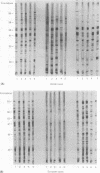
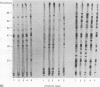
Abstract
AIMS--To compare the predictive value of immunoblotting and enzyme linked immunosorbent assay (ELISA) in diagnosing Lyme borreliosis. METHODS--An ELISA using a whole cell sonicate of the B31 strain of Borrelia burgdorferi was used to screen samples submitted for Lyme borreliosis serology. A total of 1222 serum samples reactive in the ELISA were tested by immunoblotting also using the B31 strain. Patients with other spirochaetal diseases were tested by both methods to assess specificity, while those with erythema migrans were used to evaluate sensitivity. Subjects with different clinical conditions, which may have been associated with Lyme borreliosis, were tested using both techniques. RESULTS--Only 16.3% of serum samples from patients submitted for Lyme borreliosis serology which were reactive by ELISA were confirmed as positive by immunoblotting. This is unlikely to represent a sensitivity problem as 51% of samples from 53 patients with erythema migrans were detected by immunoblotting compared with only 28% by ELISA. Patients whose samples were negative by ELISA were also negative by immunoblotting. Serum samples from patients with relapsing fever were reactive in both ELISA and by immunoblotting, but for other test groups immunoblotting offered increased specificity. CONCLUSIONS--Not all ELISA results could be confirmed by immunoblotting. Yet immunoblotting was both more sensitive and specific than ELISA techniques. As a result of these observations all ELISA results should be serologically confirmed by immunoblotting. Though immunoblotting is not suited to large scale screening of samples, it can be used satisfactorily in conjunction with ELISA methods to improve the predictive value of serological tests for Lyme borreliosis.
Full text
PDF


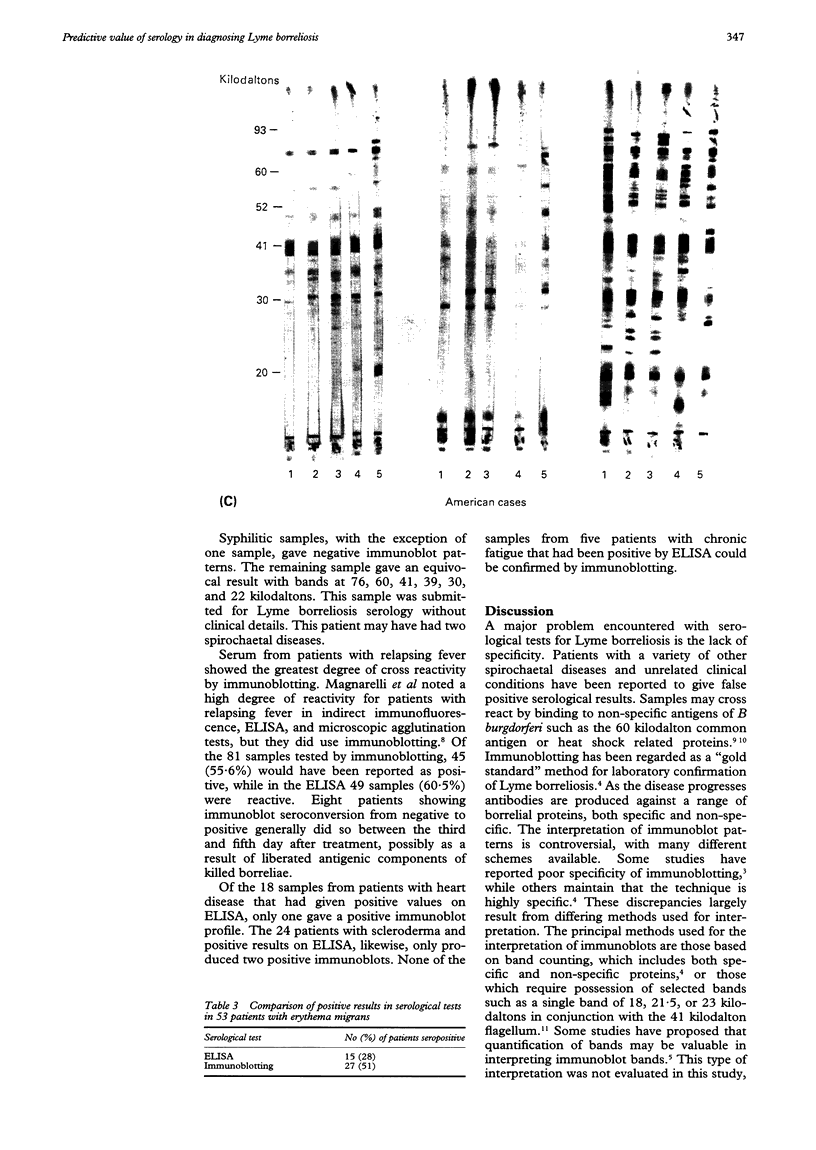


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baig S., Olsson T., Link H. Predominance of Borrelia burgdorferi specific B cells in cerebrospinal fluid in neuroborreliosis. Lancet. 1989 Jul 8;2(8654):71–74. doi: 10.1016/s0140-6736(89)90314-0. [DOI] [PubMed] [Google Scholar]
- Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J. C., Assous M., Grimont P. A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Carreiro M. M., Laux D. C., Nelson D. R. Characterization of the heat shock response and identification of heat shock protein antigens of Borrelia burgdorferi. Infect Immun. 1990 Jul;58(7):2186–2191. doi: 10.1128/iai.58.7.2186-2191.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S. J., Wright D. J. Comparison of immunofluorescence and enzyme linked immunosorbent assays for diagnosing Lyme disease. J Clin Pathol. 1989 Aug;42(8):869–871. doi: 10.1136/jcp.42.8.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S. J., Wright D. J., Luckhurst V. H. Simplified method for the interpretation of immunoblots for Lyme borreliosis. FEMS Immunol Med Microbiol. 1993 Apr;6(4):281–285. doi: 10.1111/j.1574-695X.1993.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Dattwyler R. J., Volkman D. J., Luft B. J., Halperin J. J., Thomas J., Golightly M. G. Seronegative Lyme disease. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med. 1988 Dec 1;319(22):1441–1446. doi: 10.1056/NEJM198812013192203. [DOI] [PubMed] [Google Scholar]
- Fahrer H., van der Linden S. M., Sauvain M. J., Gern L., Zhioua E., Aeschlimann A. The prevalence and incidence of clinical and asymptomatic Lyme borreliosis in a population at risk. J Infect Dis. 1991 Feb;163(2):305–310. doi: 10.1093/infdis/163.2.305. [DOI] [PubMed] [Google Scholar]
- Grodzicki R. L., Steere A. C. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J Infect Dis. 1988 Apr;157(4):790–797. doi: 10.1093/infdis/157.4.790. [DOI] [PubMed] [Google Scholar]
- Hansen K., Bangsborg J. M., Fjordvang H., Pedersen N. S., Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988 Aug;56(8):2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Hindersson P., Pedersen N. S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988 Feb;26(2):338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M., Hovind-Hougen K., Svenungsson B., Stiernstedt G. Cultivation and characterization of spirochetes from cerebrospinal fluid of patients with Lyme borreliosis. J Clin Microbiol. 1990 Mar;28(3):473–479. doi: 10.1128/jcm.28.3.473-479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Barbour A. G. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J Infect Dis. 1989 Jan;159(1):43–49. doi: 10.1093/infdis/159.1.43. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Johnson R. C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987 Jul;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Preac-Mursic V., Weber K., Pfister H. W., Wilske B., Gross B., Baumann A., Prokop J. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection. 1989 Nov-Dec;17(6):355–359. doi: 10.1007/BF01645543. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]