Abstract
AIMS--To study the expression and importance (if any) of p53 protein in gall bladder carcinoma and its precursor lesions. METHODS--Immunohistochemical staining was performed on formalin fixed, paraffin wax embedded histological sections with an anti-human p53 monoclonal antibody (DO-7; Dako Corporation M7001) (24 carcinomas, one adenocarcinoma in situ, six dysplasias, three adenomas and four cases of chronic cholecystitis). Invasive, in situ, and dysplastic areas as well as normal-looking epithelium were sought. Nuclear staining was assessed according to intensity and extent of positive cells. Both variables were graded on a scale of 1-3 and aggregate p53 scores were obtained (range: 0, 2-6). Only p53 scores of > or = 3 were regarded as significant. RESULTS--Clinically important amounts of p53 were expressed in 92% of invasive carcinomas, 86% of carcinoma in situ, and 28% of dysplastic areas. None of the adenomas contained clinically important amounts of p53. Normal epithelium, present in all the cases, did not express p53 except in one case of moderately differentiated adenocarcinoma (p53 score 3). In general, there was no difference in the prevalence of p53 protein expression between dysplasias associated with, and those unassociated with invasive disease. There was a tendency for higher grade carcinomas to express more p53 protein. CONCLUSIONS--The distribution of p53 protein in invasive carcinomas and the adjacent dysplastic and preinvasive lesions suggests that it is more commonly expressed than previously thought. The fact that p53 protein is also expressed in cases of dysplasia and carcinoma in situ unassociated with invasive malignancy lends further support to the contention that p53 gene mutations may have a role in the pathogenesis of gall bladder cancer. Expression of p53 protein may possibly be an indication of likely disease progression from dysplasia, to carcinoma in situ, to invasive disease.
Full text
PDF

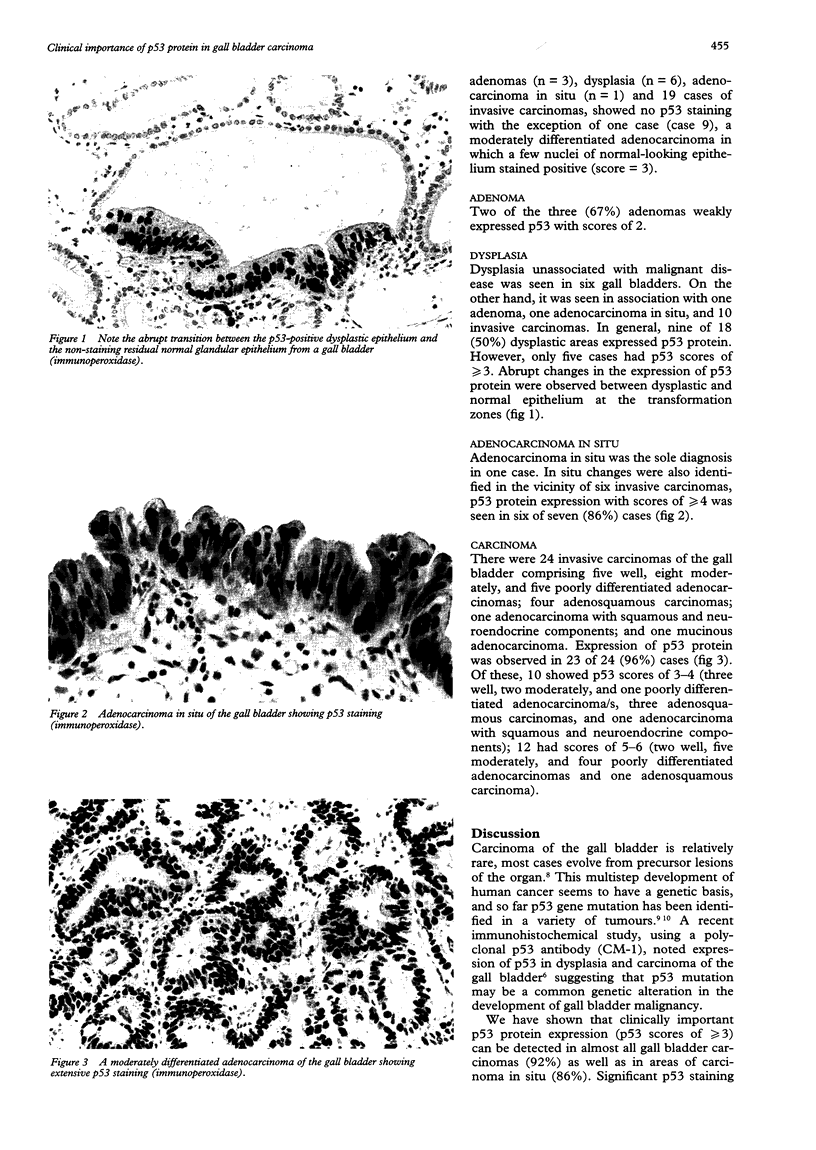

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albores-Saavedra J., Alcántra-Vazquez A., Cruz-Ortiz H., Herrera-Goepfert R. The precursor lesions of invasive gallbladder carcinoma. Hyperplasia, atypical hyperplasia and carcinoma in situ. Cancer. 1980 Mar 1;45(5):919–927. doi: 10.1002/1097-0142(19800301)45:5<919::aid-cncr2820450514>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Bártek J., Bártková J., Lukás J., Stasková Z., Vojtesek B., Lane D. P. Immunohistochemical analysis of the p53 oncoprotein on paraffin sections using a series of novel monoclonal antibodies. J Pathol. 1993 Jan;169(1):27–34. doi: 10.1002/path.1711690106. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Oren M. Overproduction of p53 antigen makes established cells highly tumorigenic. Nature. 1985 Jul 11;316(6024):158–160. doi: 10.1038/316158a0. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Han K. A., Kulesz-Martin M. F. Altered expression of wild-type p53 tumor suppressor gene during murine epithelial cell transformation. Cancer Res. 1992 Feb 1;52(3):749–753. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Kamel D., Päkkö P., Nuorva K., Vähäkangas K., Soini Y. p53 and c-erbB-2 protein expression in adenocarcinomas and epithelial dysplasias of the gall bladder. J Pathol. 1993 May;170(1):67–72. doi: 10.1002/path.1711700111. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Porter P. L., Gown A. M., Kramp S. G., Coltrera M. D. Widespread p53 overexpression in human malignant tumors. An immunohistochemical study using methacarn-fixed, embedded tissue. Am J Pathol. 1992 Jan;140(1):145–153. [PMC free article] [PubMed] [Google Scholar]
- Scott N., Sagar P., Stewart J., Blair G. E., Dixon M. F., Quirke P. p53 in colorectal cancer: clinicopathological correlation and prognostic significance. Br J Cancer. 1991 Feb;63(2):317–319. doi: 10.1038/bjc.1991.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soini Y., Päkkö P., Nuorva K., Kamel D., Lane D. P., Vähäkangas K. Comparative analysis of p53 protein immunoreactivity in prostatic, lung and breast carcinomas. Virchows Arch A Pathol Anat Histopathol. 1992;421(3):223–228. doi: 10.1007/BF01611179. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D. P53 in tumour pathology: can we trust immunocytochemistry? J Pathol. 1992 Apr;166(4):329–330. doi: 10.1002/path.1711660402. [DOI] [PubMed] [Google Scholar]





