Abstract
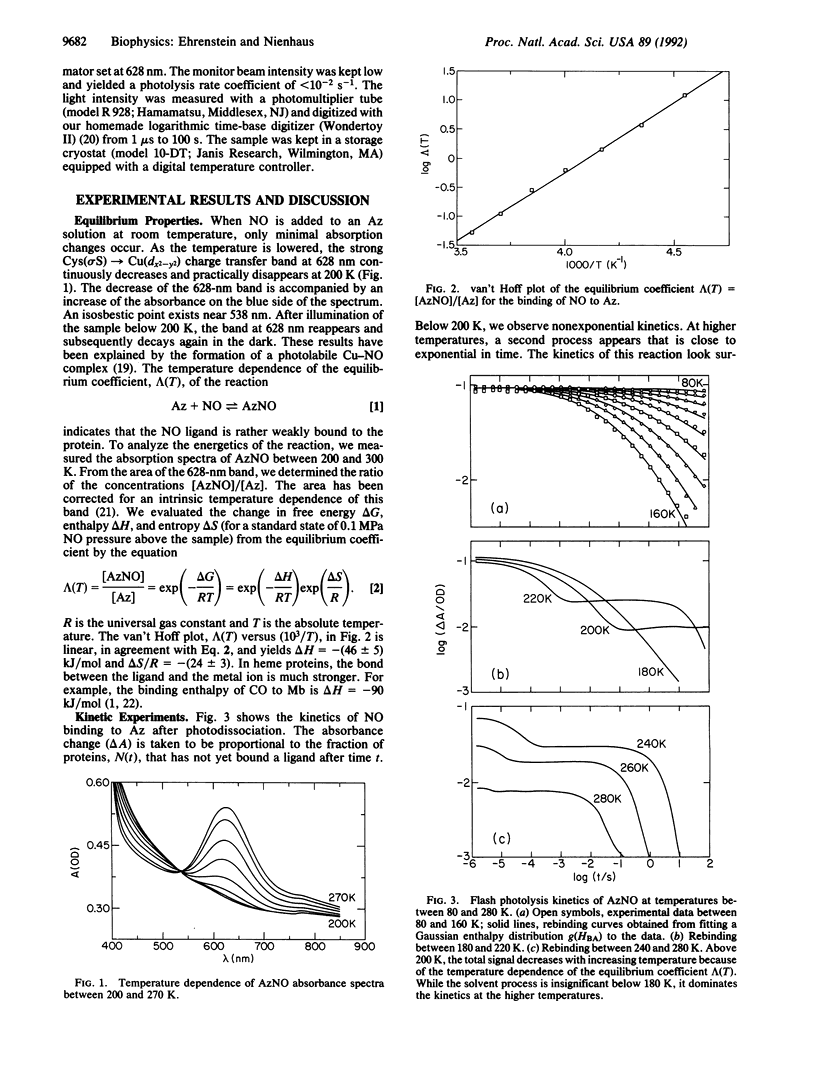
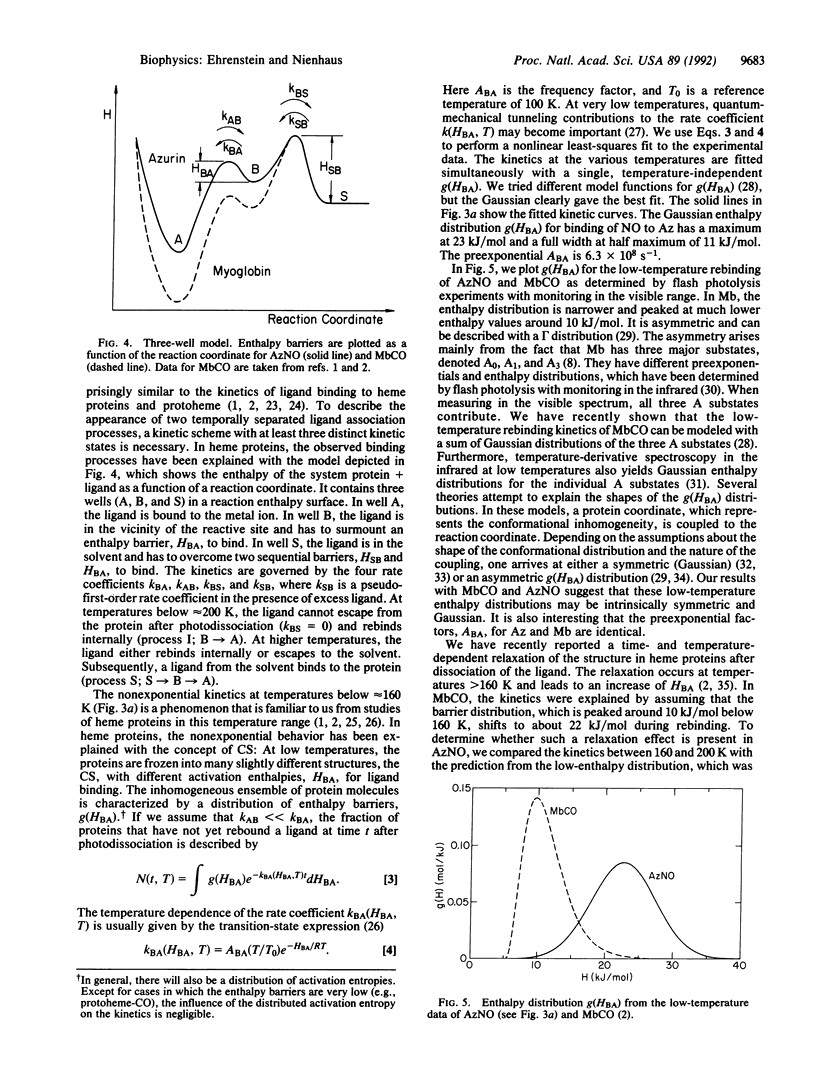
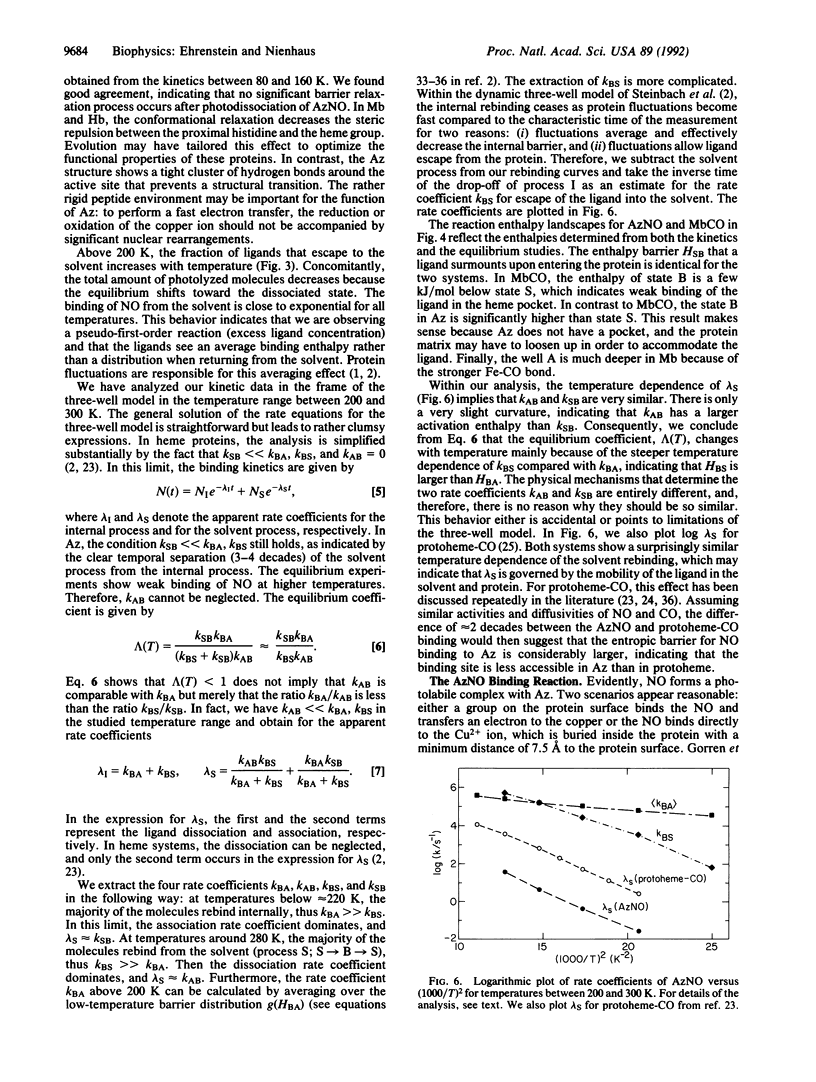
Azurin is a small blue copper protein in the electron transfer chain of denitrifying bacteria. It forms a photolabile complex with nitric oxide (NO) at low temperatures. We studied the temperature dependence of the ligand binding equilibrium and the kinetics of the association reaction after photodissociation over a wide range of temperature (80-280 K) and time (10(-6)-10(2) s). The nonexponential rebinding below 200 K is independent of the NO concentration and is interpreted as internal recombination. The rebinding can be modeled with the Arrhenius law by using a single preexponential factor of 6.3 x 10(8) s-1 and a Gaussian distribution of enthalpy barriers centered at 23 kJ/mol with a width of 11 kJ/mol. Above 200 K, a slower, exponential rebinding process appears. The dependence of the kinetics on the NO concentration characterizes this reaction as bimolecular rebinding. The binding kinetics of NO to azurin show impressive analogies to the binding of carbon monoxide to myoglobin. We conclude that conformational substates occur not only in heme proteins but also in proteins with different active sites and secondary structures.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Stenkamp R. E., Sieker L. C., Jensen L. H. A crystallographic model for azurin a 3 A resolution. J Mol Biol. 1978 Jul 25;123(1):35–47. doi: 10.1016/0022-2836(78)90375-3. [DOI] [PubMed] [Google Scholar]
- Alberding N., Chan S. S., Eisenstein L., Frauenfelder H., Good D., Gunsalus I. C., Nordlund T. M., Perutz M. F., Reynolds A. H., Sorensen L. B. Binding of carbon monoxide to isolated hemoglobin chains. Biochemistry. 1978 Jan 10;17(1):43–51. doi: 10.1021/bi00594a007. [DOI] [PubMed] [Google Scholar]
- Alcala J. R., Gratton E., Prendergast F. G. Resolvability of fluorescence lifetime distributions using phase fluorometry. Biophys J. 1987 Apr;51(4):587–596. doi: 10.1016/S0006-3495(87)83383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundsen A. R., Whelan J., Bosnich B. Biological analogues. On the nature of the binding sites of copper-containing proteins. J Am Chem Soc. 1977 Sep 28;99(20):6730–6739. doi: 10.1021/ja00462a042. [DOI] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Aqualino A, Brill AS, Bryce GF, Gerstman BS. Correlated distributions in g and A tensors at a biologically active low-symmetry cupric site. Phys Rev A. 1991 Oct 15;44(8):5257–5271. doi: 10.1103/physreva.44.5257. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Berendzen J., Braunstein D. Temperature-derivative spectroscopy: a tool for protein dynamics. Proc Natl Acad Sci U S A. 1990 Jan;87(1):1–5. doi: 10.1073/pnas.87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill A. S. Activation of electron transfer reactions of the blue proteins. Biophys J. 1978 Apr;22(1):139–142. doi: 10.1016/S0006-3495(78)85479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. F., Chance M. R., Friedman J. M. Linkage of functional and structural heterogeneity in proteins: dynamic hole burning in carboxymyoglobin. Science. 1987 Oct 16;238(4825):373–376. doi: 10.1126/science.3659921. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E., Cordone L. Optical absorption spectra of azurin and stellacyanin in glycerol/water and ethylene glycol/water solutions in the temperature range 290-20 K. Biophys Chem. 1990 Nov;38(3):213–224. doi: 10.1016/0301-4622(90)87003-4. [DOI] [PubMed] [Google Scholar]
- Dlott D. D., Frauenfelder H., Langer P., Roder H., DiIorio E. E. Nanosecond flash photolysis study of carbon monoxide binding to the beta chain of hemoglobin Zürich [beta 63(E7)His leads to Arg]. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6239–6243. doi: 10.1073/pnas.80.20.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Gorren A. C., de Boer E., Wever R. The reaction of nitric oxide with copper proteins and the photodissociation of copper-NO complexes. Biochim Biophys Acta. 1987 Nov 5;916(1):38–47. doi: 10.1016/0167-4838(87)90208-1. [DOI] [PubMed] [Google Scholar]
- Hartmann H., Parak F., Steigemann W., Petsko G. A., Ponzi D. R., Frauenfelder H. Conformational substates in a protein: structure and dynamics of metmyoglobin at 80 K. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4967–4971. doi: 10.1073/pnas.79.16.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutnik C. M., Szabo A. G. Confirmation that multiexponential fluorescence decay behavior of holoazurin originates from conformational heterogeneity. Biochemistry. 1989 May 2;28(9):3923–3934. doi: 10.1021/bi00435a045. [DOI] [PubMed] [Google Scholar]
- Keyes M. H., Falley M., Lumry R. Studies of heme proteins. II. Preparation and thermodynamic properties of sperm whale myoglobin. J Am Chem Soc. 1971 Apr 21;93(8):2035–2040. doi: 10.1021/ja00737a031. [DOI] [PubMed] [Google Scholar]
- Leeuwen F. X., Wever R., Gelder B. F., Avigliano L., Mondovi B. The interaction of nitric oxide with ascorbate oxidase. Biochim Biophys Acta. 1975 Oct 22;403(2):285–291. doi: 10.1016/0005-2744(75)90058-3. [DOI] [PubMed] [Google Scholar]
- Nar H., Messerschmidt A., Huber R., van de Kamp M., Canters G. W. Crystal structure analysis of oxidized Pseudomonas aeruginosa azurin at pH 5.5 and pH 9.0. A pH-induced conformational transition involves a peptide bond flip. J Mol Biol. 1991 Oct 5;221(3):765–772. doi: 10.1016/0022-2836(91)80173-r. [DOI] [PubMed] [Google Scholar]
- Nienhaus G. U., Mourant J. R., Frauenfelder H. Spectroscopic evidence for conformational relaxation in myoglobin. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2902–2906. doi: 10.1073/pnas.89.7.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris G. E., Anderson B. F., Baker E. N. Structure of azurin from Alcaligenes denitrificans at 2.5 A resolution. J Mol Biol. 1983 Apr 15;165(3):501–521. doi: 10.1016/s0022-2836(83)80216-2. [DOI] [PubMed] [Google Scholar]
- Ormos P., Ansari A., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Sauke T. B., Steinbach P. J., Young R. D. Inhomogeneous broadening in spectral bands of carbonmonoxymyoglobin. The connection between spectral and functional heterogeneity. Biophys J. 1990 Feb;57(2):191–199. doi: 10.1016/S0006-3495(90)82522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. L. A model of protein conformational substates. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3670–3672. doi: 10.1073/pnas.82.11.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Chu K., Frauenfelder H., Johnson J. B., Lamb D. C., Nienhaus G. U., Sauke T. B., Young R. D. Determination of rate distributions from kinetic experiments. Biophys J. 1992 Jan;61(1):235–245. doi: 10.1016/S0006-3495(92)81830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamann T. J., Frank P., Willis L. J., Loehr T. M. Normal coordinate analysis of the copper center of azurin and the assignment of its resonance Raman spectrum. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6396–6400. doi: 10.1073/pnas.79.20.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]


