Abstract
Lycopene β-cyclases are key enzymes located at the branch point of the carotenoid biosynthesis pathway. However, the transcriptional regulatory mechanisms of LCYb1 in citrus with abundant carotenoid accumulation are still unclear. To understand the molecular basis of CsLCYb1 expression, we isolated and functionally characterized the 5′ upstream sequences of CsLCYb1 from citrus. The full-length CsLCYb1 promoter and a series of its 5′ deletions were fused to the β-glucuronidase (GUS) reporter gene and transferred into different plants (tomato, Arabidopsis and citrus callus) to test the promoter activities. The results of all transgenic species showed that the 1584 bp upstream region from the translational start site displayed maximal promoter activity, and the minimal promoter containing 746 bp upstream sequences was sufficient for strong basal promoter activity. Furthermore, the CsLCYb1 promoter activity was developmentally and tissue-specially regulated in transgenic Arabidopsis, and it was affected by multiple hormones and environmental cues in transgenic citrus callus under various treatments. Finer deletion analysis identified an enhancer element existing as a tandem repeat in the promoter region between -574 to -513 bp and conferring strong promoter activity. The copy numbers of the enhancer element differed among various citrus species, leading to the development of a derived simple sequence repeat marker to distinguish different species. In conclusion, this study elucidates the expression characteristics of the LCYb1 promoter from citrus and further identifies a novel enhancer element required for the promoter activity. The characterized promoter fragment would be an ideal candidate for genetic engineering and seeking of upstream trans-acting elements.
Keywords: citrus, carotenoid, lycopene β-cyclase, promoter, cis-element, enhancer
Introduction
Carotenoids are a class of 40-carbon terpenoid molecules present in most tissues of higher plants. They are one type of the most important secondary metabolites, and play a variety of roles in many biological processes in plants, including flowering and fruit coloration (Bartley and Scolnik, 1995), light harvesting for photosynthesis (Frank and Cogdell, 1996; Robert et al., 2004), protection from excessive light (Müller et al., 2001), defense against biotic and abiotic stresses (Ramel et al., 2012), and production of apocarotenoid hormones such as abscisic acid (ABA) and strigolactone (Creelman et al., 1987; Alder et al., 2012). As an important dietary component for human body, carotenoids provide precursors for vitamin A synthesis (Lakshman and Okoh, 1993; DellaPenna and Pogson, 2006) and reduce the risks of cardiovascular diseases, cancers and age-related diseases (Fraser and Bramley, 2004; Krinsky and Johnson, 2005; Botella-Pavía and Rodríguez-Concepción, 2006). Therefore, it is of great interest to understand the biosynthesis of these isoprenoids in plants.
Carotenoid metabolism is a complicated pathway involving the expression of many genes, which are regulated by various factors, such as developmental cues and environmental conditions (Liu et al., 2015; Nisar et al., 2015). Although a number of genes related to carotenoid synthesis and degradation have been isolated and analyzed, only a few studies have addressed the regulatory mechanisms of these processes. Recently, only three types of transcription factors, namely RAP2.2 (APETALA2/ethylene-responsive; Welsch et al., 2007), PIFs (phytochrome-interacting factors; Toledo-Ortiz et al., 2010) and RIN (ripening-inhibitor; Vrebalov et al., 2002; Fujisawa et al., 2013), have been identified to directly interact with the promoters of carotenogenic genes and regulate their expression. To gain insight into the complex regulatory mechanisms of this metabolic pathway and to unravel the underlying upstream interacting factors, functional characterization of the gene promoters is of great importance.
To date, some promoters of the genes in the carotenoid biosynthetic pathway have been analyzed and some progresses have been achieved. The analysis of the Arabidopsis phytoene synthase (PSY) promoter identified a G-box-like element involved in light induction and discrimination between different light qualities, and also identified a novel cis-acting element ATCTA which contributes to strong basal promoter activity (Welsch et al., 2003). The tomato phytoene desaturase (Pds) promoter developmentally drives high GUS expression in the organs where chromoplasts are formed, and promotes gene transcription in green tissues in response to end-product regulation (Corona et al., 1996). The Arabidopsis carotenoid cleavage dioxygenases 7 (AtCCD7) promoter exhibits a vascular-specific expression pattern in transgenic plants (Liang et al., 2011), while the β-carotene hydroxylase (AtBCH) promoter shows strong constitutive expression in dicot plants (Liang et al., 2009). The functional characterization of the Gentiana lutea zeaxanthin epoxidase (GlZEP) promoter in transgenic tomato plants showed that GlZEP-GUS expression is closely associated with fruit development and chromoplast differentiation, suggesting an evolutionarily conserved link between ZEP and the differentiation of organelles that store carotenoid pigments (Yang et al., 2012). Imai et al. (2013) isolated the promoter of the carotenoid cleavage dioxygenase 4a-5 gene of Chrysanthemum morifolium (CmCCD4a-5) and assessed its petal-specific promoter activity.
Lycopene β-cyclases are key enzymes functioning at the branch point of the carotenoid biosynthesis pathway and converting upstream red lycopene to downstream bright yellow α-/β-carotene (Cunningham et al., 1996). As an important economic fruit crop, citrus contains abundant carotenoids, and the carotenoid content and composition vary greatly among different species (Fanciullino et al., 2006; Xu C.-J. et al., 2006; Xu J. et al., 2006). Previous studies have reported that the carotenoid accumulation in citrus is closely related to the transcript levels of Lycopene β-cyclase genes (Kato et al., 2004). There are two types of lycopene β-cyclase genes (here designated as LCYb1 and LCYb2, respectively) in citrus. LCYb1 is predominantly expressed in leaf tissues, while LCYb2 is mainly expressed in fruit tissues and shows a marked induction during fruit development (Alquézar et al., 2009; Mendes et al., 2011). It has been demonstrated that a relatively low transcript level of LCYb2 (also named as β-LCY2 / LCYB2) results in lycopene accumulation in red grapefruit (Citrus paradisi) (Alquézar et al., 2009; Mendes et al., 2011; Alquezar et al., 2013). However, studies on ‘HongAnliu’ sweet orange (a red-flesh mutant of ‘Anliu,’ C. sinensis) revealed that the down-regulation of both LCYb (LCYb1) and capsanthin capsorubin synthase (CCS) (LCYb2) may be responsible for the abnormal lycopene accumulation in the mutant (Xu et al., 2009; Yu et al., 2012). Zhang et al. (2012b) further elucidated that only CitLCYb1 participates in the formation of α-carotene during the green stage in the flavedo, and that the high expression levels of both CitLCYb1 and CitLCYb2 during the orange stage play an important role in the accumulation of β, β-xanthophylls in citrus fruits. Apart from the roles in fruit color development, the high expression levels of LCYb1 in leaf tissues suggest that this gene also participates in photosynthesis and other biological processes, which are crucial to the survival of plants. Additionally, since LCYb1 is nearly present in all plant species and is an evolutionarily ancient and conserved gene, study of citrus LCYb1 promoter will not only help us to understand the transcriptional regulatory mechanism of LCYb1 in citrus, but also promote the understanding of LCYb1 in other species. Although the promoters of LCYb2 have been isolated and functionally analyzed in tomato (Dalal et al., 2010) and watermelon (Bang et al., 2014), little information is available regarding the LCYb1 promoter.
The objectives of the present study were to isolate and functionally characterize the CsLCYb1 promoter from sweet orange (C. sinensis) as well as to analyze the LCYb1 promoters from different citrus species. This study will contribute to understanding the expression characteristics of LCYb1 promoters and is expected to help future transcriptional regulation studies of LCYb1 expression in citrus.
Materials and Methods
Plant Materials
The materials included four genotypes of pummelo (C. grandis; White-flesh Guanxi pummelo, Red-flesh Guanxi pummelo, Huanong red pummelo, HB pummelo), three genotypes of grapefruit (C. paradisi; Star Ruby grapefruit, Marsh grapefruit, and Flame grapefruit), four genotypes of sweet orange (C. sinensis; Washington navel orange, Cara Cara navel orange, Anliu sweet orange, HongAnliu sweet orange), and three genotypes of mandarin (C. reticulata; Bendizao mandarin, Qingjiang ponkan, Mangshan wild tangerine). Leaves of all these citrus varieties were obtained from the National Center of Citrus Breeding, Huazhong Agricultural University, Wuhan, China. The tissues were frozen in liquid nitrogen and stored at -80°C until use. Tomato (Lycopersicon esculentum cv Ailsa Craig) and Arabidopsis (Arabidopsis thaliana, ecotype Col-0) plants were grown under standard greenhouse conditions. Embryogenic callus used in this study was derived from Marsh grapefruit and subcultured on solid MT (Murashige and Tucker) basal medium containing 50 g L-1 sucrose under normal conditions (16 h light/8 h dark cycles at 25°C).
Promoter Cloning and Sequence Analysis
The CsLCYb1 cDNA sequence (orange1.1t00772) was used as a query to search the C. sinensis genomic database1 (Xu et al., 2013) and the 5′ upstream genomic sequence (about 2 kb) was retrieved (chrUn:9346020..9348020). Specific primers for promoter isolation were designed based on the reference sequence (Supplementary Table S1). Briefly, genomic DNA was extracted from leaves of Anliu sweet orange, White-flesh Guanxi pummelo, Marsh grapefruit and Bendizao mandarin using the CTAB (cetyltrimethylammonium bromide) method (Cheng et al., 2003). PCR reactions were performed under the following conditions: 95°C for 3 min, followed by 32 cycles at 95°C for 10 s, 55°C for 20 s and 72°C for 1 min, and a final 7 min extension at 72°C. The PCR products were gel-purified and cloned into the pMD18-T vector (TaKaRa, Dalian, China) for sequencing. The first nucleotide acid of the CsLCYb1 mRNA was set as the transcription start site (TSS). Promoter regions and plant regulatory motifs were searched using the Softberry TSSP and Nsite-PL program2. A search for putative cis-elements in the promoter sequence was performed by using the PLACE3 (Higo et al., 1999) and PlantCARE4 (Lescot et al., 2002) databases. The LCYb1 promoter in mandarin was retrieved from the Citrus clementina genome database 5. Multiple sequence alignments were performed using the ClustalX2 and GeneDoc programs.
Vector Construction
The entire CsLCYb1 promoter region (-1584 bp from the ATG start codon) and its five deletions (gradually truncated from the 5′ end of the CsLCYb1 promoter) were amplified by PCR from the pMD18-T basic vector containing the 5′ full-length flanking sequence. Specific primers with EcoRI and NcoI restriction sites were designed (Supplementary Table S1). The amplified fragments were double digested and inserted into the corresponding site of the plasmid pCAMBIA1301 (CAMBIA, Canberra, Australia) in the upstream of the β-glucuronidase (GUS) reporter gene, replacing the CaMV35S promoter (the cauliflower mosaic virus 35S promoter). The six vectors were designated as LP (-1584), LP1 (-1255), LP2 (-1045), LP3 (-746), LP4 (-406), and LP5 (-247), respectively. The CaMV35S::GUS fusion in pCAMBIA1301 was used as a positive expression control (designated as 35S).
Internal deletion vector construction: Double stranded DNA, (GTGACTGAAATCATCAACCCTTGATGAACATCCTTTGCTATTGGGCATGAATGGAGAAGGAAGAAAATGAG (ATTGAAGGAAGAAAAATGAG)n CGTGAAGGAGGAAAAGTGAGAAGAAAAAAAATTATATATTTTTTAATT), was synthesized to yield two fragments denoted as W1 (n = 1) and W2 (n = 2), respectively. Then, two pairs of primers (LMa-F and LMa-R; LMb-F and LMb-R) were used for PCR amplification of the full-length CsLCYb1 promoter sequences to obtain two fragments denoted as LMa and LMb, respectively. Next, overlapping PCR was performed with three fragments (LMa, LMb, W1 or W2) simultaneously as templates and with two oligonucleotides (LPF and LPR containing EcoRI and NcoI sites at their 5′-ends) as primers. The obtained fragments were double digested and subcloned into the correspondingly enzymatic sites of the pCAMBIA1301 plasmid to yield two internal deletion vectors WP1 and WP2. The promoter-GUS vectors are schematically represented in Figures 2 and 6. All constructs were verified by sequencing and then transformed into the Agrobacterium tumefaciens stain GV3101 by the freeze-thaw method. The generated constructs were subsequently transformed into plants to test promoter activities.
FIGURE 2.
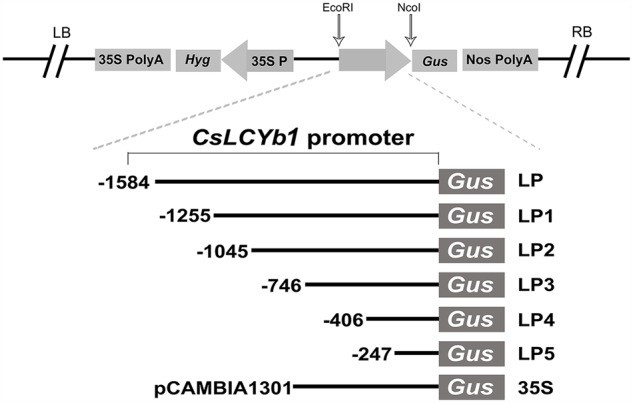
Schematic representation of the CsLCYb1 promoter::GUS vectors construction. These constructs are based on the pCAMBIA1301 vector. LB, left border; 35S PolyA, Cauliflower Mosaic Virus 35S terminator; Hyg, hygromycin resistance gene; 35S P, Cauliflower Mosaic Virus 35S promoter; GUS, β-glucuronidase reporter gene; Nos PolyA, nopaline synthase terminator; RB, right border. Hollow arrows indicate the positions of the promoter insertion in the vectors. The promoters contain the full-length sequence (LP) and its five 5′ truncated fragments (LP1, LP2, LP3, LP4, and LP5). Numbers indicate the sequence length from the first base of the ATG.
FIGURE 6.
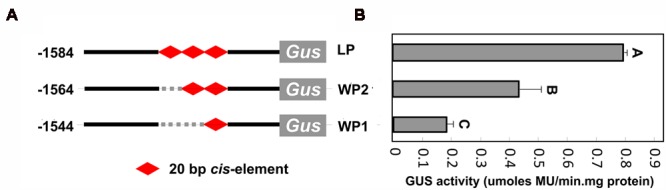
Finer deletion analysis of the 20 bp fragment. (A) Schematic representation of the internal deletion promoter constructs. Numbers indicate the sequence length from the first base of the ATG. (B) Quantitative GUS assays of different constructs in stably transformed citrus callus.
Plant Transformation
Tomato transient transformation was performed according to the method described by Orzaez et al. (2006) with minor modification. Agrobacterium cultures (0.5 mL) from individual colony were grown at 28°C for 24 h in LB liquid medium supplemented with kanamycin (100 mg L-1) and rifampicin (25 mg L-1), then transferred to 50 mL induction medium (LB medium plus 20 mM acetosyringone, 10 mM MES, pH 5.6) containing corresponding antibiotics and grown again. In the following day, the bacterial cells were sedimented by centrifugation and re-suspended in infiltration medium (10 mM MgCl2, 10 mM MES, 20 mM acetosyringone, pH 5.6) to an OD600 of approximately 1.0, and then incubated at room temperature with gentle agitation (20 rpm) for about 2 h. Cultures were collected with a syringe, and then injected into detached tomato fruits (L. esculentum cv Ailsa Craig) at a total volume of 600 μl. Three days later, the injected fruits were cut into slices for histochemical GUS staining.
Arabidopsis transformation was done using the floral dip method established by Clough and Bent (1998). Two generations of the transformed plant were selected on MS (Murashige and Skoog) medium supplemented with 25 mg l-1 of hygromycin, then were transferred to soil, and finally were grown in the greenhouse at 22°C under a 16 h light/8 h dark photoperiod. The positive transformations were further confirmed through PCR amplification of genomic DNA by using the primer sets, respectively (Supplementary Table S1). The estimation of transgene copy numbers in transgenic Arabidopsis was conducted according to the method described by Weng et al. (2004). The results are shown in Supplementary Figure S3. Finally, approximately 10 independent homozygous T2 transgenic lines with single-copy insertion of each promoter were used for subsequent GUS assays, and the wild type Arabidopsis plants were used as the negative control. Different tissues, including roots, stems, leaves, flowers, and fruits, were collected from each selected plant growing in soil for about 45 days for tissue-specific expression assay. Seedlings of the transgenic lines containing the full-length promoter construct (LP) were collected during five developmental stages for developmental expression assay. Seedlings of other transgenic lines were only collected on day 24 after seed germination to compare the promoter activities among different truncated fragments.
Embryogenic callus transformation was performed with the method described by Li et al. (2002). Positive transgenic lines were screened and subcultured with the method described by Cao et al. (2012). Transgenic callus was selected on solid MT (Murashige and Tucker) basal medium containing 50 mg l-1 of hygromycin and 250 mg L-1 of cefotaxim. PCR amplification was used to further confirm the positive lines. Each independent line was propagated on solid MT basal medium containing 50 g L-1 sucrose under normal conditions (16 h light/8 h dark cycles at 25°C). Twenty-day-old callus was harvested for GUS assays or various stimuli treatments.
Stress Treatments
In various stimuli treatments, transgenic callus was cultured on solid MT medium for about 20 days at 25°C, followed by culturing for 4 days in liquid MT medium with shaking. The stable cell suspension cultures in a good state were immersed in MT liquid medium supplemented with 100 μM ABA, 100 μM auxin (IAA), 100 μM gibberellin (GA), 100 μM salicylic acid (SA), 100 μM Methyl Jasmonate (JA), 100 μM Kinetin (KT), 10% (w/v) sucrose (Suc), 10% (w/v) glucose (Glu), 200 mM NaCl for 12 h under light. Callus cells in MT liquid medium without any supplement were used as negative control. All parallel samples were grown under the same conditions. After treatment, all samples were frozen in liquid nitrogen and used for subsequent GUS assays. Three biological replicates were performed for each set of treatments.
GUS Assays
Histochemical staining and fluorometric assays were performed according to the method proposed by Jefferson et al. (1987).
Various tissues were submerged in X-gluc buffer [100 mM phosphate buffer (pH 7.0), 1 mM 5-bromo-4-chloro-3-indolyl-glucuronide (X-gluc) solution, 0.1% Triton X-100, 10 mM EDTA, 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, and 20% methanol] overnight at 37°C. After staining, the tissues were kept in 70% ethanol until the chlorophyll was removed, and then photographed with a digital camera or under a stereomicroscope (Leica MZFL III). All the experiments were repeated ten times for each construct.
Quantitative GUS assays were performed as follows. The samples were frozen in liquid nitrogen and ground into powder. Protein was extracted with GUS extraction buffer (50 mM phosphate buffer, pH 7.0; 10 mM EDTA; 0.1% Triton X-100; 0.1% Sodium Dodecyl Sulfate; and 10 mM β-mercaptoethanol). After centrifugation, the supernatant of various extraction liquids was used for the subsequent protein quantification and fluorometric assays. Protein concentrations were determined using the BCA Protein Assay Kit (Beyotime Biotechnology, China). Fluorometric assays were performed in microtiter plates at 37°C in the presence of 1 mM 4-methylumbelliferyl glucuronide (MUG, Sigma–Aldrich). The appearance of 4-methylumbelliferone (MU) was monitored using a Tecan InfiniteTM M200 plate reader at 365 nm excitation and 455 nm emission. GUS enzyme activity was expressed as umoles of 4-MU per min per mg protein. Three replicates were performed for each sample.
Simple Sequence Repeat (SSR) Screening
Total genomic DNA was extracted from leaf samples of different citrus varieties. SSR screening primers (LSSR-F and LSSR-R) were designed according to above isolated LCYb1 promoter sequences (Supplementary Table S1). The SSR amplification reactions were conducted according to the protocol described by Chai et al. (2013). The amplification products were firstly checked by agarose gel electrophoresis. Then, PCR products were separated by polyacrylamide gel electrophoresis and visualized by silver staining following the protocol developed by Ruiz et al. (2000).
Statistical Analysis
The data were presented as mean ± SD of three independent experiments. Statistical analyses were done using the One-way ANOVA test on the Microsoft Excel program (Microsoft Office, 2010). The difference with a P-value <0.05 (∗/Lowercase letters) or <0.01(∗∗/Uppercase letters) was considered as significant.
Results
Isolation and Sequence Analysis of the CsLCYb1 Promoter
The CsLCYb1 genomic DNA sequence and its 5′ flanking region were downloaded from the genomic database of sweet orange with the full length CsLCYb1 cDNA (orange1.1t00772) as a query sequence. The 1584 bp fragment located in the upstream from the ATG start codon was obtained through PCR-based method and tentatively designated as the full-length promoter of CsLCYb1 (Figure 1). The TSS was located at -263 bp upstream from the ATG (the position of the ATG start codon was designated as 0). Bioinformatics analysis revealed that the CsLCYb1 promoter was a typical eukaryotic promoter containing a potential TATA box at -292 bp, a CAAT-box at -356 bp, and many TA-rich enhancer elements. A large number of hormone-responsive elements were predicted in the promoter, such as the ATCTA-motif in response to ethylene, the CGTCA-motif to Jasmonate, the GARE motif to gibberellin, the TCA motif to SA and the TGA motif to auxin. We also discovered some stress-responsive elements, such as the ARE-motif involved in anaerobic induction, the CATGTG-motif in dehydration response, the E-box in defense signaling, and the MYB-binding sites in drought inducibility. In addition, the CsLCYb1 promoter carried numerous light-responsive elements, such as the Box 4, Box II, CATT-motif, GA-motif, GAG-motif, SP1 and TCCC-motif. Among these motifs, the GA-motif characterized by the AGATT sequence existed as a tandem repeat in the promoter region between -588 and -522 bp. We also compared the cis-elements in the CsLCYb1 promoter with those in the previously isolated CsPSY promoter (Zeng et al., 2013) and CitCRISO promoter (Eun et al., 2015) (Accession No. KJ751507) in citrus. Many common cis-acting elements were discovered, such as the CGTCA-motif that is involved in the MeJA-responsiveness and the SP1 element that responds to light. Some of the relevant cis-elements and their relative positions in the upstream of the ATG start codon are listed in Supplementary Table S2. Notably, a pair of reverse complementary sequences that were not completely symmetrical were discovered in the regions from -1409 to -1348 bp and from -384 to -318 bp.
FIGURE 1.
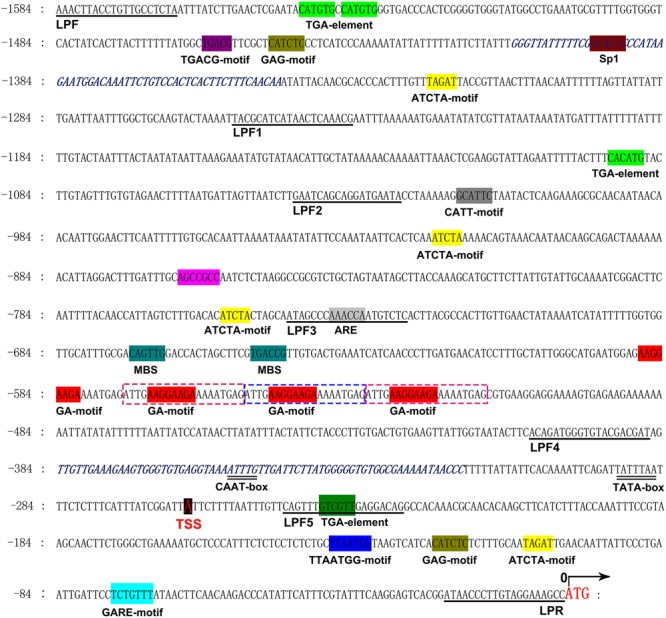
The 5′ upstream promoter sequences of the CsLCYb1 gene. Numbers indicate the positions relative to the ATG start codon (0). The putative TATA-box (double underlined), CAAT-box (double underlined), transcriptional start site (TSS, highlighted), and some cis-elements (highlighted) are labeled under the sequences. Primers for amplifying a series of 5′ truncated fragments are also underlined and labeled. The pair of reverse complementary sequences are italic in blue color. The 20 bp tandem repeat sequences are dot outlined.
Transient Expression Assay of CsLCYb1 Promoter in Tomato
Firstly, we applied a transient expression method to identify whether the cloned CsLCYb1 promoter sequence was active. Tomato fruits at the mature green stages were injected with bacterial cultures carrying each promoter::GUS construct, respectively. Fruits were harvested 3 days later and transverse sections were stained for GUS expression. As expected, we found strong GUS staining in the fruits transformed with the 35S construct, while no GUS expression was detected in the wild type without transformation. GUS staining of the full-length promoter construct LP and the truncated promoter constructs LP1, LP2, and LP3 was evident in columella and placental tissues but not in seeds. The intensity of GUS staining was similar among LP, LP1, and LP3, while LP2 showed relatively lower GUS intensity compared with the above three constructs. However, in transgenic tomato LP4 and LP5, only the vascular bundles were stained (Figure 3).
FIGURE 3.
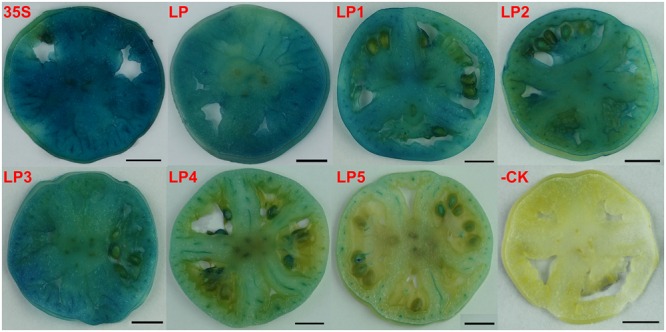
Histochemical GUS staining of tomato green fruit. Transgenic lines carrying the GUS reporter gene under the control of the CaMV35S promoter were used as the positive control (35S) and untransformed tomato was used as the negative control (-CK). LP, LP1, LP2, LP3, LP4, and LP5 represent transgenic lines under the control of the full-length CsLCYb1 promoter and its five 5′ truncated fragments, respectively. Bars, 1 cm.
Spatial and Temporal Expression Patterns of CsLCYb1 Promoter in Arabidopsis
To elucidate the spatial and temporal expression patterns of the CsLCYb1 promoter, we examined stable Arabidopsis transgenic plants. Homozygous single-insertion T2 lines of each construct were used for histochemical staining and quantitative GUS assays. Different tissues (roots, stems, leaves, flowers, and fruits) from each construct were subjected to histochemical GUS staining (Figure 4). GUS staining was observed in all tested tissues of the CaMV35S construct, but not in the wild type control. GUS expression of LP, LP1, LP2, and LP3 constructs was apparently detectable in leaves. No significant difference in GUS staining intensity was found among LP, LP1 and LP3, while the staining intensity of LP2 was relatively lower than that of the above-mentioned three constructs. Little or no GUS staining was found in the leaves of LP4 and LP5. In addition, several tissues of some constructs were slightly stained, such as the root of LP, flower of LP4, stem ends of LP1 and fruit ends of LP, LP1, LP3, and LP4. Epidermal hairs in the stems of LP, LP1, LP2, LP3, and LP4 were also stained.
FIGURE 4.
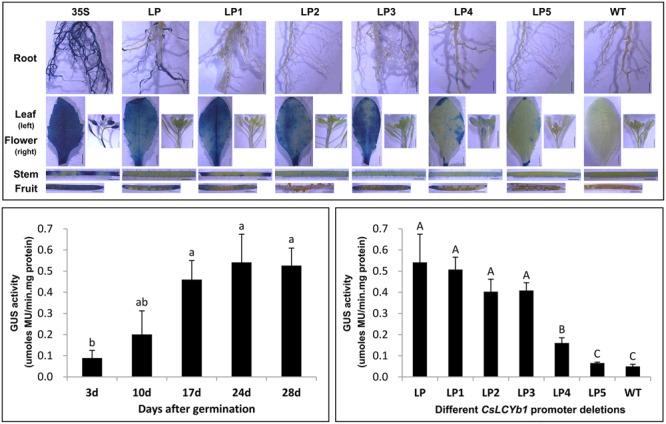
GUS assays of transgenic Arabidopsis plants. (Up) Qualitative GUS staining. Tissues (root, leave, flower, stem, and fruit) from each construct were separately subjected to histochemical GUS staining. Transgenic lines carrying the GUS reporter gene under the control of the CaMV35S promoter were used as the positive control (35S) and untransformed Arabidopsis were used as the negative control (WT). Bars, 2 mm. (Down) Quantitative GUS assays of transgenic Arabidopsis seedlings carrying the full-length promoter construct (LP) during seedling development (at bottom left) and transgenic Arabidopsis seedlings carrying different promoter constructs (at bottom right). Leaves were harvested on day 24 after seeding. Data are means ± SD of three independent experiments. Lowercase letters indicate significant differences at P < 0.05. Uppercase letters indicate significant differences at P < 0.01.
Glucuronidase enzyme activities were quantified by fluorometric 4-MUG assay at different developmental stages of seedling in the full length promoter transgenic lines (Figure 4). The results showed that the promoter activities increased along with the seedling development, reached the maximum on day 24, and subsequently decreased on day 28. Then, GUS expression of different promoter constructs was compared on day 24. In accordance with the results of GUS staining assay, the LP construct carrying the full-length sequence of the CsLCYb1 promoter produced the highest level of GUS expression in leaf tissues. With deletions of the 5′ fragments, the promoter activity gradually decreased. However, no significant difference in GUS activity was found among LP, LP1, LP2, and LP3. By comparison, the GUS activities of LP4 and LP5 were remarkably reduced. The GUS activity of LP4 was about fourfold lower than that of LP, and that of LP5 was too low to be detected.
Responses of Different Promoter Constructs to Various Stimuli in Citrus Callus
Previously we detected the transcripts of CsLCYb1 in citrus callus (data not shown). In order to eliminate the possible effect of heterogeneous expression on promoter activity, we stably transformed the promoter constructs into citrus callus, respectively. The expression level of each construct in transgenic citrus callus was also evaluated by histochemical GUS staining (Figure 5). The results showed that GUS staining was obviously visible in callus transformed with constructs LP, LP1, LP2, and LP3, suggesting that these fragments could functionally drive GUS expression in callus. The callus transformed with construct LP4 was slightly stained, indicating possible promoter activity of this fragment in callus. Pale blue was observed only in a few cells of transgenic callus with LP5.
FIGURE 5.
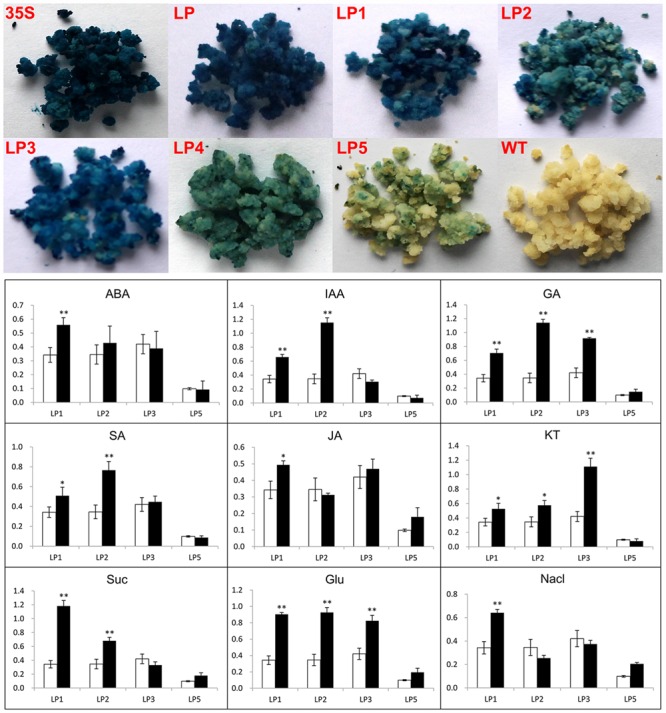
GUS assays of transgenic citrus callus. (Up) Qualitative GUS staining. Citrus callus carrying different promoter constructs were separately subjected to histochemical GUS staining. Transgenic lines carrying the GUS reporter gene under the control of the CaMV35S promoter were used as the positive control (35S) and untransformed callus was used as the negative control (WT). (Down) Quantitative GUS assays of different promoter deletions in stably transformed citrus callus under various treatments, including abscisic acid (ABA), auxin (IAA), gibberellin (GA), salicylic acid (SA), Methyl Jasmonate (JA), Kinetin (KT), sucrose (Suc), glucose (Glu), and NaCl. Data are means ± SD of three independent experiments. Significant differences between values are indicated by asterisk (∗P < 0.05, ∗∗P < 0.01).
Although the roles of phytohormones, glucose (sucrose or mannitol) and various other stimuli in carotenoid accumulation have been studied, little has been known about the molecular mechanisms that regulate carotenoid metabolism and gene expression. To fully reveal the regulation, we analyzed CsLCYb1 promoter function in detail by GUS expression assay under various stimuli treatments. Based on the GUS staining results, we speculated that the sequence region from LP1 (-1255) to LP4 (-406) was likely to significantly contribute to the regulation of the CsLCYb1 promoter activity. Thereby, we tested the expression levels of constructs LP1, LP2, and LP3 under various stimuli including hormones, sugar and salt stress to examine the responses of different 5′ sequence regions. GUS expression level driven by LP5 promoter was also tested under all conditions. As shown in Figure 5, LP1 promoter activity was significantly induced under ABA and JA treatments. In contrast, the promoter activities of LP2 and LP3 were not strongly affected by both ABA and JA. IAA treatment induced the activities of LP1 and LP2 promoter (1.9- and 3.3-fold, respectively), and SA treatment also induced their activities (1.5- and 2.2-fold, respectively). However, the LP3 construct did not show any significant differences in promoter activity under IAA and SA treatments. When callus cells were incubated with GA, we observed significant induction of GUS expression. Compared with under normal conditions, the promoter activities of LP1, LP2, and LP3 were increased to 2.1-, 3.3-, and 2.2-fold, respectively. Under KT treatment, GUS expression driven by either LP1 or LP2 was increased to about 1.6-fold. Notably, a deletion from LP2 to LP3 resulted in a sharp increase of GUS activity to 1.1 μmol MU min-1 mg-1 protein (2.6-fold). Both sucrose and glucose treatments significantly enhanced the promoter activities of LP1 and LP2. However, GUS expression of LP3 was only promoted by glucose, not by sucrose. Additionally, we analyzed the promoter activities under NaCl treatment to determine whether the promoter activity responded to salt stress. The results showed that the promoter activity of LP1 was significantly increased, while the activities of LP2 and LP3 were decreased to some extent, even though no significant differences were observed.
Finer Deletion Analysis of the CsLCYb1 Promoter
Since a deletion from LP3 to LP4 resulted in a significant reduction in promoter activity, we speculated that an enhancer (or enhancers) may be located in this region. Further sequence analysis revealed the existence of a 20 bp fragment (ATTGAAGGAAGAAAAATGAG) in the region as a tandem repeat (between -574 and -513 bp upstream from the ATG). A search of the PLACE database for the potential cis-elements in the 20 bp sequence identified five reported cis-elements: Inr-element (YTCANTYY), CAAT-box (CAAT), GT1-motif (GAAAAA), GT-element (GRWAAW), and pollen-specific element (AGAAA). In order to verify whether the 20 bp fragment was essential for promoter function, we performed finer deletion analysis. Additional vectors with the deletion of one or two copies of the 20 bp fragment were constructed and transformed into citrus callus to test the promoter activities. Compared with the complete CsLCYb1 promoter, the deletion of one copy caused the promoter activity to dramatically decrease to 55%, while the promoter activity with the deletion of two copies dropped to approximately 23% (Figure 6). Taken together, these data clearly indicated that the 20 bp fragment acted as a positive cis-acting regulatory element to affect promoter activity.
Sequence Analysis of LCYb1 Promoters from Other Citrus Species
In order to further understand the sequence characteristics of LCYb1 promoter, we isolated promoters of LCYb1 alleles from other citrus species. Due to the high heterozygosity in citrus genome, most of the gene loci have two different alleles termed as a and b, respectively. CsLCYb1 in sweet orange has two different coding sequences (data not shown), while we got only one promoter sequence named as pCsLCYb1. We tried our best but failed to get the other one. However, in pummelo and grapefruit, we obtained two different promoter sequences named as pCgLCYb1a and pCgLCYb1b, pCpLCYb1a and pCpLCYb1b, respectively (Supplementary Figure S1). Multiple sequence alignment revealed that these promoter sequences differed in the copy numbers of the 20 bp enhancer element in addition to the differences in several single nucleotide polymorphisms (SNPs; Supplementary Figure S2). We retrieved the LCYb1 promoter in mandarin (named as pCrLCYb1) from the C. clementina genome databases and found that a large fragment was inserted in the enhancer region of the LCYb1 promoter (Supplementary Figure S1). Interestingly, partial sequences of the large fragment were reversely complementary to a citrus tristeza virus (CTV) resistance gene according to the NCBI blast search, and the insertion resulted in the remaining of only one copy number of the 20 bp enhancer element in the promoter. The sequence characteristics of LCYb1 promoters from four citrus clades are schematically represented in Figure 7A.
FIGURE 7.
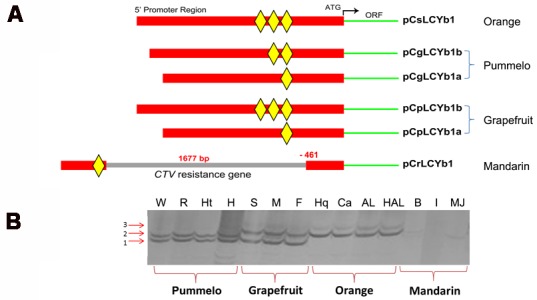
Analysis of LCYb1 promoters from various citrus species. (A) Schematic representation of promoter structure of LCYb1 from four citrus species. Green lines represent the coding sequences of LCYb1 genes. Red lines represent the promoter sequences of LCYb1. Gray lines represent the inserted large fragment. The inserted position and fragment size are indicated. Yellow rhombuses represent the 20 bp enhancer elements. (B) SSR screening of different LCYb1 promoters from various citrus varieties. Numbers on the left denote the three electrophoretic bands. Four citrus species including pummelo, grapefruit, sweet orange and mandarin were detected. W, White-flesh Guanxi pummelo; R, Red-flesh Guanxi pummelo; Ht, Huanong red pummelo; H, HB pummelo; S, Star Ruby grapefruit; M, Marsh grapefruit; F, Flame grapefruit; Hq, Washington navel orange; Ca, Cara Cara navel orange; AL, Anliu sweet orange; HAL, HongAnliu sweet orange; B, Bendizao mandarin; I, Qingjiang ponkan; MJ, Mangshan wild tangerine.
To further confirm the association between the copy numbers of the 20 bp enhancer element and genetic evolution of citrus species, a pair of primers was designed to develop a derived SSR (simple sequence polymorphism) DNA molecular marker (Supplementary Table S1). The primers LSSR-F and LSSR-R were used to amplify the promoter enhancer regions from four clades of citrus species (pummelo, mandarin, orange and grapefruit). Through the polyacrylamide gel electrophoresis method, three electrophoretic bands were separated clearly as shown in Figure 7B. According to the corresponding copy numbers, we defined these three bands as 1, 2, and 3. Pummelo had bands 1 and 2, while grapefruit had bands 1 and 3. Sweet orange only contained one band (3), while no band was found for mandarin. These results indicated that the SSR markers based on the 20 bp enhancer element could be used to distinguish different citrus species.
Discussion
Lycopene β-cyclases are key enzymes catalyzing the cyclization of the linear trans-lycopene to produce the cyclic α- and β-carotenes in the carotenoid biosynthetic pathway (Cunningham et al., 1996). There are two Lycopene β-cyclase genes (LCYb1 and LCYb2) in citrus. Functional analysis showed that both enzymes participate in the formation of β-carotene, and play important roles in fruit ripening and plant development (Alquézar et al., 2009; Xu et al., 2009; Mendes et al., 2011; Yu et al., 2012; Zhang et al., 2012b, 2013). Although the chromoplast-specific LCYb2 is mainly responsible for the carotenogenesis in fruits, the role of LCYb1 in leaf tissues and flavedo at green stage cannot be neglected. To elucidate the molecular basis of LCYb1 gene expression and investigate the upstream interacting factors, we cloned the LCYb1 promoter from citrus and analyzed its characteristics in detail for the first time. Even though CsLCYb1 was found to be highly expressed in leaf tissues, its expression was also detected in other tissues including unripe fruit and citrus callus, as demonstrated by previous studies (Xu et al., 2009; Gao et al., 2011; Cao et al., 2012; Yu et al., 2012; Zhang et al., 2012b). We also previously detected the transcripts of CsLCYb1 in these tissues (data not shown). Due to the difficulty of transformation, long life cycle (at least 1 year from sowing to fruit ripening), and the difficulty of GUS measurement of citrus species, we investigated the promoter activities by transient expression assay in tomato fruit and stable transformation method in Arabidopsis plants. These two methods had been successfully used for studying the promoter function of carotenogenic genes, such as the tomato phytoene desaturase (SlPDS; Corona et al., 1996) and chromoplast-specific Lycopene β-cyclase (SlCYC-B; Dalal et al., 2010), the C. morifolium carotenoid cleavage dioxygenase 4a-5 gene (CmCCD4a-5; Imai et al., 2013), the Crocus sativu carotenoid cleavage dioxygenase (CsCCD; Ahrazem et al., 2010), the Arabidopsis carotenoid cleavage dioxygenase (AtCCD7; Liang et al., 2011), the C. unshiu carotenoid isomerase (CuCRTISO; Eun et al., 2015), and the G. lutea zeaxanthin epoxidase (GlZEP; Yang et al., 2012). Tomato transient assay is an efficient and simple way, while Arabidopsis stable transformation is appropriate for temporal and tissue-specific detection. Further promoter detection in transgenic citrus callus can exclude the effect of heterogeneous background on promoter activity. Therefore, it is reasonable and appropriate to analyze CsLCYb1 promoter function simultaneously in transgenic tomato green fruit, Arabidopsis plants and citrus callus.
Expression Characteristics of Different CsLCYb1 Promoter Deletions
Promoter deletion analyses performed, respectively, in tomato, Arabidopsis and citrus callus produced similar results (Figures 3–5), suggesting that the three analyzed species shared some transcription factors binding to the promoter. A similar finding was reported based on the functional analysis of a dahlia mosaic virus subgenomic transcript (DaMVSgt) promoter in transient protoplasts, transgenic tobacco and Arabidopsis plants (Banerjee et al., 2015). The 1584 bp upstream region from the translation start site displayed the maximum promoter activity, and the minimal promoter LP3 containing 746 bp upstream sequences was sufficient to drive strong GUS gene expression. Therefore, the minimal promoter LP3 could be a useful tool in genetic engineering. The truncated fragment LP4 containing core promoter element (TATA-box, CAAT-box, and TSS) exhibited very weak promoter activity (Figures 1 and 5). However, the shortest fragment LP5, which did not contain any core promoter element, also drove very little GUS expression in transgenic callus (Figure 5). One explanation may be that the actual positions of core elements in the promoter regions are not consistent with the bioinformatics predictions, which needs to be verified by more experiments. Another reason could be that the promoter sequences containing no core elements such as TATA-box still have promoter activity as demonstrated previously (Burke and Kadonaga, 1996; Nakamura et al., 2002). In the promoter expression assays, the GUS staining intensity of LP2 was slightly lower than that of LP1 and LP3 in tomato and Arabidopsis; however, this minor difference did not reach significant level as revealed by the quantitative results (Figures 3 and 4).
Expression Patterns of CsLCYb1 Promoter in Response to Various Exogenous and Endogenous Factors
Previous studies reported that the CsLCYb1 transcripts accumulate predominantly in leaf and fruit flavedo which contain high proportions of chloroplasts (Alquézar et al., 2009; Mendes et al., 2011; Zhang et al., 2012b). This study investigated the expression patterns of CsLCYb1 promoter by stable genetic transformation in Arabidopsis. The results showed that promoter activity was highly correlated with seedling development and that GUS staining was observed clearly in leaf tissues, while little or no GUS staining was observed in other tissues (fruits, flowers, stems, and roots; Figure 4). A similar result was observed in transgenic Arabidopsis expressing the citrus PSY promoter-GUS construct (Zeng et al., 2013). These findings indicate that the CsLCYb1 promoter is developmentally and tissue-specifically regulated. The expression patterns of CsLCYb1 promoter determined by GUS expression were similar to the endogenous gene expression profiles in citrus, confirming that the CsLCYb1 promoter is strictly regulated.
As secondary metabolites, carotenoids play vital roles in plant stress resistance. Previous studies have revealed that when plants suffer from environmental stresses, the expression of the genes for carotenoid biosynthesis will increase to produce more antioxidative components for enhancing plant resistance (Giuliano et al., 1993; Fanciullino et al., 2014). In addition to environmental cues, the elegant modulation of carotenoid metabolism is also tightly coordinated by endogenous signals, such as hormone levels. The hormonal regulation network of carotenoid metabolism has been reviewed previously (Osorio et al., 2013; Liu et al., 2015). This study investigated the responses of different CsLCYb1 promoter fragments to various stimuli in transgenic citrus callus. The results showed that the promoter activity of CsLCYb1 was induced by most stimuli, such as GA, IAA, and Glu (Figure 5). Some results are consistent with the change of CsLCYb1 gene expression under the same treatments (Zhang et al., 2012a; Su et al., 2015). Zeng et al. (2013) also reported that the promoter activity of citrus PSY (the key rate-limiting enzyme in the carotenoid biosynthetic pathway) was affected by various stimuli. Additionally, we found that the GUS expression levels driven by different CsLCYb1 promoter deletions were influenced by NaCl treatment (Figure 5), suggesting that the promoter activity of CsLCYb1 could respond to salt stress. Very weak GUS staining was observed at the mechanical cut ends of some transgenic Arabidopsis tissues (Figure 4), indicating that the promoter activity of CsLCYb1 may be induced by oxidative stress resulting from mechanical wound. Similar results were observed by Bouvier et al. (1998), who reported that the promoter activity of the CCS (which also has the function of lycopene β-cyclase) gene was dramatically activated by various ROS progenitors under different oxidative stress conditions. The stress-related and hormone-responsive cis-elements predicted in the promoter sequences (Supplementary Table S2) may partially explain the response of CsLCYb1 promoter to various stimuli. However, this explanation remains to be further verified. LCYb1 is highly expressed in green tissues, and is involved in plant photosynthesis and photoprotection. Previous studies reported that the mRNA transcripts of LCYb1 in citrus are largely enhanced by light (Gao et al., 2011; Ma et al., 2012, 2015). This study predicted many light-responsive elements in the promoter of CsLCYb1 (Supplementary Table S2), suggesting that the promoter activity of CsLCYb1 is likely affected by light. Overall, the above analyses illustrate that CsLCYb1 promoter responds to various exogenous and endogenous factors, and that the regulation of this promoter is a complex process.
Identification of a Novel Enhancer Element Conferring Strong Promoter Activity
Promoter deletion analyses performed in three types of transgenic species all demonstrated that a deletion from LP3 to LP4 resulted in a significant reduction of promoter activity. Finer deletion analysis revealed that a 20 bp fragment existing as a tandem repeat in the region between LP3 and LP4 is an enhancer element conferring strong promoter activity to the minimal promoter, since the reduced copy number of the 20 bp fragment in the full-length promoter resulted in considerable decrease of GUS expression (Figure 6). A similar finding was previously reported, which suggested that four tandem repeats of a 20 bp sequence in the promoter of the melon cucumisin gene are sufficient to confer fruit-specific gene expression pattern to the minimal promoter, and that the 20 bp sequence contains a regulatory enhancer (Yamagata et al., 2002). Bustos et al. (2010) reported that the fusion of four tandem copies of a P1BS element (PHOSPHATE STARVATION RESPONSE REGULATOR 1, PHR1 binding sequences) to a 35S minimal promoter is sufficient to confer Pi inducibility to the reporter gene. In the future work, we will fuse the enhancer element to the upstream of a 35S minimum promoter to observe whether the enhancer element activates the 35S minimum promoter activity.
In silico analysis of the 20 bp sequence identified several interesting cis-elements (Inr-element, GT-element, GT-1 motif, and GA-motif, etc.). Previous studies have reported that Inr-elements and GT-elements are present in the promoter of many light-regulated genes, and the GT-1 motifs are present in the promoter of stress-induced genes (Zhou, 1999; Nakamura et al., 2002; Park et al., 2004). These results further indicate that the novel enhancer element may respond to light and stresses. The GA-motif was also found in the promoter of G. lutea lycopene β-cyclase gene (JQ417648), suggesting a common regulatory mechanism. Additionally, the deletion of the 20 bp fragment may disrupt adjacent cis-elements, such as the ARR (Arabidopsis response regulator) transcription factor binding site (NGATT) existing in the enhancer region as four copies. The ARR proteins belong to the GARP superfamily, two members of which have recently been reported to be related to carotenogenesis. One member is the GOLDEN2-LIKE (GLK) gene, which controls the dominant Uniform ripening (U) locus of tomato fruit. Tomato carrying the u mutation produced fruit with defective chloroplasts and low levels of sugar and lycopene (Powell et al., 2012). The other member is the ARABIDOPSIS PSEUDO RESPONSR REGULATOR2-like (APRR2-like) gene, which affects plastid number and size in tomato fruit, and enhances the levels of chlorophyll in immature fruit and carotenoids in red ripe fruit (Pan et al., 2013). Welsch et al. (2003, 2007) identified an enhancer element ATCTA in the phytoene synthase promoter from Arabidopsis and further discovered that the transcription factor RAP2.2 (a member of the APETALA2 (AP2)/ethylene-responsive element-binding protein) interacting with the SINAT2 (SEVEN IN ABSENTIA OF ARABIDOPSIS2, a RING finger protein) bound to the ATCTA element to coordinately regulate AtPSY expression. These analyses indicate that the enhancer element identified in this study could be used as a good candidate to seek upstream trans-acting factors.
Differences in Copy Number of Enhancer Element in LCYb1 Promoters from Different Citrus Species
Previous studies of the ODORANT1 (ODO1) gene, a key regulator in the volatile benzenoid pathway in petals, identified an enhancer region which distinguished a fragrant from a non-fragrant petunias cultivar (Van Moerkercke et al., 2011). This study also investigated the sequence characteristics of LCYb1 promoter from different citrus species, which have different carotenoid contents and compositions. The results showed that the copy numbers of the 20 bp enhancer element were different among these species (Figure 7A). These differences may affect the expression level of LCYb1 gene, thus resulting in carotenoid diversity among different citrus species. However, this speculation needs to be confirmed by more experiments. Sweet orange is a natural hybrid of pummelo and mandarin (Xu et al., 2013). In sweet orange, we only successfully isolated one LCYb1 promoter sequence, which is relatively more similar with the promoter from pummelo. The failure to clone the promoter of the other allele originating from mandarin may be due to the insertion of a large fragment of a CTV resistance gene. CTV is one of the most severe diseases of citrus in the world (Bar-Joseph et al., 1989). Previous studies have reported that most of mandarin species are relatively more resistant to certain CTV strains compared with pummelo and grapefruit (Garnsey et al., 1987; Moreno et al., 2008). This study discovered the insertion of a CTV resistance gene fragment only in mandarin, while not in pummelo and grapefruit. These findings suggest that the fragment insertion may be associated with the differences in CTV resistance among citrus species, which deserves further exploration. Due to the sexual compatibility between species, high frequency of bud mutations, long history of cultivation and wide dispersion, the taxonomy and phylogeny of citrus are still complicated, controversial and confusing (Nicolosi et al., 2000; Garcia-Lor et al., 2013). The sequence polymorphisms identified in the LCYb1 promoters may provide potential molecular markers to investigate the diversity and relationship among citrus species. Indeed, a derived SSR marker based on the copy numbers of the 20 bp enhancer element in LCYb1 promoters could distinguish mandarin, pummelo, grapefruit and orange species as shown by the study of 14 citrus species (Figure 7B), which provides an ideal molecular marker to study the genetic relationship between different species.
Conclusion
This study elucidates the expression characteristics of the LCYb1 promoter from citrus, thus facilitating the understanding of the complex regulatory mechanisms of LCYb1 expression in higher plants. The identified novel enhancer element is required for promoter activity; besides, it also can be used as a marker to distinguish different citrus species. These data give a clue to further study of the differences in gene expression among species. The promoter cloned and functionally validated in this study would be an ideal candidate for genetic engineering and seeking of upstream trans-acting elements.
Author Contributions
SL performed the major experiments and wrote the manuscript. YZ and KZ, assisted in the experiments. XZ give suggestions for writing improvement. QX designed the experiment. XD proposed and supervised the research, and provided valuable comments on the manuscript. All authors read and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research was financially supported by National Natural Science Foundation of China (31330066 and 31521092) and China Agriculture Research System (CARS-27).
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01367
References
- Ahrazem O., Trapero A., Gomez M. D., Rubio-Moraga A., Gomez-Gomez L. (2010). Genomic analysis and gene structure of the plant carotenoid dioxygenase 4 family: a deeper study in Crocus sativus and its allies. Genomics 96 239–250. 10.1016/j.ygeno.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Alder A., Jamil M., Marzorati M., Bruno M., Vermathen M., Bigler P., et al. (2012). The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335 1348–1351. 10.1126/science.1218094 [DOI] [PubMed] [Google Scholar]
- Alquezar B., Rodrigo M. J., Lado J., Zacarías L. (2013). A comparative physiological and transcriptional study of carotenoid biosynthesis in white and red grapefruit (Citrus paradisi Macf.). Tree Genet. Genomes 9 1257–1269. 10.1007/s11295-013-0635-7 [DOI] [Google Scholar]
- Alquézar B., Zacarías L., Rodrigo M. J. (2009). Molecular and functional characterization of a novel chromoplast-specific lycopene β-cyclase from Citrus and its relation to lycopene accumulation. J. Exp. Bot. 60 1783–1797. 10.1093/jxb/erp048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee J., Sahoo D. K., Raha S., Sarkar S., Dey N., Maiti I. B. (2015). A region Containing an as-1 element of Dahlia Mosaic Virus (DaMV) subgenomic transcript promoter plays a key role in green tissue-and root-specific expression in plants. Plant Mol. Biol. Rep. 33 532–556. 10.1007/s11105-014-0766-5 [DOI] [Google Scholar]
- Bang H., Yi G., Kim S., Leskovar D., Patil B. S. (2014). Watermelon lycopene β-cyclase: promoter characterization leads to the development of a PCR marker for allelic selection. Euphytica 200 363–378. 10.1007/s10681-014-1158-5 [DOI] [Google Scholar]
- Bar-Joseph M., Marcus R., Lee R. F. (1989). The continuous challenge of Citrus tristeza virus control. Annu. Rev. Phytopathol. 27 291–316. 10.1146/annurev.py.27.090189.001451 [DOI] [Google Scholar]
- Bartley G. E., Scolnik P. A. (1995). Plant carotenoids: pigments for photoprotection, visual attraction, and human health. Plant Cell 7 1027–1038. 10.2307/3870055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella-Pavía P., Rodríguez-Concepción M. (2006). Carotenoid biotechnology in plants for nutritionally improved foods. Plant Physiol. 126 369–381. 10.1105/tpc.110.073866 [DOI] [Google Scholar]
- Bouvier F., Backhaus R. A., Camara B. (1998). Induction and control of chromoplast-specific carotenoid genes by oxidative stress. J. Biol. Chem. 273 30651–30659. 10.1074/jbc.273.46.30651 [DOI] [PubMed] [Google Scholar]
- Burke T. W., Kadonaga J. T. (1996). Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 10 711–724. 10.1101/gad.10.6.711 [DOI] [PubMed] [Google Scholar]
- Bustos R., Castrillo G., Linhares F., Puga M. I., Rubio V., Perez-Perez J., et al. (2010). A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 6:e1001102 10.1371/journal.pgen.1001102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Zhang J., Xu J., Ye J., Yun Z., Xu Q., et al. (2012). Comprehending crystalline β-carotene accumulation by comparing engineered cell models and the natural carotenoid-rich system of citrus. J. Exp. Bot. 63 4403–4417. 10.1093/jxb/ers115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai L., Biswas M. K., Yi H., Guo W., Deng X. (2013). Transferability, polymorphism and effectiveness for genetic mapping of the Pummelo (Citrus grandis Osbeck) EST-SSR markers. Sci. Hortic. 155 85–91. 10.1186/1471-2164-9-287 [DOI] [Google Scholar]
- Cheng Y.-J., Guo W.-W., Yi H.-L., Pang X.-M., Deng X. (2003). An efficient protocol for genomic DNA extraction fromCitrus species. Plant Mol. Biol. Rep. 21 177–178. 10.1007/BF02774246 [DOI] [Google Scholar]
- Clough S. J., Bent A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Corona V., Aracri B., Kosturkova G., Bartley G. E., Pitto L., Giorgetti L., et al. (1996). Regulation of a carotenoid biosynthesis gene promoter during plant development. Plant J. 9 505–512. 10.1046/j.1365-313X.1996.09040505.x [DOI] [PubMed] [Google Scholar]
- Creelman R. A., Gage D. A., Stults J. T., Zeevaart J. A. (1987). Abscisic acid biosynthesis in leaves and roots of Xanthium strumarium. Plant Physiol. 85 726–732. 10.1104/pp.85.3.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F. X., Pogson B., Sun Z., McDonald K. A., DellaPenna D., Gantt E. (1996). Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 8 1613–1626. 10.1105/tpc.8.9.1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal M., Chinnusamy V., Bansal K. C. (2010). Isolation and functional characterization of lycopene β-cyclase (CYC-B) promoter from Solanum habrochaites. BMC Plant Biol. 10:61 10.1186/1471-2229-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaPenna D., Pogson B. J. (2006). Vitamin synthesis in plants: tocopherols and carotenoids. Annu. Rev. Plant Biol. 57 711–738. 10.1146/annurev.arplant.56.032604.144301 [DOI] [PubMed] [Google Scholar]
- Eun C.-H., Kim S.-U., Kim I.-J. (2015). The promoter from the Citrus unshiu carotenoid isomerase gene directs differential GUS expression in transgenic Arabidopsis. Mol. Breed. 35 1–12. 10.1007/s11032-015-0310-9 [DOI] [Google Scholar]
- Fanciullino A., Bidel L., Urban L. (2014). Carotenoid responses to environmental stimuli: integrating redox and carbon controls into a fruit model. Plant Cell Environ. 37 273–289. 10.1111/pce.12153 [DOI] [PubMed] [Google Scholar]
- Fanciullino A.-L., Dhuique-Mayer C., Luro F., Casanova J., Morillon R., Ollitrault P. (2006). Carotenoid diversity in cultivated citrus is highly influenced by genetic factors. J. Agric. Food Chem. 54 4397–4406. 10.1021/jf0526644 [DOI] [PubMed] [Google Scholar]
- Frank H. A., Cogdell R. J. (1996). Carotenoids in photosynthesis. Photochem. Photobiol. 63 257–264. 10.1111/j.1751-1097.1996.tb03022.x [DOI] [PubMed] [Google Scholar]
- Fraser P. D., Bramley P. M. (2004). The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 43 228–265. 10.1016/j.plipres.2003.10.002 [DOI] [PubMed] [Google Scholar]
- Fujisawa M., Nakano T., Shima Y., Ito Y. (2013). A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 25 371–386. 10.1105/tpc.112.108118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Xu J., Liu X., Liu B., Deng X. (2011). Light effect on carotenoids production and expression of carotenogenesis genes in citrus callus of four genotypes. Acta Physiol. Plant. 33 2485–2492. 10.1007/s11738-011-0793-x [DOI] [Google Scholar]
- Garcia-Lor A., Curk F., Snoussi-Trifa H., Morillon R., Ancillo G., Luro F., et al. (2013). A nuclear phylogenetic analysis: SNPs, indels and SSRs deliver new insights into the relationships in the ’true citrus fruit trees’ group (Citrinae, Rutaceae) and the origin of cultivated species. Ann. Bot. 111 1–19. 10.1093/aob/mcs227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnsey S. M., Barrett H. C., Hutchison D. J. (1987). Identification of citrus tristeza virus resistance in citrus relatives and its potential applications. Phytophylactica 19 187–191. [Google Scholar]
- Giuliano G., Bartley G. E., Scolnik P. A. (1993). Regulation of carotenoid biosynthesis during tomato development. Plant Cell 5 379–387. 10.2307/3869719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K., Ugawa Y., Iwamoto M., Korenaga T. (1999). Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 27 297–300. 10.1093/nar/27.1.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai A., Takahashi S., Nakayama K., Satoh H. (2013). The promoter of the carotenoid cleavage dioxygenase 4a-5 gene of Chrysanthemum morifolium (CmCCD4a-5) drives petal-specific transcription of a conjugated gene in the developing flower. J. Plant Physiol. 170 1295–1299. 10.1016/j.jplph.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. (1987). GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Ikoma Y., Matsumoto H., Sugiura M., Hyodo H., Yano M. (2004). Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit. Plant Physiol. 134 824–837. 10.1104/pp.103.031104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky N. I., Johnson E. J. (2005). Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 26 459–516. 10.1016/j.mam.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Lakshman M., Okoh C. (1993). [23] Enzymatic conversion of all-trans-β-carotene to retinal. Methods Enzymol. 214 256–269. 10.1016/0076-6879(93)14070-Y [DOI] [PubMed] [Google Scholar]
- Lescot M., Déhais P., Thijs G., Marchal K., Moreau Y., Van de Peer Y., et al. (2002). PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30 325–327. 10.1093/nar/30.1.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Shi W., Deng X. (2002). Agrobacterium -mediated transformation of embryogenic calluses of Ponkan mandarin and the regeneration of plants containing the chimeric ribonuclease gene. Plant Cell Rep. 21 153–156. 10.1007/s00299-002-0492-6 [DOI] [Google Scholar]
- Liang Y. S., Bae H.-J., Kang S.-H., Lee T., Kim M. G., Kim Y.-M., et al. (2009). The Arabidopsis beta-carotene hydroxylase gene promoter for a strong constitutive expression of transgene. Plant Biotechnol. Rep. 3 325–331. 10.1007/s11816-009-0106-7 [DOI] [Google Scholar]
- Liang Y. S., Jeon Y. A., Lim S. H., Kim J. K., Lee J. Y., Kim Y. M., et al. (2011). Vascular-specific activity of the Arabidopsis carotenoid cleavage dioxygenase 7 gene promoter. Plant Cell Rep. 30 973–980. 10.1007/s00299-010-0999-1 [DOI] [PubMed] [Google Scholar]
- Liu L., Shao Z., Zhang M., Wang Q. (2015). Regulation of carotenoid metabolism in tomato. Mol. Plant 8 28–39. 10.1016/j.molp.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Ma G., Zhang L., Kato M., Yamawaki K., Kiriiwa Y., Yahata M., et al. (2012). Effect of blue and red LED light irradiation on beta-cryptoxanthin accumulation in the flavedo of citrus fruits. J. Agric. Food Chem. 60 197–201. 10.1021/jf203364m [DOI] [PubMed] [Google Scholar]
- Ma G., Zhang L., Kato M., Yamawaki K., Kiriiwa Y., Yahata M., et al. (2015). Effect of the combination of ethylene and red LED light irradiation on carotenoid accumulation and carotenogenic gene expression in the flavedo of citrus fruit. Postharvest Biol. Technol. 99 99–104. 10.1016/j.postharvbio.2014.08.002 [DOI] [Google Scholar]
- Mendes A. F., Chen C., Gmitter F. G., Moore G. A., Costa M. G. (2011). Expression and phylogenetic analysis of two new lycopene β-cyclases from Citrus paradisi. Physiol. Plant 141 1–10. 10.1111/j.1399-3054.2010.01415.x [DOI] [PubMed] [Google Scholar]
- Moreno P., Ambros S., Albiach-Marti M. R., Guerri J., Pena L. (2008). Citrus tristeza virus: a pathogen that changed the course of the citrus industry. Mol Plant Pathol. 9 251–268. 10.1111/j.1364-3703.2007.00455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P., Li X.-P., Niyogi K. K. (2001). Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125 1558–1566. 10.1104/pp.125.4.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Tsunoda T., Obokata J. (2002). Photosynthesis nuclear genes generally lack TATA-boxes: a tobacco photosystem I gene responds to light through an initiator. Plant J. 29 1–10. 10.1046/j.0960-7412.2001.01188.x [DOI] [PubMed] [Google Scholar]
- Nicolosi E., Deng Z. N., Gentile A., Malfa S. L., Continella G., Tribulato E. (2000). Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor. Appl. Genet. 100 1155–1166. 10.1007/s001220051419 [DOI] [Google Scholar]
- Nisar N., Li L., Lu S., Khin N. C., Pogson B. J. (2015). Carotenoid metabolism in plants. Mol. Plant 8 68–82. 10.1016/j.molp.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Orzaez D., Mirabel S., Wieland W. H., Granell A. (2006). Agroinjection of tomato fruits. A tool for rapid functional analysis of transgenes directly in fruit. Plant Physiol. 140 3–11. 10.1104/pp.105.068221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S., Scossa F., Fernie A. R. (2013). Molecular regulation of fruit ripening. Front. Plant Sci. 4:198 10.3389/fpls.2013.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Bradley G., Pyke K., Ball G., Lu C., Fray R., et al. (2013). Network inference analysis identifies an APRR2-like gene linked to pigment accumulation in tomato and pepper fruits. Plant Physiol. 161 1476–1485. 10.1104/pp.112.212654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. C., Kim M. L., Kang Y. H., Jeon J. M., Yoo J. H., Kim M. C., et al. (2004). Pathogen-and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol. 135 2150–2161. 10.1104/pp.104.041442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell A. L., Nguyen C. V., Hill T., Cheng K. L., Figueroa-Balderas R., Aktas H., et al. (2012). Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 336 1711–1715. 10.1126/science.1222218 [DOI] [PubMed] [Google Scholar]
- Ramel F., Birtic S., Ginies C., Soubigou-Taconnat L., Triantaphylidès C., Havaux M. (2012). Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. U.S.A. 109 5535–5540. 10.1073/pnas.1115982109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Horton P., Pascal A. A., Ruban A. V. (2004). Insights into the molecular dynamics of plant light-harvesting proteins in vivo. Trends Plant Sci. 9 385–390. 10.1016/j.tplants.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Ruiz C., Breto M. P., Asins M. (2000). A quick methodology to identify sexual seedlings in citrus breeding programs using SSR markers. Euphytica 112 89–94. 10.1023/A:1003992719598 [DOI] [Google Scholar]
- Su L., Diretto G., Purgatto E., Danoun S., Zouine M., Li Z., et al. (2015). Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol. 15:114 10.1186/s12870-015-0495-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Ortiz G., Huq E., Rodriguez-Concepcion M. (2010). Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. U.S.A. 107 11626–11631. 10.1073/pnas.0914428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Moerkercke A., Haring M. A., Schuurink R. C. (2011). The transcription factor EMISSION OF BENZENOIDS II activates the MYB ODORANT1 promoter at a MYB binding site specific for fragrant petunias. Plant J. 67 917–928. 10.1111/j.1365-313X.2011.04644.x [DOI] [PubMed] [Google Scholar]
- Vrebalov J., Ruezinsky D., Padmanabhan V., White R., Medrano D., Drake R., et al. (2002). A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296 343–346. 10.1126/science.1068181 [DOI] [PubMed] [Google Scholar]
- Welsch R., Maass D., Voegel T., DellaPenna D., Beyer P. (2007). Transcription factor RAP2.2 and its interacting partner SINAT2: stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiol. 145 1073–1085. 10.1104/pp.107.104828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch R., Medina J., Giuliano G., Beyer P., von Lintig J. (2003). Structural and functional characterization of the phytoene synthase promoter from Arabidopsis thaliana. Planta 216 523–534. 10.1007/s00425-002-0885-3 [DOI] [PubMed] [Google Scholar]
- Weng H. B., Pan A., Yang L., Zhang C., Liu Z., Zhang D. (2004). Estimating number of transgene copies in transgenic rapeseed by real-time PCR assay with HMG I/Y as an endogenous reference gene. Plant Mol. Biol. Rep. 22 289–300. 10.1007/BF02773139 [DOI] [Google Scholar]
- Xu C.-J., Fraser P. D., Wang W.-J., Bramley P. M. (2006). Differences in the carotenoid content of ordinary citrus and lycopene-accumulating mutants. J. Agric. Food Chem. 54 5474–5481. 10.1021/jf060702t [DOI] [PubMed] [Google Scholar]
- Xu J., Tao N., Liu Q., Deng X. (2006). Presence of diverse ratios of lycopene/β-carotene in five pink or red-fleshed citrus cultivars. Sci. Hortic. 108 181–184. 10.4238/2015.December.23.32 [DOI] [Google Scholar]
- Xu Q., Chen L.-L., Ruan X., Chen D., Zhu A., Chen C., et al. (2013). The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 45 59–66. 10.1038/ng.2472 [DOI] [PubMed] [Google Scholar]
- Xu Q., Yu K., Zhu A., Ye J., Liu Q., Zhang J., et al. (2009). Comparative transcripts profiling reveals new insight into molecular processes regulating lycopene accumulation in a sweet orange (Citrus sinensis) red-flesh mutant. BMC Genomics 10:540 10.1186/1471-2164-10-540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Yonesu K., Hirata A., Aizono Y. (2002). TGTCACA motif is a novel cis-regulatory enhancer element involved in fruit-specific expression of thecucumisin gene. J. Biol. Chem. 277 11582–11590. 10.1074/jbc.M109946200 [DOI] [PubMed] [Google Scholar]
- Yang Q., Yuan D., Shi L., Capell T., Bai C., Wen N., et al. (2012). Functional characterization of the Gentiana lutea zeaxanthin epoxidase (GlZEP) promoter in transgenic tomato plants. Transgenic Res. 21 1043–1056. 10.1007/s11248-012-9591-5 [DOI] [PubMed] [Google Scholar]
- Yu K., Xu Q., Da X., Guo F., Ding Y., Deng X. (2012). Transcriptome changes during fruit development and ripening of sweet orange (Citrus sinensis). BMC Genomics 13:10 10.1186/1471-2164-13-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W. F., Huang M., Wang X. P., Ampomah-Dwamena C., Xu Q., Deng X. X. (2013). Identification and functional characterization of the promoter of a phytoene synthase from sweet orange (Citrus sinensis Osbeck). Plant Mol. Biol. Rep. 31 64–74. 10.1007/s11105-012-0477-8 [DOI] [Google Scholar]
- Zhang J. C., Zhou W. J., Xu Q., Tao N. G., Ye J. L., Guo F., et al. (2013). Two Lycopene beta-Cyclases genes from sweet orange (Citrus sinensis L. Osbeck) encode enzymes with different functional efficiency during the conversion of Lycopene-to-provitamin A. J. Integr. Agric. 12 1731–1747. 10.1016/S2095-3119(13)60366-4 [DOI] [Google Scholar]
- Zhang L., Ma G., Kato M., Yamawaki K., Takagi T., Kiriiwa Y., et al. (2012a). Regulation of carotenoid accumulation and the expression of carotenoid metabolic genes in citrus juice sacs in vitro. J. Exp. Bot. 63 871–886. 10.1093/jxb/err318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ma G., Shirai Y., Kato M., Yamawaki K., Ikoma Y., et al. (2012b). Expression and functional analysis of two lycopene β-cyclases from citrus fruits. Planta 236 1315–1325. 10.1007/s00425-012-1690-2 [DOI] [PubMed] [Google Scholar]
- Zhou D.-X. (1999). Regulatory mechanism of plant gene transcription by GT-elements and GT-factors. Trends Plant Sci. 4 210–214. 10.1016/S1360-1385(99)01418-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


