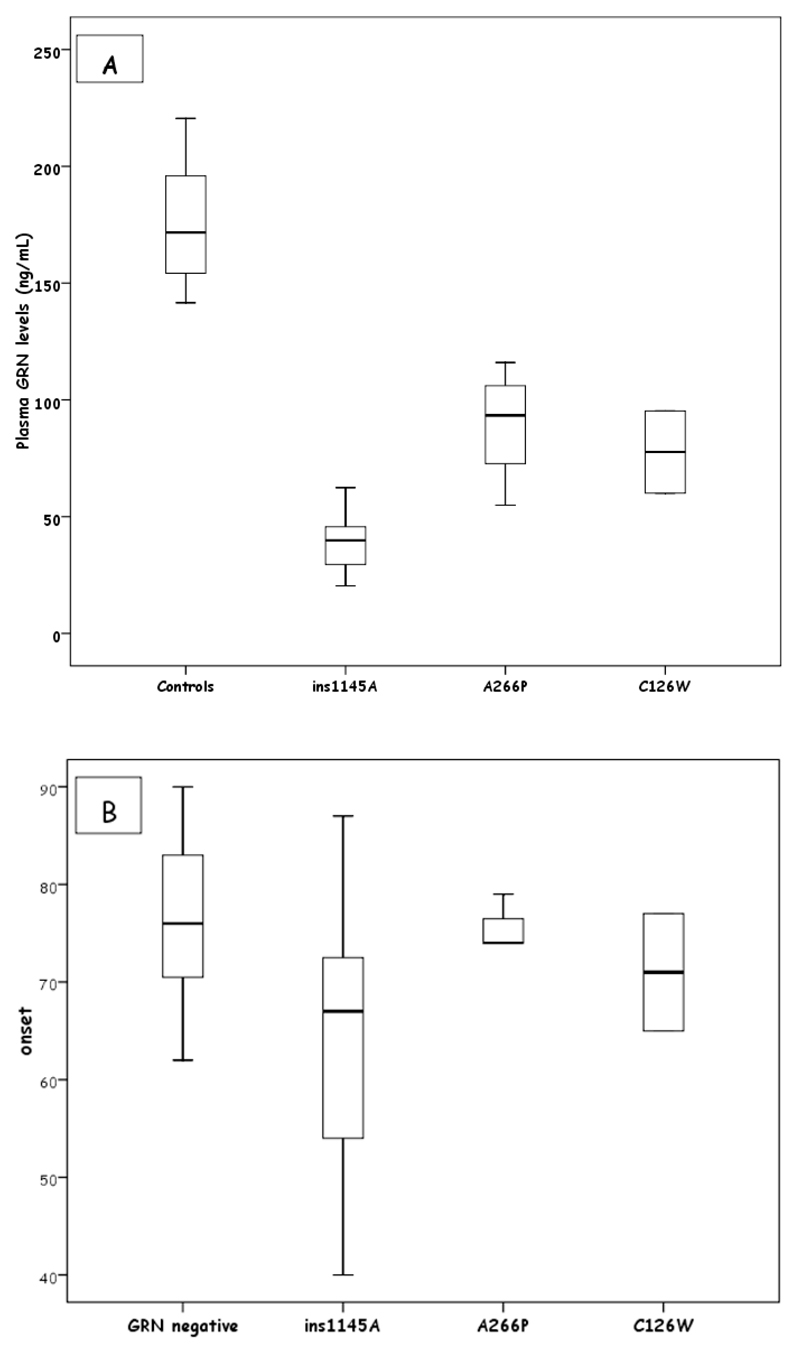
Plasma progranulin protein levels were 38.6±12.7 (20-62) ng/ml in carriers of the c.1145insA (11 available patients), 89.4±25.4 (54-116) ng/ml in carriers of the A266P (3 patients), 77.7±24.9 (60-95) for the C126W carriers (two patients) and 175.7±25.4 (142-221) in controls, demonstrating reduced levels of progranulin for all mutations, according to a previously established cut-off for loss of function mutations (≤ 110.9 ng/ml), (Finch et al., 2009; Ghidoni et al., 2012) and range for missense mutations (110-140 ng/ml) (Finch et al., 2009) (Figure 1A). Of note, carriers of the C126W mutation had progranulin levels similar to those with loss of function mutations.
Figure 1.
A. Plasma GRN levels in FTD mutated patients and controls; B. Age at onset in GRN_positive and GRN_negative patients
3.4. Comparison of clinical phenotypes among GRN_positive and GRN_negative patients
Few differences in the FTD phenotype were detected between GRN_positive and GRN_negative patients. A difference in the age at onset (ANOVA test p=0.04) was evident among the four groups [c.1145insA carriers (64.2±12.5 years); A266P carriers (75.7±2.9 years); C126W carriers (71±8.5 years); GRN_negative(75.9±9.0 years), Figure 1B]. After Bonferroni correction only the difference in age of onset between the carriers of the c.1145insA and the GRN_negative patients was significant (p=0.037).
3.4.1. Onset
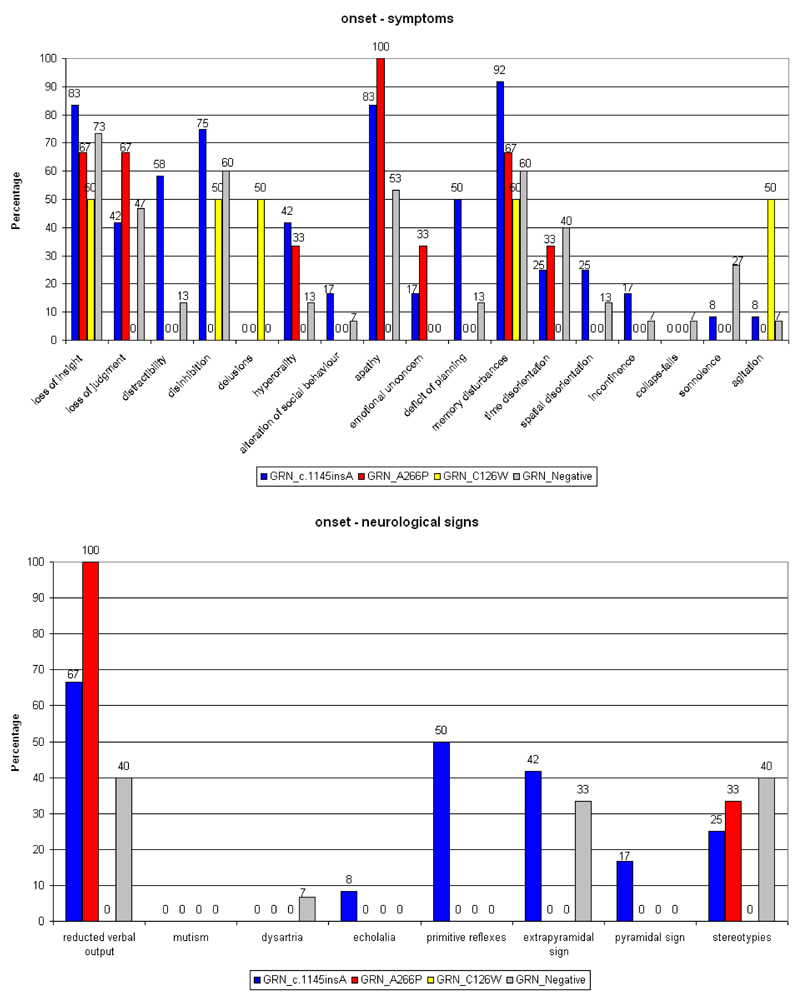
Patients bearing the c.1145insA were more distracted (p=0.01), with slight impairment of planning (p=0.06), and had a tendency for greater behavioural disinhibition when compared with GRN_negative cases or with carriers of the A266P or C126W mutations. Reduction of verbal output and apathy were common to all but one group (C126W) in different percentages. Patients with the c.1145insA patients showed primitive reflexes and extrapyramidal signs early (50% and 42% respectively); neurological signs were absent in the other GRN_positive patients. GRN_negative patients had 33% rigidity and stereotypies (Figure 2).
Figure 2.
Symptoms and neurological signs at onset in the GRN mutations carriers (in the FTD patients)
3.4.2. Manifest stage
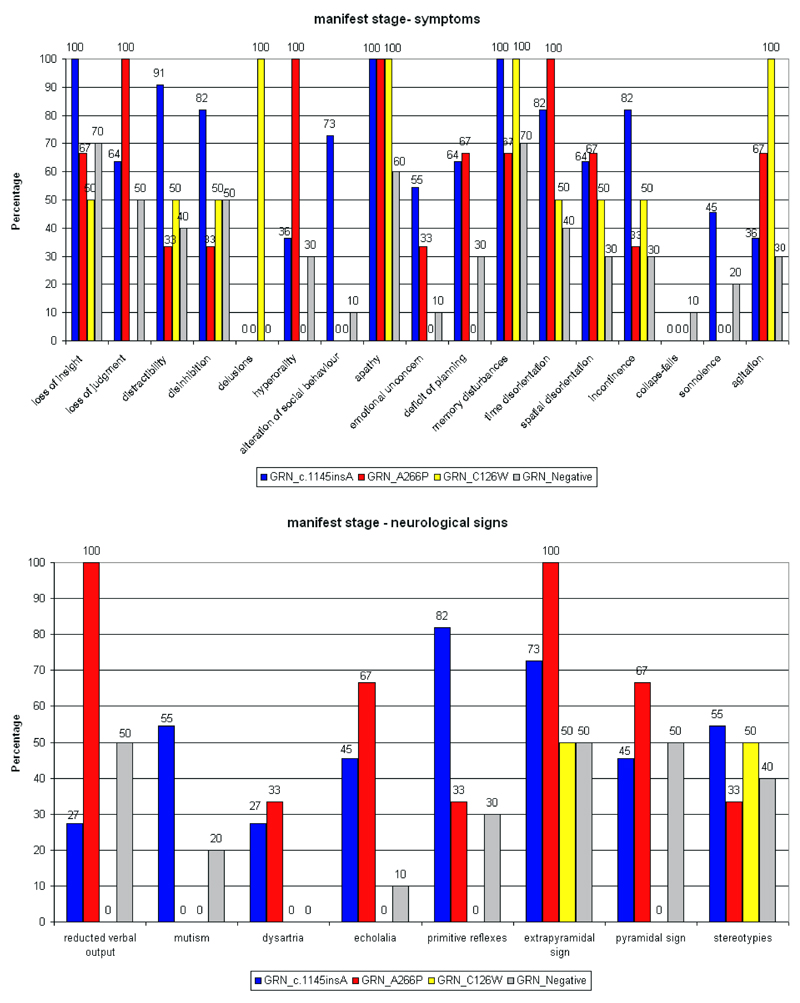
The c.1145insA carriers were more distracted and disinhibited with greater alteration of social behaviour; language disorder evolved to complete mutism; A266P carriers showed apathy in 100% of cases, emotional unconcern, greater hyperorality, extrapyramidal signs, pyramidal signs in 67% of cases; C126W patients presented with paranoid delusion, disinhibition, agitation and extrapyramidal signs (Figure 3).
Figure 3.
Symptoms and neurological signs at manifest stage in the GRN mutation carriers (in FTD patients)
4. Discussion
We describe the occurrence of dementia in an isolated Southern Italian population using a two-phase community-based door-to-door study. The overall prevalence of dementia was 5.9%. Surprisingly, FTD was the most common type of dementia (FTD 60%, AD 10%, VaD 6.7%, other forms of dementia 23.3%), which differs from other studies where AD was identified to be the most common (Andreasen et al., 1999; Ratnavalli et al., 2002). We are unable to provide an explanation for the low incidence of AD in this population. Our analysis detected 18 FTD patients out of 509 subjects, with an estimated overall prevalence of 35 per 100,000 inhabitants (3.5%, Table 1). We cannot formally compare our results with published data because it is the first door-to-door epidemiological study. A prevalence of 15 FTD patients per 100,000 in a population between 45 and 64 years of age was previously reported2, whereas the prevalence of FTD in the Netherlands (Rosso et al., 2003) was 1.1 in 100,000 with a maximum of 9.4 per 100,000 for ages 60–69. An Italian study reported 17.6 FTD per 100,000 inhabitants (Borroni et al., 2010). However, the high FTD frequency that we found could be attributed to the specific genetic background of the investigated population. FTD patients were carefully clinically characterized in the second phase of the study, although neuropathological data were lacking. Patients underwent structural and functional brain imaging and had a follow-up evaluation to further confirm diagnosis. To exclude vascular dementia, VRFs were collected and the results were not different compared to a general population over 65 of age from the same ethnic and geographic background (www.cuore.iss.it/fat_rischio/regioni/calabria/calabria.html). Although we were unable to clinically reinforce the diagnosis of FTD with a LCR biomarker study to distinguish between FTD and the frontal variant of AD, we are confident in the diagnosis because through the door-to-door study we identified patients at the beginning of the disease thus minimizing the possibility of misdiagnosis. Notably, the FTD clinical phenotype was similar for GRN_positive and GRN_negative patients.
The overall genetic contribution of GRN mutations in our FTD cohort was 53% (17/32) increasing to 71.4 % in patients with family history of dementia (15/21). The prevalence of GRN mutations was higher than in a large world-wide series (5-11% of FTLD cases (Gass et al., 2006; Le Ber et al., 2007; van der Zee et al., 2007) and 20-25% of FTLD patients with family history of dementia (Gass et al., 2006; Le Ber et al., 2007; van der Zee et al., 2007) or a clinical FTLD series from the Italian population (1.64%) (Borroni et al., 2010).
The high prevalence of GRN mutations in the Biv. population could be partially explained by a founder effect as it happens in an isolated population similar to C9orf72 in Finland (Renton et al., 2011)This phenomenon could be particularly evident in this village since isolated areas tend to have higher levels of consanguinity and parental isonomy (Cizza et al., 2011). Hence, taking into account our knowledge of the particular context (Bruni et al., 2010) the higher prevalence of GRN mutations may relate to a founder effect or could be due to patient selection; it is possible that the door-to-door study allowed us to identify the disease early and therefore better clinically characterize the FTD phenotype. Notably, the frequency of GRN mutations we found was similar to that of pathologically confirmed ubiquitin-positive cases, about 60-70% of GRN-related FTLD (van der Zee et al., 2007).
The three GRN mutations identified are different: the c.1145insA is a frameshift mutation, whereas the A266P and the C126W are missense mutations with the C126W affecting a progranulin cysteine residue. Biochemically, the c.1145insA causes a decrease of 50% in GRN mRNA, likely by nonsense-mediated mRNA decay (Baker et al., 2007; Cruts et al., 2007); on the other hand, we demonstrated that mutant GRN A266P mRNA was not degraded as expected from missense mutations that impair secretion of the progranulin protein (Le Ber et al., 2007; Shankaran et al., 2008). Our data demonstrated that the c.1145insA causes a strong reduction in the amount of protein in plasma; the A266P induces a slight decrease of protein, whereas C126W shows an intermediate level of progranulin between the other two mutations. For the c.1145insA and A266P mutations, our data are in agreement with the cut-off (≤110.9 ng/ml) reported for plasma progranulin levels for loss of function (Finch et al., 2009; Ghidoni et al., 2012) and the range (110-140 ng/ml) reported for missense mutations (Finch et al., 2009), respectively.
For instance, the reduced levels of plasma progranulin for the C126W mutation are in line with data reported for mutations affecting cysteine residues such as R432C, which significantly impair secretion, reducing plasma progranulin by ~45% (Shankaran et al., 2008). Other mutations affecting cysteine residues (C521Y) cause impaired elastase cleavage of progranulin into granulins (GRNs) and do not influence plasma progranulin levels (Wang et al., 2010). Indeed, it is possible that not all mutations involving cysteine residues have the same pathogenic mechanism; it is also possible that various pathogenic effects coexist, some of which are already known (haploinsufficency and impaired elastase cleavage of progranulin into granulins) and others are unknown, such as the turnover rate of the progranulin protein. The c.1145insA and A266P mutations were detected in familial FTD patients, belonging to two branches of the same large kindred clearly segregating with the disease (Bruni et al., 2007). The C126W was identified in two “apparently sporadic” FTD patients genealogically linked in the 18th century. The genetic and biochemical differences we found could have had consequences on the FTD clinical phenotype, although only few differences were evident between GRN_positive and GRN_negative patients.
The entire FTD cohort showed the broad clinical spectrum of FTD. GRN mutations are known to be associated with highly variable phenotype (Beck et al., 2008; Le ber et al., 2008). Age of onset, in our dataset, was significantly lower in c.1145insA carriers than A266P or C126W carriers and GRN_negative patients. The age of onset data indicate a correlation with progranulin plasma levels. However, within the c.1145insA mutation patients, the wide range of onset does not correlate with progranulin plasma dosage and it is not explained by any of the potential candidate modifier genes, suggesting that other genetic or environmental factors could contribute to the phenotype.
Although the clinical FTD picture was roughly the same for all GRN_positive and GRN_negative patients, the clinical criteria (Brun et al., 1994) allowed us to detect three distinct FTD phenotypes correlated to each GRN mutation. Carriers of c.1145insA carriers showed a more severe “frontal Gestalt” with marked distractibility, disinhibition, a greater impairment of social awareness, and language impairment manifesting to complete mutism (behavioural type). A266P carriers showed affective symptoms with apathy in 100% of cases, emotional unconcern and verbal output reduction that remained stable for the entire course of the disease (affective type), while C126W carriers presented with paranoid delusions and agitation (delirious type). Also from a neurological point of view there are some neurological differences: primitive reflexes are observed early only in c.1145insA carriers whereas early extrapyramidal signs characterize both c.1145insA carriers and GRN_negative patients, in contrast with findings reporting these signs in late stages of FTD (Diehl-Schmid et al., 2007; Padovani et al., 2007). Overall c.1145insA carriers were more impaired than carriers of the other mutations. This is likely due to the severe progranulin dysfunction causing an earlier and more significant frontal lobe involvement.
In conclusion, we report the unusually high prevalence of FTD and discuss the clinical phenotype associated with different GRN mutations.
Supplementary Material
Supplemental Data: Appendix e-1; figure e-1; figure e-2; figure e-3
5. Acknowledgments
The authors thank patients and families for the interest and generous participation in their research effort; the Associazione per la Ricerca Neurogenetica-ONLUS Lamezia Terme for invaluable help in assisting persons and families. This study was partially supported by finalized projects of the Italian Health Ministery: 1) DGRST n° 4/2760-P/I.9.ab, 2007; 2) RFPS-2006-7-334858, 2006. Moreover, this work in part was supported by grants from Canadian Institutes of Health Research, the Ontario Research Fund, Weston foundation (ER, PSH), the Howard Hughes Medical Institute, The Wellcome Trust Medical Research Council, the Alzheimer Society of Ontario (PSH).
Footnotes
Disclosure statement
All authors report no disclosures and no actual or potential conflicts of interest.
References
- Andreasen N, Blennow K, Sjodin C, Winblad B, Svardsudd K. Prevalence and Incidence of Clinically diagnoses memory impairments in a geographically defined general population in Sweden. Neuroepidemiology. 1999;18:144–155. doi: 10.1159/000026206. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Beck J, Rohrer JD, Campbell T, Isaacs A, Morrison KE, Goodall EF, Warrington EK, Stevens J, Revesz T, Holton J, Al-Sarraj S, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain. 2008;131(Pt3):706–720. doi: 10.1093/brain/awm320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Alberici A, Grassi M, Turla M, Zanetti O, Bianchetti A, Dalla Volta G, Rozzini R, Gilberti N, Bellelli G, Padovani A. Is frontotemporal lobar degeneration a rare disorder? Evidence from a preliminary study in Brescia county, Italy. J Alzheimer Dis. 2010;19:111–116. doi: 10.3233/JAD-2010-1208. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund B, Gustafson L. Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni AC, Momeni P, Bernardi L, Tomaino C, Frangipane F, Elder J, Kawarai T, Sato C, Pradella S, Wakutani Y, Anfossi M, et al. Heterogeneity within a large kindred with frontotemporal dementia: A novel progranulin mutation. Neurology. 2007;69:140–147. doi: 10.1212/01.wnl.0000265220.64396.b4. [DOI] [PubMed] [Google Scholar]
- Bruni AC, Bernardi L, Colao R, Rubino E, Smirne N, Frangipane F, Terni B, Curcio SA, Mirabelli M, Clodomiro A, Di Lorenzo R, et al. Worldwide distribution of PSEN1 Met146Leu mutation: a large variability for a founder mutation. Neurology. 2010;74(10):798–806. doi: 10.1212/WNL.0b013e3181d52785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizza G, Bernardi L, Smirne N, Maletta R, Tomaino C, Costanzo A, Gallo M, Lewis JG, Geracitano S, Grasso MB, Potenza G, et al. Clinical manifestations of highly prevalent corticosteroid-binding globulin mutations in a village in southern Italy. J Clin Endocrinol Metab. 2011;96(10):E1684–1693. doi: 10.1210/jc.2011-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442(7105):920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl-Schmid J, Schūlte-Overberg J, Hartmann J, Förstl H, Kurz A, Häussermann P. Extrapyramidal signs, primitive reflexes and incontinence in fronto-temporal dementia. Eur J Neurol. 2007;14(8):860–864. doi: 10.1111/j.1468-1331.2007.01773.x. [DOI] [PubMed] [Google Scholar]
- Fenoglio C, Galimberti D, Cortini F, Kauwe JS, Cruchaga C, Venturelli E, Villa C, Serpente M, Scalabrini D, Mayo K, Piccio LM, et al. rs5848 Variant Influences GRN mRNA Levels in Brain and Peripheral Mononuclear Cells in Patients with Alzheimer's Disease. J Alzheimers Dis. 2009;18(3):603–612. doi: 10.3233/JAD-2009-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch N, Baker M, Crook R, Swanson K, Kuntz K, Surtees R, Bisceglio G, Rovelet-Lecrux A, Boeve B, Petersen RC, Dickson DW, et al. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain. 2009;132(Pt 3):583–591. doi: 10.1093/brain/awn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch N, Carrasquillo MM, Baker M, Rutherford NJ, Coppola G, Dejesus-Hernandez M, Crook R, Hunter T, Ghidoni R, Benussi L, Crook J, et al. TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology. 2011;76(5):467–474. doi: 10.1212/WNL.0b013e31820a0e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R, Josephs K, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- Ghani M, Pinto D, Lee JH, Grinberg Y, Sato C, Moreno D, Scherer SW, Mayeux R, George-Hyslop P, Rogaeva E. Genome-wide survey of large rare copy number variations in Alzheimer’s disease among Caribbean Hispanics. G3 (Bethesda) 2012;2(1):71–78. doi: 10.1534/g3.111.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghidoni R, Stoppani E, Rossi G, Piccoli E, Albertini V, Paterlini A, Glionna M, Pegoiani E, Agnati LF, Fenoglio C, Scarpini E, et al. Optimal Plasma Progranulin Cut off Value for Predicting Null Progranulin Mutations in Neurodegenerative Diseases: A Multicenter Italian Study. Neurodegener Dis. 2012;9(3):121–127. doi: 10.1159/000333132. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry. 2003;74:1206–1209. doi: 10.1136/jnnp.74.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Ishikawa T, Tanabe H. Epidemiology of frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2004;17:265–268. doi: 10.1159/000077151. [DOI] [PubMed] [Google Scholar]
- Le Ber I, van der Zee J, Hannequin D, Gijselinck I, Campion D, Puel M, Laquerrière A, De Pooter T, Camuzat A, Van den Broeck M, Dubois B, et al. Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat. 2007;28(9):846–855. doi: 10.1002/humu.20520. [DOI] [PubMed] [Google Scholar]
- Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, Rovelet-Lecrux A, Hahn-Barma V, van der Zee J, Clot F, Bakchine S, Puel M, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 2008;131(Pt3):732–746. doi: 10.1093/brain/awn012. [DOI] [PubMed] [Google Scholar]
- Padovani A, Agosti C, Premi E, Bellelli G, Borroni B. Extrapyramidal symptoms in Frontotemporal Dementia: prevalence and clinical correlations. Neurosci Lett. 2007;422(1):39–42. doi: 10.1016/j.neulet.2007.05.049. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43(6):815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hadges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SM, Donker Kaat L, Baks T, Joosse M, de Koning I, Pijnenburg Y, de Jong D, Dooijes D, Kamphorst W, Ravid R, Niermeijer MF, et al. Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126:2016–2022. doi: 10.1093/brain/awg204. [DOI] [PubMed] [Google Scholar]
- Shankaran SS, Capell A, Hruscha AT, Fellerer K, Neumann M, Schmid B, Haass C. Missense mutations in the progranulin gene linked to frontotemporal lobar degeneration with ubiquitin-immunoreactive inclusions reduce progranulin production and secretion. J Biol Chem. 2008;283(3):1744–1753. doi: 10.1074/jbc.M705115200. [DOI] [PubMed] [Google Scholar]
- van der Zee J, Le Ber I, Maurer-Stroh S, Engelborghs S, Gijselinck I, Camuzat A, Brouwers N, Vandenberghe R, Sleegers K, Hannequin D, Dermaut B, et al. Mutations other than null mutations producing a pathogenic loss of progranulin in frontotemporal dementia. Hum Mutat. 2007;28(4):416. doi: 10.1002/humu.9484. [DOI] [PubMed] [Google Scholar]
- Wang J, Van Damme P, Cruchaga C, Gitcho MA, Vidal JM, Seijo-Martínez M, Wang L, Wu JY, Robberecht W, Goate A. Pathogenic cysteine mutations affect progranulin function and production of mature granulins. J Neurochem. 2010;112(5):1305–1315. doi: 10.1111/j.1471-4159.2009.06546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.