Abstract
The onion maggot, Delia antiqua, is a devastating pest of liliaceous crops and current control measures fail to avert pesticide residues, threats to agroecosystem, and costly expenditures. Insect growth regulators (IGRs) are used as trypetid pest chemosterilants for their suppression on adult fertility and fecundity, but their effects on onion flies are unknown. Here, three IGRs (lufenuron, cyromazine, pyriproxyfen) were incorporated into baits to evaluate their effects on onion fly survival, fecundity, fertility, susceptibility of adults in different ages and offspring development. Lufenuron and cyromazine did not affect survival of new-emerged adults, but lufenuron inhibited adult fertility without affecting fecundity, and cyromazine reduced fertility and fecundity. Differently, pyriproxyfen enhanced fecundity within 10 days after treatment, while it reduced adult survival without affecting fertility. The fertility of younger adults was affected by lufenuron and cyromazine whereas the fecundity was affected with cyromazine and pyriproxyfen. For offspring of onion flies treated with lufenuron or cyromazine, most of larvae died within 5 days after hatch, but surviving larvae pupated and emerged normally. Pyriproxyfen did not affect offspring larval survival or pupation but affected pupal emergence. Thus, lufenuron and cyromazine could be potential chemosterilants for onion flies.
Delia antiqua (Meigen) (Diptera: Anthomyiidae) is a devastating crop pest distributed widely in many temperate countries such as Canada, Mexico, United States, China and Japan1. Its larva, onion maggot, feeds on bulb onions, garlics as well as other liliaceous crops, which leads to rot of the damaged parts2. If not controlled, it can reduce onion yield by as much as 80%3,4 and lead to garlic economic loss up to 50–70%5.
As the crop damage is directly caused by onion maggot feeding, present control methods are mainly targeted at the larva. Common methods for larval control are insecticides application in the furrow at planting6, seed coating treatments for onions and application of pesticides into irrigation water in garlic and onion fields4,6,7,8. The most widely used insecticides are organophosphates, carbamates and neonicotinoids6,9,10. Other methods targeting onion flies (adults) include spraying pesticides on crop leaves11, bait trapping stations12,13,14,15, repelling female oviposition with plastic strips16 and the male sterile technique17. However, application of chemicals to the crop results in pesticide contamination, which may cause food poisoning. Besides, large scale applications of chemical pesticides in the field could pose a great threat to agroecological environments18. In addition, repelling female oviposition with plastic strips and the male sterile technique cost too much. Thus, new management methods have to be developed as an alternative to these traditional ones for onion maggot control.
Recently, a group of chemicals called insect growth regulators (IGRs) have been used in baits to control trypetid and some dipteran pests as these chemicals inhibit adult reproduction19,20,21,22. Those chemicals have been incorporated into baits, fed to dipteran pests and shown excellent sterilizing effects on dipteran adults. For example, lufenuron reduces fertility of the Mexican fruit fly (Anastrepha ludens), the West Indian fruit fly (A. oblique), the sapote fruit fly (A. serpentine), the guava fruit fly (A. striata), the Mediterranean fruit fly (Ceratitis capitata), Bactrocera dorsalis, the melon fly (B. cucurbitae), the olive fruit fly (B. oleae) and the solanum fruit fly (B. latifrons) when ingested by adults19,20,23. Cyromazine also leads to sterility when ingested by adults of C. capitata24,25, Musca domestica22,26, and Lucilia cuprina27. In addition, lufenuron suppressed C. capitata population in field experiments28,29,30,31,32, and pyriproxyfen has been used to manage tsetse fly33,34,35 as well as Haematobia irritans36 in the field too. However, those studies mainly focus on the effects of IGRs on fertility and fecundity, while other parameters such as adult survival and offspring development are scarcely studied.
The onion fly adult needs to feed after emergence to meet the requirement of reproductive system development37, which suggests that baits containing pesticides could be used in onion maggot management. Given that IGRs showed excellent sterilization effects on dipteran pests, these chemicals might also be active against onion flies. The aim of this work is to investigate the effect of several selected IGRs used in baits on onion fly adult survival, fecundity, fertility, susceptibility of adults in different ages and offspring development, which would provide support information for them being used as onion fly chemosterilants. With lufenuron, pyriproxyfen and cyromazine selected as representative IGRs, this study also tried to compare the effect of IGRs with different modes of action on onion fly reproduction. Lufenuron is a benzoylurea chitin-synthesis inhibitor which interferes with the deposition of new cuticle during moulting38. Pyriproxyfen is a juvenile hormone mimic which prevents maturation of larvae to adults39. Cyromazine is a chitin-synthesis inhibitor with supposed ecdysone activity40,41.
Results
Survival, fecundity and fertility when 1-day old onion flies were fed with different doses of lufenuron, pyriproxyfen and cyromazine
Survival of the onion fly was not affected by the 72 h treatment of lufenuron at doses of 100, 500 or 1000 mg kg−1 during the 30-day period (Table 1). Differently, survival of onion flies treated by pyriproxyfen at doses of 100, 500 or 1000 mg kg−1 showed no significant difference compared to control on day 5 and 10 after emergence. The survival decreased significantly and was directly related to pyriproxyfen dose compared to the control on day 15, 20, 25 and 30 after emergence (Table 1). For cyromazine, the 72 h treatment at doses of 100, 500 and 1000 mg kg−1 did not affect survival compared to the control at each time point during the experimental period (Table 1).
Table 1. Survival of onion flies treated by selected IGRs.
| IGRs | Timea | Dose (mg kg−1) |
F | df | P | |||
|---|---|---|---|---|---|---|---|---|
| Control | 100 | 500 | 1000 | |||||
| Lufenuron | 5 | 98.57 ± 3.78a | 98.57 ± 3.78a | 95.71 ± 7.87a | 98.57 ± 3.78a | 0.545 | (3, 24) | 0.656 |
| 10 | 98.57 ± 3.78a | 97.14 ± 4.88a | 95.71 ± 7.87a | 97.14 ± 4.88a | 0.308 | (3, 24) | 0.820 | |
| 15 | 97.14 ± 4.88a | 97.14 ± 4.88a | 95.71 ± 7.87a | 95.71 ± 5.35a | 0.138 | (3, 24) | 0.936 | |
| 20 | 95.71 ± 5.35a | 94.28 ± 7.87a | 94.28 ± 7.87a | 92.85 ± 7.56a | 0.182 | (3, 24) | 0.908 | |
| 25 | 90.00 ± 8.16a | 91.42 ± 6.90a | 88.57 ± 6.90a | 84.28 ± 12.72a | 0.824 | (3, 24) | 0.494 | |
| 30 | 84.28 ± 5.35a | 85.71 ± 12.72a | 77.14 ± 12.53a | 75.71 ± 12.72a | 1.383 | (3, 24) | 0.272 | |
| Pyriproxyfen | 5 | 98.57 ± 3.78a | 98.57 ± 3.78a | 98.57 ± 3.78a | 98.57 ± 3.78a | 0.333 | (3, 24) | 0.801 |
| 10 | 95.71 ± 7.87a | 92.85 ± 4.88a | 91.42 ± 9.00a | 90.00 ± 5.77a | 0.833 | (3, 24) | 0.489 | |
| 15 | 90.00 ± 10.00a | 72.85 ± 7.56b | 67.14 ± 14.96bc | 52.85 ± 7.56c | 15.087 | (3, 24) | <0.01 | |
| 20 | 90.00 ± 10.00a | 67.14 ± 9.51b | 48.57 ± 6.90c | 25.71 ± 5.35d | 78.357 | (3, 24) | <0.01 | |
| 25 | 87.14 ± 9.51a | 57.14 ± 9.51b | 35.71 ± 13.97c | 17.14 ± 9.51d | 54.245 | (3, 24) | <0.01 | |
| 30 | 77.14 ± 7.56a | 25.71 ± 9.76b | 11.42 ± 10.69c | 4.29 ± 7.87c | 92.217 | (3, 24) | <0.01 | |
| Cyromazine | 5 | 98.57 ± 3.78a | 97.14 ± 7.56a | 97.14 ± 7.56a | 95.71 ± 7.87a | 0.200 | (3, 24) | 0.895 |
| 10 | 97.14 ± 4.88a | 94.28 ± 9.76a | 94.28 ± 11.33a | 94.28 ± 7.87a | 0.185 | (3, 24) | 0.906 | |
| 15 | 94.28 ± 7.87a | 92.85 ± 9.51a | 91.42 ± 12.14a | 90.00 ± 11.54a | 0.220 | (3, 24) | 0.882 | |
| 20 | 88.57 ± 12.14a | 85.71 ± 13.97a | 85.71 ± 13.97a | 81.42 ± 15.73a | 0.309 | (3, 24) | 0.819 | |
| 25 | 85.71 ± 9.76a | 82.85 ± 12.53a | 80.00 ± 11.54a | 78.57 ± 13.45a | 0.496 | (3, 24) | 0.689 | |
| 30 | 82.85 ± 7.56a | 77.14 ± 7.56a | 75.71 ± 12.72a | 72.85 ± 12.53a | 1.143 | (3, 24) | 0.352 | |
Within each row in the table above, values (survival, %, mean ± s.e., n = 7) were analyzed with ANOVA, and different letters denote significant differences (Tukey’s HSD test, p < 0.05). “a”means the day after emergence.
Fecundity of onion flies was not affected by lufenuron at doses of 100, 500 and 1000 mg kg−1 compared to the control at each time point (Table 2). Pyriproxyfen showed multiple effects on onion fly fecundity. Particularly, fecundity of the onion fly on 10 day after emergence was stimulated significantly to 32.72 ± 9.73 and 34.14 ± 12.04 by pyriproxyfen at doses of 100 and 500 mg kg−1, respectively, which was significantly higher than that of the control (18.72 ± 4.19), while 1000 mg kg−1 of this IGR did not affect fecundity (Table 2). Fecundity on day 15 after emergence was not affected by pyriproxyfen at doses of 100 and 500 mg kg−1, while 1000 mg kg−1 reduced fecundity significantly (Table 2). Effects of pyriproxyfen on onion fly fecundity were similar on day 20, 25 and 30 after emergence (Table 2), i.e., fecundity decreased significantly in response to increasing pyriproxyfen doses compared to the control. Onion fly fecundity was significantly decreased by the 72 h treatment of cyromazine at doses of 100, 500 and 1000 mg kg−1, and it was directly related to cyromazine doses compared to the control (Table 2). On day 30 after emergence, fecundity of cyromazine-treated onion fly was reduced to 116.40 ± 7.55, 94.48 ± 19.26, 74.04 ± 14.74 at doses of 100, 500 and 1000 mg kg−1, respectively, which was significantly lower than that of the control (164.10 ± 15.40).
Table 2. Fecundity of onion flies treated by selected IGRs.
| IGRs | Timea | Dose (mg kg−1) |
F | df | P | |||
|---|---|---|---|---|---|---|---|---|
| Control | 100 | 500 | 1000 | |||||
| Lufenuron | 5 | −b | − | − | − | − | − | − |
| 10 | 13.00 ± 4.62a | 12.25 ± 3.43a | 12.06 ± 3.07a | 15.28 ± 5.07a | 0.898 | (3, 24) | 0.457 | |
| 15 | 53.37 ± 8.27a | 46.46 ± 6.56a | 46.80 ± 7.36a | 54.37 ± 7.19a | 2.277 | (3, 24) | 0.105 | |
| 20 | 77.61 ± 11.77a | 70.40 ± 8.78a | 73.85 ± 8.52a | 76.61 ± 14.57a | 0.584 | (3, 24) | 0.631 | |
| 25 | 107.00 ± 21.41a | 100.10 ± 14.83a | 97.75 ± 15.02a | 105.50 ± 23.13a | 0.371 | (3, 24) | 0.774 | |
| 30 | 132.40 ± 28.07a | 114.20 ± 13.57a | 116.00 ± 15.79a | 122.30 ± 27.47a | 0.952 | (3, 24) | 0.431 | |
| Pyriproxyfen | 5 | − | − | − | − | − | − | − |
| 10 | 18.72 ± 4.19b | 32.72 ± 9.73a | 34.14 ± 12.04a | 15.80 ± 3.55b | 9.216 | (3, 24) | <0.01 | |
| 15 | 50.82 ± 9.22a | 60.95 ± 8.04a | 49.52 ± 8.29a | 29.22 ± 10.30b | 15.244 | (3, 24) | <0.01 | |
| 20 | 88.27 ± 9.74a | 84.72 ± 11.82a | 58.04 ± 15.41b | 35.90 ± 6.47c | 32.910 | (3, 24) | <0.01 | |
| 25 | 120.50 ± 13.79a | 113.40 ± 14.09a | 61.58 ± 9.18b | 36.78 ± 10.85c | 77.868 | (3, 24) | <0.01 | |
| 30 | 155.80 ± 14.71a | 121.20 ± 14.67b | 64.08 ± 9.17c | 45.54 ± 9.92d | 118.173 | (3, 24) | <0.01 | |
| Cyromazine | 5 | − | − | − | − | − | − | − |
| 10 | 19.81 ± 3.00a | 10.48 ± 3.47b | 8.83 ± 2.79b | 2.64 ± 1.40c | 45.840 | (3.24) | <0.01 | |
| 15 | 69.65 ± 12.61a | 42.15 ± 10.44b | 39.13 ± 7.48b | 19.89 ± 5.13c | 33.565 | (3, 24) | <0.01 | |
| 20 | 104.70 ± 15.44a | 79.33 ± 8.50b | 74.90 ± 16.28b | 36.89 ± 13.29c | 29.157 | (3, 24) | <0.01 | |
| 25 | 133.30 ± 21.81a | 106.80 ± 12.16b | 95.09 ± 20.10b | 63.38 ± 17.51c | 17.651 | (3, 24) | <0.01 | |
| 30 | 164.10 ± 15.40a | 116.40 ± 7.55b | 94.48 ± 19.26c | 74.04 ± 14.74c | 47.412 | (3, 24) | <0.01 | |
Within each row in the table above, values (fecundity, mean ± s.e., n = 7) were analyzed with ANOVA, and different letters denoted significant differences (Tukey’s HSD test, p < 0.05). “a”means the day after emergence. “b”mean onion flies did not oviposit on day 5 after emergence.
The onion fly fertility was significantly decreased by lufenuron at doses of 100, 500 and 1000 mg kg−1, and it was directly related to doses compared to the control till day 25 after emergence (Table 3). At a dose of 1000 mg kg−1, lufenuron reduced fertility to 13.09 ± 4.36%, 16.19 ± 2.61%, 27.61 ± 2.33%, 38.57 ± 3.61% and 60.57 ± 2.71% on day 5, 10, 15, 20, 25, and 30, respectively, which were all significantly lower than that of the control. On the contrary, pyriproxyfen treatments showed no effects on fertility of the onion fly at each time point (Table 3). Compared to the control, cyromazine significantly decreased the fertility of onion flies on day 5 and 10 after emergence, while these inhibition effects disappeared on day 15, 20, 25, and 30 after emergence (Table 3).
Table 3. Fertility of onion flies treated by selected IGRs.
| IGRs | Timea | Dose (mg kg−1) |
F | df | p | |||
|---|---|---|---|---|---|---|---|---|
| Control | 100 | 500 | 1000 | |||||
| Lufenuron | 5 | 98.57 ± 2.99a | 36.99 ± 4.07b | 23.85 ± 2.90c | 13.09 ± 4.36d | 762.228 | (3, 24) | <0.01 |
| 10 | 95.71 ± 4.60a | 37.66 ± 4.73b | 31.47 ± 5.28c | 16.19 ± 2.61d | 553.649 | (3, 24) | <0.01 | |
| 15 | 95.71 ± 5.92a | 59.28 ± 4.60b | 43.33 ± 3.73c | 27.61 ± 2.33d | 307.798 | (3, 24) | <0.01 | |
| 20 | 83.57 ± 3.61a | 77.57 ± 6.05a | 64.85 ± 6.55b | 38.57 ± 3.61c | 89.835 | (3, 24) | <0.01 | |
| 25 | 85.14 ± 3.07a | 85.42 ± 2.35a | 80.47 ± 8.23a | 60.57 ± 2.71b | 42.184 | (3, 24) | <0.01 | |
| 30 | 82.47 ± 5.10a | 82.80 ± 5.33a | 84.42 ± 2.21a | 81.61 ± 10.20a | 0.246 | (3, 24) | 0.864 | |
| Pyriproxyfen | 5 | 92.19 ± 4.69a | 93.66 ± 5.06a | 93.85 ± 5.79a | 90.47 ± 4.16a | 0.664 | (3, 24) | 0.582 |
| 10 | 85.00 ± 6.31a | 88.90 ± 3.35a | 85.00 ± 9.36a | 84.14 ± 7.00a | 0.654 | (3, 24) | 0.588 | |
| 15 | 91.42 ± 3.90a | 87.47 ± 6.54a | 86.14 ± 6.00a | 86.76 ± 6.44a | 1.153 | (3, 24) | 0.348 | |
| 20 | 86.90 ± 6.70a | 81.47 ± 8.26a | 85.66 ± 6.75a | 86.00 ± 8.76a | 0.669 | (3, 24) | 0.579 | |
| 25 | 90.00 ± 5.09a | 83.85 ± 9.49a | 86.76 ± 6.31a | 88.57 ± 7.02a | 0.885 | (3, 24) | 0.463 | |
| 30 | 87.14 ± 8.70a | 83.80 ± 8.03a | 80.95 ± 8.97a | 86.66 ± 8.61a | 0.757 | (3, 24) | 0.529 | |
| Cyromazine | 5 | 90.47 ± 7.86a | 82.33 ± 8.54a | 31.85 ± 9.06b | 9.76 ± 8.52c | 148.406 | (3, 24) | <0.01 |
| 10 | 92.85 ± 6.36a | 85.09 ± 8.20a | 52.85 ± 8.46b | 22.71 ± 9.55c | 106.351 | (3, 24) | <0.01 | |
| 15 | 89.80 ± 7.43a | 92.38 ± 5.35a | 91.52 ± 7.57a | 89.42 ± 6.10a | 0.320 | (3, 24) | 0.811 | |
| 20 | 85.95 ± 7.51a | 87.61 ± 5.52a | 84.09 ± 4.54a | 85.00 ± 7.07a | 0.424 | (3, 24) | 0.737 | |
| 25 | 83.80 ± 4.38a | 85.57 ± 4.35a | 84.19 ± 4.59a | 85.04 ± 4.38a | 0.238 | (3, 24) | 0.869 | |
| 30 | 85.04 ± 4.23a | 84.52 ± 4.88a | 85.47 ± 5.24a | 85.00 ± 4.08a | 0.057 | (3, 24) | 0.982 | |
Within each row in the table above, values (fertility, %, mean ± s.e., n = 7) were analyzed with ANOVA, and different letters denote significant differences (Tukey’s HSD test, p < 0.05). “a”means the day after emergence.
Fecundity and fertility of onion flies which were treated in different ages by lufenuron, pyriproxyfen and cyromazine
Fecundity of 1-day, 4-day and 7-day old onion flies treated with 0, 50 or 500 mg kg−1 of lufenuron was not significantly different with each other (Table 4). Pyriproxyfen decreased fecundity of onion fly adults treated in different ages. Specifically, under the treatment of pyriproxyfen, the onion fly fecundity decreased significantly as the treated adult age decreased (Table 4). Fecundity of 7-day, 4-day and 1-day old onion flies when treated with pyriproxyfen at doses of 50 mg kg−1 was 135.84 ± 14.53, 115.48 ± 7.82 and 89.23 ± 7.87, respectively. With the treatment of pyriproxyfen at doses of 500 mg kg−1, fecundity of 7-day, 4-day and 1-day old onion flies was 136.92 ± 11.24, 95.87 ± 9.58 and 67.95 ± 7.32, respectively. Similar with pyriproxyfen, inhibition effects of cyromazine on onion fly fecundity decreased significantly as the treated adult age increased (Table 4). For onion flies treated by cyromazine at a dose of 50 mg kg−1, fecundity of 1-day, 4-day and 7-day old onion flies was 95.75 ± 11.19, 149.98 ± 13.42 and 153.63 ± 18.99, respectively. For onion flies treated by cyromazine at a dose of 500 mg kg−1, fecundity of 1-day, 4-day and 7-day old onion flies was 64.02 ± 13.68, 102.87 ± 7.85 and 150.77 ± 14.10, respectively.
Table 4. Fecundity of 1-day, 4-day, and 7-day old onion flies when treated by selected IGRs.
| Adult age | IGRs Dose (mg kg−1) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lufenuron |
Pyriproxyfen |
Cyromazine |
|||||||
| Control | 50 | 500 | Control | 50 | 500 | Control | 50 | 500 | |
| 1-day | 121.54 ± 13.04a | 121.33 ± 10.47a | 123.19 ± 13.47a | 133.73 ± 11.34a | 89.23 ± 7.87c | 67.95 ± 7.32c | 178.07 ± 10.15a | 95.75 ± 11.19b | 64.02 ± 13.68c |
| 4-day | 125.55 ± 20.64a | 124.11 ± 16.25a | 124.92 ± 16.86a | 135.37 ± 11.10a | 115.48 ± 7.81b | 95.87 ± 9.58b | 180.63 ± 10.91a | 149.48 ± 13.42a | 102.87 ± 7.85b |
| 7-day | 125.04 ± 15.47a | 123.08 ± 16.22a | 112.95 ± 12.58a | 131.01 ± 10.70a | 135.84 ± 14.53a | 136.92 ± 11.24a | 176.44 ± 10.87a | 153.63 ± 18.99a | 150.77 ± 14.10a |
| F | 0.119 | 0.065 | 1.409 | 0.279 | 34.222 | 93.017 | 0.275 | 32.856 | 88.635 |
| df | 2, 18 | 2, 18 | 2, 18 | 2, 18 | 2, 18 | 2, 18 | 2, 18 | 2, 18 | 2, 18 |
| p | 0.889 | 0.937 | 0.270 | 0.760 | <0.001 | <0.001 | 0.762 | <0.001 | <0.001 |
Within each column in the table above, values (fecundity, mean ± s.e., n = 7) were analyzed with ANOVA, and different letters denote significant differences (Tukey’s HSD test, p < 0.05).
As it was shown in Table 5, fertility of 1-day, 4-day and 7-day old onion flies fed with baits containing lufenuron was significantly different with each other, and fertility of treated onion fly decreased significantly as the adult age increased. For onion flies treated by 50 mg kg−1 of lufenuron, fertility of 1-day, 4-day and 7-day old adult flies was 79.29 ± 4.46%, 66.14 ± 4.67% and 49.71 ± 5.53%, respectively. For onion flies treated with 500 mg kg−1 of lufenuron, fertility of 1-day, 4-day and 7-day old adult flies was 36.86 ± 5.21%, 18.43 ± 4.61% and 5.14 ± 5.18%, respectively. Under the treatment of pyriproxyfen, fertility of 1-day, 4-day and 7-day old onion flies was not significantly different with each other (Table 5). Inhibition effects of cyromazine on onion flies increased significantly as adult age increased (Table 5). Fertility of 7-day old onion fly was decreased to 8.57 ± 6.27% by the 72 h treatment of cyromazine at a dose of 500 mg kg−1, which was significantly lower than that of 4-day old (32.29 ± 6.16%) and 1-day old (58.57 ± 6.73%) onion flies.
Table 5. Fertility of 1-day, 4-day, and 7-day old onion flies when treated by selected IGRs.
| Adult age | IGRs Dose (mg kg−1) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lufenuron |
Pyriproxyfen |
Cyromazine |
|||||||
| Control | 50 | 500 | Control | 50 | 500 | Control | 50 | 500 | |
| 1-day | 94.29 ± 6.07a | 79.29 ± 4.46a | 36.86 ± 5.21a | 92.14 ± 8.09a | 90.54 ± 10.51a | 89.29 ± 13.67a | 96.43 ± 7.48a | 91.86 ± 8.21a | 58.57 ± 6.73a |
| 4-day | 95.00 ± 7.64a | 66.14 ± 4.67b | 18.43 ± 4.61b | 97.14 ± 7.56a | 92.86 ± 10.75a | 92.73 ± 10.72a | 95.71 ± 6.07a | 73.00 ± 6.27b | 32.29 ± 6.16b |
| 7-day | 94.29 ± 7.32a | 49.71 ± 5.53c | 5.14 ± 5.18c | 91.43 ± 10.69a | 95.00 ± 9.57a | 92.86 ± 9.06a | 94.29 ± 8.38a | 55.71 ± 6.73c | 8.57 ± 6.27c |
| F | 0.024 | 63.773 | 70.798 | 0.440 | 0.329 | 0.224 | 0.153 | 45.133 | 107.258 |
| df | 2, 18 | 2, 18 | 2, 18 | 2, 18 | 2, 18 | 2, 18 | 2, 18 | 2, 18 | 2, 18 |
| p | 0.976 | <0.001 | <0.001 | 0.859 | 0.724 | 0.801 | 0.859 | <0.001 | <0.001 |
Within each column in the table above, values (fertility, %, mean ± s.e., n = 7) were analyzed with ANOVA, and different letters denote significant differences (Tukey’s HSD test, p < 0.05).
Offspring larval development of onion flies treated by lufenuron, pyriproxyfen and cyromazine
Offspring larval survival on day 5 after hatch of 50 mg kg−1 lufenuron-treated onion flies was significantly reduced to 55.00 ± 11.93%, which was lower than that of the control (84.29 ± 8.38%, Table 6). Pyriproxyfen did not affect offspring larval survival on day 5 after hatch when parent flies were treated at a dose of 50 mg kg−1 (Table 6). Cyromazine reduced offspring larval survival on day 5 after hatch to 78.86 ± 6.64% when parent flies were fed with 50 mg kg−1 of cyromazine, which was significantly lower than that of the control (92.00 ± 6.93%, Table 6).
Table 6. Effects on larval development when onion fly adults were fed with IGRs.
| % | IGRs | Dose (mg kg−1) |
df | t | p | |
|---|---|---|---|---|---|---|
| Control | 50 | |||||
| 5-day larval survival | Lufenuron | 84.29 ± 8.38 | 55.00 ± 11.93* | 12 | 5.314 | <0.001 |
| Pyriproxyfen | 85.29 ± 10.83 | 85.29 ± 8.75 | 12 | −0.670 | 0.515 | |
| Cyromazine | 92.00 ± 6.93 | 78.86 ± 6.64* | 12 | 3.622 | 0.003 | |
| 15-day larval survival | Lufenuron | 87.00 ± 6.58 | 87.71 ± 11.61 | 12 | −0.142 | 0.890 |
| Pyriproxyfen | 95.14 ± 8.47 | 91.57 ± 9.02 | 12 | 0.764 | 0.460 | |
| Cyromazine | 95.86 ± 4.10 | 94.43 ± 7.76 | 12 | 0.430 | 0.675 | |
| Pupation rate | Lufenuron | 97.57 ± 4.24 | 97.14 ± 4.88 | 12 | 0.175 | 0.864 |
| Pyriproxyfen | 97.14 ± 7.56 | 99.14 ± 2.27 | 12 | −0.607 | 0.512 | |
| Cyromazine | 98.57 ± 3.78 | 97.14 ± 7.56 | 12 | 0.447 | 0.663 | |
| Emergence rate | Lufenuron | 98.57 ± 3.78 | 97.14 ± 4.88 | 12 | 0.612 | 0.552 |
| Pyriproxyfen | 97.14 ± 7.56 | 63.57 ± 7.48* | 12 | 8.352 | <0.001 | |
| Cyromazine | 97.14 ± 7.56 | 94.29 ± 7.82 | 12 | 0.693 | 0.502 | |
Within each row in the table above, values (%, mean ± s.e., n = 7) were compared by using an independent-samples t test. “*” denotes significant differences (independent-samples t test, p < 0.05).
Offspring larval survival on day 15 after hatch was not affected significantly when parent flies were fed with sugar baits containing 50 mg kg−1 of lufenuron, pyriproxyfen or cyromazine, respectively (Table 6).
Offspring pupation rate was not affected significantly when parent flies were fed with sugar baits containing 50 mg kg−1 of lufenuron, pyriproxyfen or cyromazine, respectively (Table 6).
Emergence rate of offspring pupae when parent flies were fed with sugar baits containing 50 mg kg−1 of lufenuron or cyromazine was not affected compared to the control (Table 6). However, it was significantly reduced to 63.57 ± 7.48% with pyriproxyfen, which was significantly lower than that of the control (97.14 ± 7.56%).
Discussion
Although insect growth regulators have been used as chemosterilants for fruit flies19,20,22,23,26,28,29,30,31,32, this is the first laboratory attempt to use IGRs in adult-targeted baits to control onion maggot damage. In this study, IGRs with different modes of action were chosen to represent 3 kinds of the most widely used IGRs insecticides at present. In addition, detailed life parameters of the onion fly including adult survival, fecundity, fertility, susceptibility of adult in different ages and offspring development were observed, which would provide sufficient justification for IGRs being used as chemosterilants. Results in this study showed that these chemicals in sugar baits inhibited onion fly reproduction in various ways.
The most remarkable effect of lufenuron on onion flies was the significant inhibition of fertility when adult flies were fed it (Tables 3 and 5). Similar effects had been reported on C. capitata23,42, A. ludens20, A. obliqua20, A. serpentina20, A. striata20, B. dorsalis23, B. latifron23, M. domestica43 and B. oleae19. In this study, the hooked mouth part could be observed with stereoscopic microscopes in unhatched eggs produced by lufenuron-treated onion flies, which indicated that the embryo had developed completely even though the larva failed to get out of the egg shell (Supplementary, Fig. S1). Lufenuron had been reported to interfere with the deposition of new cuticle in insects38. Thus, the developed embryo cuticle containing chitin maybe affected by lufenuron, being less rigid, is unable to act as an effective exoskeleton for the attached musculature. This effect was similar to another benzoylphenyl urea chitin-synthesis inhibitor diflubenzuron which decreased fertility of the housefly44. Another interesting aspect was the effect of lufenuron and the age of adult flies on fertility. Results showed that older adult onion flies were more susceptible to IGRs resulting in decreased fertility (Table 5). Similar effects had been reported on C. capitata42 and M. domestica43,45. This effect may result from incorporation of relatively more lufenuron into forming eggs in older adults, while these chemicals might have been metabolized and excreted before they were incorporated into eggs in younger adult females. Besides, most of offspring larvae died within 5 days of hatching when parent onion flies were treated by lufenuron, which was of great significance in crop protection as less new-born larvae could develop to third instar that are responsible for severe damage to crops.
Pyriproxyfen, a juvenile hormone analogue, shortened preoviposition period of onion flies when treated at a dose of 100 mg kg−1, while higher doses did not affect preoviposition period (Supplementary, Table S2). What’s more interesting was that it enhanced egg production during short period after non-lethal dose of treatments (Table 2). This is consistent with another species. A non-lethal dose of pyriproxyfen enhances the fecundity significantly as well as the ovarian development when Rhagoletis pomonella adults are treated by topical application46. Former researchers discussed this enhanced fecundity as hormesis19, while it is more like juvenile hormone regulation. Contrary to increased fecundity, survival of adult female was reduced significantly (Table 1). This may be energy compromise between survival and reproduction in a short period. Pyriproxyfen had no effects on fertility when 1-day old onion fly adult females were treated (Table 5). Fertility of matured Stomoxys calcitrans47, B. oleae19, and R. pomonella46 adult is not affected when treated by pyriproxyfen, while fertility of unmatured S. calcitrans47, C. capitata42 was reduced significantly. In this study, it seemed that fertility of older onion fly females was not affected by pyriproxyfen (Table 5). Although survival had been significantly reduced in this experiment and former researchers proved that pupae produced by pyriproxyfen treated female adult cannot emerge36,42,48, pyriproxyfen cannot be used in the field as farmers cannot bear potential serious damage caused by numerous larvae hatched from increased fecundity.
The most impressive effect of cyromazine on onion flies was the sharply decreased fecundity of 1-day old onion flies (Table 2). Besides, the younger treated adults were less fecund, whereas the fecundity of matured onion flies was not affected (Table 4). Similar effects had also been reported on L. sericata27,49,50, A. ludens21, B. oleae19, C. capitata24,25 and Drosophila melanogaster51. Inhibitory effects of this chemical on fecundity of dipteran insects may depend on the age of females when they are treated, and fecundity of unmatured adult female are more likely to be affected. The average preoviposition period of onion fly female is 7 days2, and fecundity of 7-day old adult females was not affected (Table 4). As for the reduced fecundity, there may be three reasons. First, cyromazine inhibited development of the reproduction system, especially the ovaries. Cyromazine prolonged the preoviposition period of newly emerged onion flies (Supplementary, Table S2), which indicated that cyromazine may inhibit vitellogenesis in the ovary. Second, eggs may developed normally in ovaries, but they could not be laid. This may result from reduced mechanical strength of onion fly female ovipositor, which had also been reported on A. ludens52. Mechanical strength of unmatured A. ludens female ovipositor is affected by cyromazine52, while its ovary development is not affected directly by cyromazine21,24. Third, eggs formed normally in female ovaries, but they were resorbed before being laid. Cyromazine is of ecdysone activity, and it has been hypothesized to be related to the development of hormone 20-hydroxyecdysone40,41. Low concentrations of ecdysteroid are essential for normal oogenesis, while there is a threshold concentration in egg chambers and that apoptosis at mid-oogenesis is induced when the ecdysteroid levels exceed that threshold53. Fruther researches need to be conducted to define mechanisms of cyromazine inhibition on fecundity.
Considering lufenuron, pyriproxyfen and cyromazine as representative chemicals, this research not only determined the effects of these 3 IGRs on onion fly reproduction but also provides support for predicting effects of IGRs with similar modes of action on onion fly reproduction. Thus, combined applications of these selected IGRs on onion flies were not conducted although it could show more significant inhibition effects on reproduction. Overall, lufenuron and cyromazine affected onion fly reproduction when these chemicals were fed to flies, which indicated that they could be used as chemosterilants for onion flies. Besides, these two chemicals didn’t affect survival of onion flies indicating that they could be of great advantages in conservation of natural enemy especially those predatory adults. Our results indicate that IGRs with similar modes of actions could be potential chemosterilants for onion fly control. There would be many advantages if these chemicals were used as chemosterilants for onion flies. First, as IGRs specifically interfere with chitin deposition which was only discovered in insect cuticle or work as specific hormones influence insect maturity and reproduction mediation54, they are of great safety to mammal and human as IGRs act specifically on arthropods. This makes them optimized chemicals to be used in baits for onion flies. Second, IGRs used in baits combined with specific attractants could avoid application of pesticides in large scale such as foliar spraying and reduce environmental impact especially on non-targeted beneficial insects. Third, some of these IGRs such as lufenuron and cyromazine could kill offspring larvae before them making threats to crops, which is of great significance in practical crop protection. In this study, inhibitory effects of IGRs on onion fly males or females were not detected respectively as we mainly focused on their practical performance in the field. As for IGRs effects on onion fly male or female, further experiments shall be conducted to illustrate mechanical details of these inhibitory effects on reproduction. Although further work have to be conducted to test their control efficiency in the field, this work may provide new options for onion maggot control.
Methods
Experiment I Effects of adult-ingested lufenuron, pyriproxyfen and cyromazine on onion fly survival, fecundity and fertility
Preliminary tests showed that the LC50 of lufenuron, pyriproxyfen and cyromazine was more than 2000 mg kg−1 at 72 h (Supplementary, Table S1). In case of unexpected onion fly death, lower treatment doses (100, 500 and 1000 mg kg−1) of these three IGRs insecticides were used to treat onion flies (emerged within 6 h) as described previously20,55. Detailed information were in supplementary methods online. Briefly, granulated sugar was impregnated with acetone or methanol-water solution containing different amount of insecticides, and the solvent was evaporated to obtain 100, 500, 1000 mg kg−1 doses of IGRs-sugar baits. Five pairs of flies were put into a cylinder-shaped glass chamber (open at both ends, L = 12 cm, ∅≈6 cm, supplementary, Fig. S2) and fed only with these IGRs-sugar baits and water for 72 h, and then these baits were replaced with milk and 5% sucrose water solution. Granulated sugar was used as control as acetone or methanol impregnation showed no effects on onion flies (Data not shown). All flies survived from the 72-hour treatment due to the low toxicity of the selected IGRs. Each group of treated onion flies (in each glass chamber) was regarded as one replication, and each treatment group was replicated 7 times.
To test effects of these selected IGRs on survival of onion flies, the number of remaining alive adults in each chamber was recorded every 24 h. Survival of onion flies in each glass chamber (each replication) at 5, 10, 15, 20, 25, and 30 d after eclosion was calculated.
To study the effect of these selected IGRs on fecundity of onion flies, eggs laid during last 24 h by females from each chamber were collected and counted every day from first oviposition up to the 30th day after eclosion. In order to obtain the quantity of eggs laid per female per day, the total number of eggs from one chamber was divided by the number of remaining females yesterday in that chamber. Fecundity, i.e., the quantity of laid eggs accumulated at 5, 10, 15, 20, 25, and 30 d after eclosion per female in each glass chamber (one replication) was calculated.
To study the effect of adult-ingested IGRs on fertility (larval hatch), 30 eggs from each chamber were sampled randomly at 5, 10, 15, 20, 25, and 30 d after treatment and placed in plastic petri dishes containing a round piece of permanently wet filter paper, covered with a black humid cloth, and maintained at 21 ± 0.5 °C and 70–75% RH. Egg hatch was checked using an Olympus stereoscopic microscope (Olympus Corporation, Japan) after 4 days of incubation (The egg period of onion flies usually lasts about 3 days). Empty egg shells were regarded as hatched larvae, and hatch percent of these 30 eggs from each glass chamber (one replication) was calculated.
Experiment II Effects of adult-ingested lufenuron, pyriproxyfen and cyromazine on fecundity and fertility of adults treated in different ages
Onion fly adults in different ages (different maturation stages) may respond differently to IGRs treatments. The average preoviposition period of the onion fly is 7 days2. Thus, 1-day old (emerged within 6 h, undeveloped reproduction system), 4-day old (emerged about 96 h, developing reproduction system) and 7-day old (emerged about 168 h, developed reproduction system) adult onion flies which had been starved for 24 h (for 4-day and 7-day old onion flies) were fed with sugar baits containing 50 and 500 mg kg−1 of lufenuron, pyriproxyfen and cyromazine. Granulated sugar was used as control. Five pairs of onion flies were put inside a glass chamber and treated, and each group of treated onion flies (in each glass chamber) was regarded as one replication. Each treatment was replicated 7 times. Detailed onion fly treatment procedure was in Experiment I and supplementary materials.
Onion flies survived from treatments and egg quantity in each glass chamber were checked and recorded every day, and fecundity at 30 d after emergence per female in each glass chamber (one replication) was calculated as described above.
To compare the response of onion fly fertility to selected IGRs when onion flies were treated in different developmental stages, 30 eggs from each chamber (each replication) were randomly collected at day 10 after treatment. The hatch percent (fertility) of these collected eggs was calculated as describe in Experiment I.
Experiment III Effects of adult-ingested lufenuron, pyriproxyfen and cyromazine on offspring larval development
Adults in different developmental stages which showed the greatest susceptibility to IGRs according to the former experiment were used in this experiment. Sugar baits containing 50 mg kg−1 of IGRs were used to obtain sufficient hatched larvae for further observation. In this experiment, onion flies were treated with the similar method as described before. Adults were put in a cage (25 × 25 × 25 cm wood-profile cages) instead of in a glass chamber. Specifically, twenty pairs of onion flies were put inside a cage and treated, and was regarded as one replication (in each cage). Granulated sugar was used as control. Each treatment was replicated 7 times. Eight days after treatment, one hundred eggs from each cage were collected randomly and incubated as described above. Three pieces of garlic were put aside the eggs as food for hatched larvae, and the larval development from each cage was observed continuously until they pupated. For each group of eggs (from one cage), fertility was recorded after 4 days of incubation. Five-day old larvae after hatch are too small to make significant threats to crops, while 15-day old larvae damage crops. Thus, we tried to evaluate the larva survival at day 5 and 15 after egg hatch as follows:
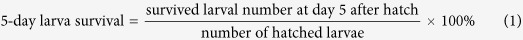 |
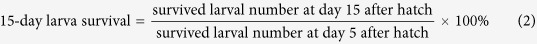 |
Besides, pupation rate and pupal emergence rate were also calculated as follows:
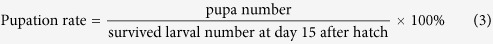 |
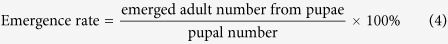 |
Statistical analysis
Prior to statistical analysis we tested all variables for normality with the Kolmogorov-Smirnov test and homogeneity of group variances with Levene’s test. In Experiment I, onion fly survival, fecundity or fertility at each time point was regarded as variable, and IGRs doses were regarded as independent variables in one-way ANOVA and followed by Tukey’s HSD multiple comparisons. In Experiment II, fecundity or fertility of onion flies treated with each insecticide dose (including the 0 mg kg−1 group, i.e., the control group) was regarded as dependent variable, and onion fly developmental stages were regarded as independent variables in one-way ANOVA and followed by Tukey’s HSD multiple comparisons. For 5-day larval survival, 15-day larval survival, pupation rate, and emergence rate in Experiment III, data were compared by using an independent-samples t test. All analyses were performed with PASW Statistics 18.0.0 (2009; SPSS Inc. Quarry Bay, HK).
Additional Information
How to cite this article: Zhou, F. et al. Sterilization Effects of Adult-targeted Baits Containing Insect Growth Regulators on Delia antiqua. Sci. Rep. 6, 32855; doi: 10.1038/srep32855 (2016).
Supplementary Material
Acknowledgments
This research was funded by “International Commonweal Scientific Research Special Fund-Research and Demonstration of Crop Root Maggot Control Technology (No. 201303027)”.
Footnotes
Author Contributions The study was jointly conceived by F.Z., Z.W., H.Z. and M.X. Experiments were designed by F.Z. and M.X.; F.Z. prepared the manuscript; H.X., X.L., X.M. G.Z. and Y.L. edited the manuscript. F.Z. and Z.W. carried out experiments.
References
- Ellis P. R. & Eckenrode C. J. Factors influencing resistance in Allium sp. to onion maggot. Bull. Entomol. Soc. Am. 25, 151–154 (1979). [Google Scholar]
- Bin C., WanSun L., Guozhong F., Bozheng H. & Tingjing L. Laboratory rearing, biological characteristics and diapause induction of the onion maggot, Delia antiqua. CQNUJ 27, 1–5 (2010). [Google Scholar]
- Nault B. A. Ecology and management of onion maggot. Onion World 23, 24–27 (2007). [Google Scholar]
- Wilson R. G., Orloff S. B. & Taylor A. G. Evaluation of insecticides and application methods to protect onions from onion maggot, Delia antiqua, and seedcorn maggot, Delia platura, damage. Crop Prot. 67, 102–108 (2015). [Google Scholar]
- Huang Y., Wang X., Zhang X., Lu F. & Lu Z. Biology and control of Delia antiqua. J. Changjiang Veg. 4, 18–19 (1995). [Google Scholar]
- Nault B. A., Straub R. W. & Taylor A. G. Performance of novel insecticide seed treatments for managing onion maggot (Diptera: Anthomyiidae) in onion fields. Crop Prot. 25, 58–65 (2006). [Google Scholar]
- Taylor A. G., Eckenrode C. & Straub R. Seed coating technologies and treatments for onion: challenges and progress. Hort Science 36, 199–205 (2001). [Google Scholar]
- Górski R. & Mielcarek M. Effectiveness of seed dressings in protection of onion against onion fly (Hylemyia antiqua Meig.). J. Plant. Prot. Res. 44, 1 (2004). [Google Scholar]
- Pandey A. K. & Namgayal D. Quantification of damage and evaluation of different insecticides against onion maggot, Delia antiqua (Meigen)(Diptera: Anthomyiidae) in Kargil district of Ladakh region. JHA 1, 62–65 (2010). [Google Scholar]
- Rawlins W. & Gonzalez D. Evaluation of Several Insecticides to Control the Onion Maggot. J. Econ. Entomol. 59, 288–290 (1966). [Google Scholar]
- Finch S., Eckenrode C. & Cadoux M. E. Behavior of onion maggot (Diptera: Anthomyiidae) in commercial onion fields treated regularly with parathion sprays. J. Econ. Entomol. 79, 107–113 (1986). [Google Scholar]
- Dindonis L. L. & Miller J. R. Onion fly trap catch as affected by release rates ofn-dipropyl disulfide from polyethylene enclosures. J. Chem. Ecol. 7, 411–418 (1981). [DOI] [PubMed] [Google Scholar]
- Harris M. O., Keller J. E. & Miller J. R. Responses to n-dipropyl disulfide by ovipositing onion flies: Effects of concentration and site of release. J. Chem. Ecol. 13, 1261–1277 (1987). [DOI] [PubMed] [Google Scholar]
- Zhou F., Xue M., Wang Z., Zhao H. & Wang S. Trapping eficiency of sticky insect boards against Delia antiqua in the fields. Plant Protection 38, 172 (2012). [Google Scholar]
- Zhou F., Wang Z., Xue M., Zhao H. & Xu H. Laboratory evaluation of attract-and-kill Insecticides for onion maggot Delia antiqua (Meigen) and their potential use in fields. JFAE 11, 1409–1413 (2013). [Google Scholar]
- Hoffmann M. P. et al. Nonwoven fiber barriers for control of cabbage maggot and onion maggot (Diptera: Anthomyiidae). J. Econ. Entomol. 94, 1485–1491 (2001). [DOI] [PubMed] [Google Scholar]
- Theunissen J., Loosjes M., Noordink J., Noorlander J. & Ticheler J. In Controlling fruit flies by the sterile-insect technique (International Atomic Energy Agency, 1975). [Google Scholar]
- Carruthers R., Whitfield G. & Haynes D. Pesticide-induced mortality of natural enemies of the onion maggot, Delia antiqua [Dip.: Anthomyiidae]. Entomophaga 30, 151–161 (1985). [Google Scholar]
- Sánchez-Ramos I., Fernández C. E., González-Núñez M. & Pascual S. Laboratory tests of insect growth regulators as bait sprays for the control of the olive fruit fly, Bactrocera oleae (Diptera: Tephritidae). Pest Manag. Sci. 69, 520–526 (2012). [DOI] [PubMed] [Google Scholar]
- Moya P. et al. Evaluation of lufenuron as a chemosterilant against fruit flies of the genus Anastrepha (Diptera: Tephritidae). Pest Manag. Sci. 66, 657–663 (2010). [DOI] [PubMed] [Google Scholar]
- Martinez A. J. & Soreno D. S. Effect of cyromazine on the oviposition of Mexican Fruit Fly (Diptera: Tephritidae) in the laboratory. J. Econ. Entomol. 84, 1540–1543 (1991). [Google Scholar]
- Alam M. J. & Moro A. N. Effect of cyromazine fed to adults on reproduction and offspring development in housefiy. J. Pestic. Sci. 25, 228–233 (2000). [Google Scholar]
- Chang C. L., Cho I. K. & Li Q. X. Laboratory evaluation of the chemosterilant lufenuron against the fruit flies Ceratitis capitata, Bactrocera dorsalis, B. cucurbitae, and B. latifrons. J. Asia-Pac. Entomol. 15, 13–16 (2012). [Google Scholar]
- Budia F. & Vinuela E. Effects of cyromazine on adult C. Capitata (Diptera: Tephritidae) on mortality and reproduction. J. Econ. Entomol. 89, 826–831 (1996). [Google Scholar]
- Jimenez-Peydro R., Gimeno-Martos C., Lopez-Ferrer J., Serrano-Delgado C. & Moreno-Marí J. Effects of the insect growth regulator cyromazine on the fecundity, fertility and offspring development of Mediterranean fruit fly, Ceratitis capitata Wied. (Dipt., Tephritidae). J. Appl. Entomol. 119, 435–438 (1995). [Google Scholar]
- El-Oshar M. A., Motoyama N., Hughes P. B. & Dauterman W. C. Studies on cyromazine in the house fly, Musca domestica (Diptera: Muscidae). J. Econ. Entomol. 78, 1203–1207 (1985). [DOI] [PubMed] [Google Scholar]
- Friedel T. & McDonell P. A. Cyromazine inhibits reproduction and larval development of the australian sheep blow fly (Diptera: Calliphoridae). J. Econ. Entomol. 78, 868–873 (1985). [DOI] [PubMed] [Google Scholar]
- Navarro-Llopis V., Sanchis-Cabanes J., Ayala I., Casaña-Giner V. & Primo-Yúfera E. Efficacy of lufenuron as chemosterilant against Ceratitis capitata in field trials. Pest Manag. Sci. 60, 914–920 (2004). [DOI] [PubMed] [Google Scholar]
- Navarro-Llopis V., Sanchis J., Primo-Millo J. & Primo-Yufera E. Chemosterilants as control agents of Ceratitis capitata (Diptera: Tephritidae) in field trials. Bull. Entomol. Res. 97, 359–368 (2007). [DOI] [PubMed] [Google Scholar]
- Alemany A., González A., Juan A. & Tur C. Evaluation of a chemosterilization strategy against Ceratitis capitata (Diptera: Tephritidae) in Mallorca island (Spain). J. Appl. Entomol. 132, 746–752 (2008). [Google Scholar]
- Bachrouch O. et al. Efficacy of the lufenuron bait station technique to control Mediterranean fruit fly (Medfly) Ceratitis capitata in citrus orchards in Northern Tunisia. TJPP 3, 35–45 (2008). [Google Scholar]
- Navarro-Llopis V., Domínguez-Ruiz J., Zarzo M., Alfaro C. & Primo J. Mediterranean fruit fly suppression using chemosterilants for area-wide integrated pest management. Pest Manag. Sci. 66, 511–519 (2010). [DOI] [PubMed] [Google Scholar]
- Langley P. A., Felton T. & Oouchi H. Juvenile hormone mimics as effective sterilants for the tsetse fly Glossina morsitans morsitans. Med. Vet. Entomol. 2, 29–35 (1988). [DOI] [PubMed] [Google Scholar]
- Hargrove J. & Langley P. Sterilizing tsetse (Diptera: Glossinidae) in the field: a successful trial. Bull. Entomol. Res. 80, 397–403 (1990). [Google Scholar]
- Hargrove J. & Langley P. A field trial of pyriproxyfen-treated targets as an alternative method for controlling tsetse (Diptera: Glossinidae). Bull. Entomol. Res. 83, 361–368 (1993). [Google Scholar]
- Bull D. L. & Meola R. W. Effect and fate of the insect growth regulator pyriproxyfen after application to the horn fly (Diptera: Muscidae). J. Econ. Entomol. 86, 1754–1760 (1993). [Google Scholar]
- Wang Y. Damages caused by onion maggot and its nuisanceless control. J. Changjiang Veg. 5, 64–64 (2010). [Google Scholar]
- van Eck W. H. Mode of action of two benzoylphenyl ureas as inhibitors of chitin synthesis in insects. Insect Biochem. 9, 295–300 (1979). [Google Scholar]
- Dhadialla T. S., Carlson G. R. & Le D. P. New insecticides with ecdysteroidal and juvenile hormone activity. Annu. Rev. Entomol. 43, 545–569 (1998). [DOI] [PubMed] [Google Scholar]
- Friedel T., Hales D. F. & Birch D. Cyromazine-induced effects on the larval cuticle of the sheep blowfly, Lucilia cuprina: Ultrastructural evidence for a possible mode of action. Pestic. Biochem. Phys. 31, 99–107 (1988). [Google Scholar]
- Van De Wouw A. P., Batterham P. & Daborn P. J. The insect growth regulator insecticide cyromazine causes earlier emergence in Drosophila melanogaster. Arch Insect Biochem. 63, 101–109 (2006). [DOI] [PubMed] [Google Scholar]
- Casaña-Giner V., Gandía-Balaguer A., Mengod-Puerta C., Primo-Millo J. & Primo-Yúfera E. Insect growth regulators as chemosterilants for Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 92, 303–308 (1999). [DOI] [PubMed] [Google Scholar]
- Guneidy N. A. & Mohammed S. S. Effect of a selected rice bran extract and a chitin synthesis inhibitor on viability of eggs of the house fly Musca domestica Linnaeus. Egypt. Acad. J. Biol. Sci. A Entomol. 5, 1–12 (2012). [Google Scholar]
- Chang S. C. & Bokovec A. B. Effects of difluhenzuron and penfluron on viability of house fly eggs. J. Econ. Entomol. 73, 285–287 (1980). [DOI] [PubMed] [Google Scholar]
- Siriwattanarungsee S., Sukontason K., Olson J., Chailapakul O. & Sukontason K. Efficacy of neem extract against the blowfly and housefly. Parasitol. Res. 103, 535–544 (2008). [DOI] [PubMed] [Google Scholar]
- Duan J. J., Prokopy R. J., Yin C. M., Bergweiler C. & Oouchi H. Effects of pyriproxyfen on ovarian development and fecundity of Rhagoletis pomonella flies. Entomol. Exp. Appl. 77, 17–21 (1995). [Google Scholar]
- Liu S. S., Li A. Y., Lohmeyer K. H. & Perez De Leon A. A. Effects of pyriproxyfen and buprofezin on immature development and reproduction in the stable fly. Med. Vet. Entomol. 26, 379–385 (2012). [DOI] [PubMed] [Google Scholar]
- Langley P. A., Felton T., Stafford K. & Oouchp H. Formulation of pyriproxyfen, a juvenile hormone mimic, for tsetse control. Med. Vet. Entomol. 4, 127–133 (1990). [DOI] [PubMed] [Google Scholar]
- Levot G. W. & Shipp E. Reduction in offspring survival of Lucilia Cuprina (wiedemann) following consumption of insect development inhibitors. Aust. J. Entomol. 23, 85–89 (1984). [Google Scholar]
- Kotze A. C. Effects of cyromazine on reproduction and offspring development in Lucilia cuprina (Diptera: Calliphoridae). J. Econ. Entomol. 85, 1614–1617 (1992). [DOI] [PubMed] [Google Scholar]
- Wilson T. G. Cyromazine toxicity to Drosophila melanogaster (Diptera: Drosophilidae) and lack of cross-resistance in natural population strains. J. Econ. Entomol. 90, 1163–1169 (1997). [DOI] [PubMed] [Google Scholar]
- Moreno D. S., Martinez A. J. & Riviello M. S. Cyromazine effects on the reproduction of Anastrepha ludens (Diptera: Tephritidae) in the laboratory and in the field. J. Econ. Entomol. 87, 202–211 (1994). [Google Scholar]
- Terashima J., Takaki K., Sakurai S. & Bownes M. Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J. Endocrinol. 187, 69 (2005). [DOI] [PubMed] [Google Scholar]
- Wright J. E. Environmental and toxicological aspects of insect growth regulators. Environ. Health Perspect. 14, 127–132 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiding J. Review of the global status and recent development of insecticide resistance in field populations of the housefly, Musca domestica (Diptera: Muscidae). Bull. Entomol. Res. 89, S9–S67 (1999). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


