Summary
Background
Sleep disturbances are common, and perhaps are even more prevalent in irritable bowel syndrome (IBS).
Aim
To determine the effect of measured sleep on: 1) IBS symptoms the following day, and IBS-specific quality of life (IBS-QOL), and 2) non-GI pain symptoms.
Methods
IBS patients’ sleep patterns were compared to healthy individuals via wrist-mounted actigraphy over 7 days. Daily bowel pain logs (severity, distress; 10-point Likert), stool pattern (Bristol scale) and supporting symptoms (e.g., bloating, urgency; 5-point Likert) were kept. Validated measures, including the GI Symptom Rating Scale-IBS, Visceral Sensitivity Index, Pittsburgh Sleep Quality Index and the IBS-Quality of Life were collected. Mediation analysis explored the relationship between sleep, mood, and bowel symptoms.
Results
50 subjects (38.6±1.0years old, 44 female; 24 IBS and 26 healthy controls) completed sleep monitoring. IBS patients slept more hours per day (7.7±0.2 vs 7.1±0.1, p=0.008), but felt less well-rested. IBS patients demonstrated more waking episodes during sleep (waking episodes; 12.1 vs 9.3, p<0.001). Waking episodes predicted worse abdominal pain (p≤0.01) and GI distress (p<0.001), but not bowel pattern or accessory IBS symptoms (p>0.3 for each). Waking episodes negatively correlated with general- and IBS-specific QOL in IBS (r= −0.58 and −0.52, p<0.001 for each). Disturbed sleep effects on abdominal pain were partially explained by mood as an intermediate.
Conclusion
Sleep disturbances are more common in IBS, and correlate with IBS-related pain, distress, and poorer IBS-related QOL. Disturbed sleep effects extend beyond the bowel, leading to worse mood and greater somatic pain in IBS patients.
Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder, affecting as many as 15% of US adults1. IBS is characterized by the hallmark symptoms of abdominal pain or discomfort and altered bowel habits, resulting in poor health-related quality of life (HRQOL) 2. IBS patients commonly endorse both affective symptoms (e.g., depression and anxiety) and somatic pain symptoms without structural explanation, such as back pain and headache.
Several lifestyle factors can modify symptom perception in the IBS patient, including diet and exercise3–6. Of particular interest to this study was the potential role of sleep in IBS symptom perception. Disordered sleep is quite common, with as many as 70 million Americans, or one-third of US adults having insomnia symptoms.7, 8 Sleep disturbances appear to be even more common in IBS, affecting as many as 50% of diagnosed individuals9, 10. While prototypical IBS symptoms can potentially have a deleterious effect on a restorative sleep pattern, recent evidence suggests that sleep disruption may directly enhance visceral hypersensitivity and GI symptoms. For example, gastroesophageal reflux disease (GERD) patients experimentally deprived of sleep have more severe esophageal symptoms when exposed to acid stimulation protocols11. Specific to functional GI disorders, a recent study in women (n=24) with IBS showed that self-reported sleep disturbances were associated with abdominal pain, anxiety and fatigue the next day12. Further, since affective disorders (anxiety and depression) are common in IBS13, and that sleep disturbances are a hallmark feature of these mood disorders14, 15, psychiatric comorbidity may play an important role in understanding the effect of sleep on IBS symptoms.
Despite previous observations thatpoor sleep is more common in IBS, little is known about the impact of disturbed sleep on individual IBS symptoms. Which specific derangements in sleep pattern may lead to expression of IBS symptoms is poorly understood. Further, we are not aware of any study which has examined the influence of sleep on mood and extraintestinal symptoms, both critical factors to the symptom severity and HRQOL in the IBS patient. We speculate that sleep disturbances in IBS patients result in a generalized hypersensitivity to pain, both visceral and somatic, via alterations in central nervous system responses to peripheral pain signals within brain regions known to modulate the affective and cognitive responses to pain, such as the homeostatic afferent processing network16. We further hypothesized that mood disturbances, also represented within much of the same brain neurocircuity,17,18 would render IBS patients particularly susceptible to the effects of disordered sleep on pain perception.
This study prospectively measured sleep quality and collected subjective sleep reports in order to determine the relationship of objective and subjective sleep measures on IBS symptoms and IBS-specific HRQOL. We also sought to examine whether any effect of sleep on pain is specific to visceral discomfort, or more generally to non-GI symptoms as well. Finally, we aimed to explore the role of mood as a potential mediator between sleep and pain symptoms.
Methods
Subjects and Clinical Characteristics
The subjects in this report were prospectively recruited from the authors’ (GSS and CPG) outpatient tertiary GI practices, as well as from campus-based advertisements from 2009 to 2013. All study participants who agreed to participate completed seven days of sleep actigraphy monitoring and daily log of GI and extraintestinal symptoms, as detailed below. In addition, comprehensive multidimensional symptom, affective state and quality of life questionnaires also were implemented. At the conclusion of the one-week study period, subjects returned their actigraph watches and daily logs for data analysis. A total of 51 individuals were approached to participate, and only one IBS patient did not complete the study. The IBS group was composed of adult patients (≥18 years old) who were both clinically diagnosed with IBS by a gastroenterologist and met Rome III diagnostic criteria19. All IBS patients had organic bowel disease excluded via a comprehensive evaluation performed at the discretion of the treating gastroenterologist. The control group was comprised of healthy individuals over 18 years of age without ROME III criteria for any functional GI disorder, prior gastrointestinal diagnoses, or active GI symptoms. Exclusion criteria for both study groups included a history of structural GI illness, prior GI surgery, major medical illness, history of alcohol or substance abuse, or history of a sleep disorder or sleep apnea. Use of sleep medications (as needed, less than once a week) prior to enrollment was acceptable, but was not permitted during study participation. IBS patients were continued on all previously prescribed medications, including antidepressants at a stable medication dose without adjustment for at least four weeks prior to enrollment. Using pilot data estimates of mean waking episodes during sleep of 10±4 in IBS and 8±4 in controls, a sample size of 50 subjects was calculated to allow a power of 0.80, alpha =0.05 to detect a statistically significant difference in waking episodes in the study groups. The study protocol was approved by the Human Research Protection Office (Institutional Review Board) at Washington University School of Medicine and Barnes-Jewish Hospital, St. Louis, Missouri.
Study Measures
Demographics and Medical History
Gender, age, race, body mass index, and marital status were recorded. Medical history included documented past medical history of IBS, other GI- and non-GI functional disorders, sleep disorders, and other GI illnesses, current medications, tobacco and alcohol use.
GI Symptom Measures
The ROME III Research Diagnostic Questionnaire was administered to establish presence of ROME III-defined functional GI disorders20. The GI Symptom Rating Scale for IBS (GSRS-IBS) was administered as a validated rating scale, consisting of fifteen items assessing prototypical IBS symptoms21. GI symptom burden within the two weeks preceding enrollment was used to evaluate symptom Severity, Bother and Frequency. GI symptom Severity and Bother were assessed with 10-cm Visual Analog Scales (VAS), using previously described methods22. Symptom frequency (total number of symptomatic days) within the preceding two weeks was quantified (0 to 14 days).
During the 7-day enrollment period, a daily bowel symptom log was completed before bedtime each evening, which recorded bowel symptom Severity and Bother (10-point Likert scale), accessory bowel symptoms (Bloating/Distention, Gas/Flatus, Mucus and Urgency along a 5-point Likert scale where 1 = “none/mild” and 5 = “severe”), the number of bowel movements over the preceding 24 hours, and a Bristol stool scale for the predominant bowel pattern that day23.
Somatic Symptom Measures
At baseline, all participants completed the Patient Health Questionnaire-15 (PHQ-15) as a validated assessment of somatic symptoms, including headache, arthralgia, back pain, and fatigue24. Further, a daily assessment of common non-GI symptoms were assessed in a symptom log completed each evening (Back/Hip pain, Headache, Neck/Shoulder pain, Achiness, Muscle/Joint pain, Fatigue, and Sexual dysfunction where 1 = “none/mild” and 5 = “severe”).
Psychological Assessments
The Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI), validated 21 question multiple-choice self-reported inventory measuring the severity of depression and anxiety over the previous 4 weeks were used to screen for these mood disorders25,26. The Visceral Sensitivity Index (VSI) was employed as a validated15-item self-report questionnaire used to measure GI specific anxiety27, 28.
Quality of Life Measures
The IBS-QOL is a validated measure of IBS-specific HRQOL29, with overall scores averaging 63.2 ±18.5 in IBS samples. The Work Productivity and Activity Impairment questionnaire for IBS (WPAI-IBS) consists of 6 items and is a validated measure used to quantify the effects of IBS on productivity and daily activities; outcomes are expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity30. The short form 36 (SF-36) was used as a validated assessment of health status and its impact on general HRQOL31, 32. The SF-36 is normalized to a maximal score of 100 (higher scores indicating better HRQOL), and divided into physical and mental domains. This assesses the role that medical conditions and pain have on physical and emotional well-being, and on limitation of day-to-day and pleasurable activities.
Sleep-specific Measures
All subjects also completed a National Sleep Foundation Daily Sleep Log (www.sleepfoundation.org) with questions regarding subjective sleep quality (estimated time to fall asleep, number of awakening at night, and sleep duration in hours). The log, completed in the morning to reflect the previous night’s sleep, also allowed free-form recording of any perceived issues which may have interrupted sleep (e.g., pain, urination, dreams, and environmental factors). The Pittsburgh Sleep Quality Index (PSQI) was completed at the conclusion of the monitoring period as a validated retrospective self-report measure of sleep quality and disturbance. Individuals with sleep problems or poor sleep quality have higher PSQI scores [Minimum Score = 0 (better); Maximum Score = 21 (worse)], with scores >5 being regarded as reflecting poorer sleep quality33.
Actigraph Recording
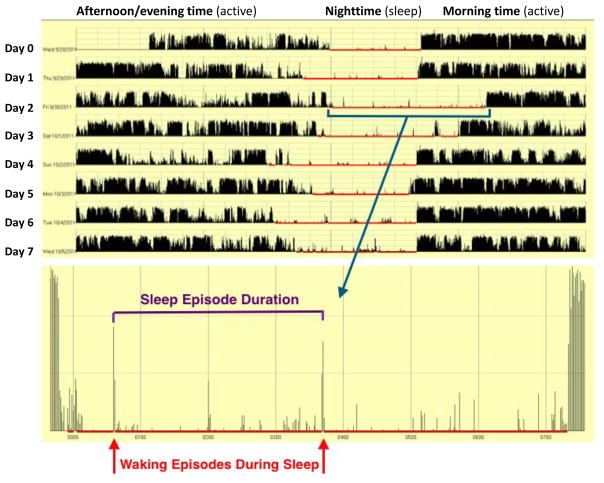
Subjects wore a wrist mounted actigraph monitor (Motionlogger, Ambulatory Monitoring, Ardsley, NY) on their non-dominant hand continuously for the duration of the 7-day study period. The uploaded data was analyzed on ActionW software version 2.7. Subjects were permitted to remove the monitor to brief periods of time (e.g. bathing), but were instructed to record these events both using the ‘Event’ button on the actigraph, and in their daily logs. Actigraphy has been validated as an objective measure of sleep when compared to polysomnography and offers the advantage of ‘real world’ testing34. Actigraphy parameters of particular interest included mean sleep episode duration, waking episodes during sleep, longest undisturbed sleep episode, and total hours of sleep per day (Figure 1). Additionally, every subject’s daily actigraph recording was manually compared to the patient’s daily sleep log by a single, blinded investigator (AP) to ensure accuracy of the automated actigraph interpretation algorithm in classifying sleep status.
Figure 1. Example of sleep actigraphy from a study subject.
Actigraph tracing from a 7-day study period (time along x-axis), and measured activity (y-axis) Sleeping hours (red) are magnified to show detail, with waking episodes during sleep (red arrows) are recorded via patterned movement. Sleep episode duration (purple interval) is defined by the time between waking episodes.
Statistical Analysis
Grouped values are reported as mean, standard error of mean, and 95% confidence intervals or medians with associated range, where appropriate. Between-group comparisons were performed using Student’s t-tests for continuous variables, or independent samples median and Mann-Whitney U testing for non-parametric measures; Chi-square or Fisher’s exact analyses were carried out on binomial data as indicated. In each case, p<0.05 was required for statistical significance. Pearson correlations were performed to establish: 1) the relationship between the objective sleep actigraphy measures and following-day reports of GI- and somatic symptoms; 2) to determine the association of subjective reports of sleep quality with the actigraphy data; and 3) to assess the relationship of anxiety measures with sleep quality. In order to conduct these analyses, each patient-day was regarded as a discrete data point. Univariate linear regression models were developed to assess the actigraphy sleep measures on overall and IBS-specific QOL, as well as reports of recent IBS symptoms. Sobel mediational analyses which included actigraphy sleep measures (waking episodes), generalized and visceral-specific anxiety, and depression measures were performed in order to explore if effect of sleep on IBS symptoms might be mediated by concomitant mood disturbances. Pre-conditionally, all of the variables to be included in the analysis were required to be significantly correlated using Pearson correlations, and linear regression models were developed in including the independent (e.g., waking episodes during sleep) and dependent variable of interest (IBS symptoms on GSRS-IBS), followed by second order models which included the mediational variables of interest (e.g., VSI, HADS anxiety) in order to determine the unstandardized regression coefficients and their standard errors. Statistical analysis was carried out using SPSS 22 software (IBM, Armonk, New York).
Results
Patient Characteristics
A total of 50 subjects (mean 38.6 ±1.0 years, 44 female) were enrolled. Baseline demographic and clinical characteristics are shown in Table 1. The median age and gender distribution between IBS patients and healthy controls were nearly identical, though systematic matching was not employed. There was no statistically significant difference between the two groups in regards to BMI, race, marital status, or tobacco use. Healthy controls were slightly more educated, and reported greater rates of employment. IBS patients had significantly higher rates of depression, general anxiety and visceral-specific anxiety symptoms as compared to the controls (p<0.001). The IBS group also reported significantly more severe, bothersome, and frequent bowel symptoms than their healthy counterparts (p<0.001 for all), with more bowel symptoms across all GSRS domains (p<0.05 for all except Constipation), poorer IBS-QOL (p<0.001) and general HRQOL (SF-36; p=0.004), as anticipated. Tricyclic medication use was higher in the IBS group (p=0.03), as expected given its common use in IBS management. This IBS study population did not statistically differ from our overall IBS clinic population with regard to demographics, baseline GI symptom severity/frequency, HRQOL, or psychological measures.
Table 1.
Baseline Demographics and Clinical Characteristics
| Irritable bowel syndrome (n=24) | Healthy controls (n=26) | p value | |
|---|---|---|---|
| Median age (range) | 44 (18 – 70) | 45 (20 – 72) | 0.60 |
| Female gender (%) | 18 (90%) | 19 (83%) | 0.67 |
| Caucasian | 12 (60%) | 13 (57%) | 0.81 |
| Married | 10 (50%) | 12 (52%) | 0.88 |
| At Least Some College Education | 16 (80%) | 23 (100%) | 0.04 |
| Employed full- or part-time | 14 (70%) | 22 (96%) | 0.04 |
| Current Tobacco Use | 3 (15%) | 1 (4.3%) | 0.23 |
| Body mass index (BMI, kg/m2) | 27.7 ±1.1 | 25.8 ±1.4 | 0.31 |
| Bowel symptom Severity [VAS]1 | 5.6 ±0.7 | 1.7 ±0.4 | <0.001 |
| Bowel Symptom Bother [VAS]1 | 5.7 ±0.7 | 1.6 ±0.4 | <0.001 |
| Bowel Symptom Frequency 2 | 6 (0 – 14) | 1 (0 – 5) | <0.001 |
| GSRS-IBS Pain | 6.7 ±3.2 | 2.8 ±1.1 | <0.001 |
| GSRS-IBS Bloat | 8.9 ±4.1 | 4.8 ±2.2 | 0.001 |
| GSRS-IBS Constipation | 4.2 ±2.9 | 2.9 ±1.5 | 0.09 |
| GSRS-IBS Diarrhea | 9.6 ±6.5 | 5.1 ±1.4 | 0.005 |
| HADS: Depression subscale | 3.8 ±2.9 | 2.1 ±1.9 | <0.001 |
| HADS: Anxiety subscale | 8.7 ±4.7 | 4.0 ±3.7 | <0.001 |
| Visceral Sensitivity Index (VSI) | 39.5 ±4.8 | 5.7 ±1.5 | <0.001 |
| Patient Health Questionnaire (PHQ-15) | 11.6 ±1.0 | 6.3 ±0.9 | <0.001 |
| SF-36 Total | 61.3 ±5.3 | 80.4 ±2.9 | 0.004 |
| Mental subscale | 61.9 ±5.1 | 61.3 ±5.3 | 0.008 |
| Physical subscale | 56.8 ±5.8 | 77.0 ±3.5 | 0.006 |
| IBS-QOL Total | 69.7 ±4.0 | 98.3 ±0.7 | <0.001 |
| WPAI-IBS (Activity impairment, % time) | 33.3% | 2.0% | <0.001 |
| Tricyclic antidepressant use | 6 (25.0%) | 1 (3.8%) | 0.03 |
| Other antidepressant use | 4 (16.7%) | 1 (3.8%) | 0.13 |
| Sleep aide use | 7 (29.2%) | 3 (11.5%) | 0.12 |
Measured along a 10-cm Visual Analog Scale (0=“Not severe/bothered at all”, 10 = “Extremely severe/bothered”)
Subjective Sleep Data
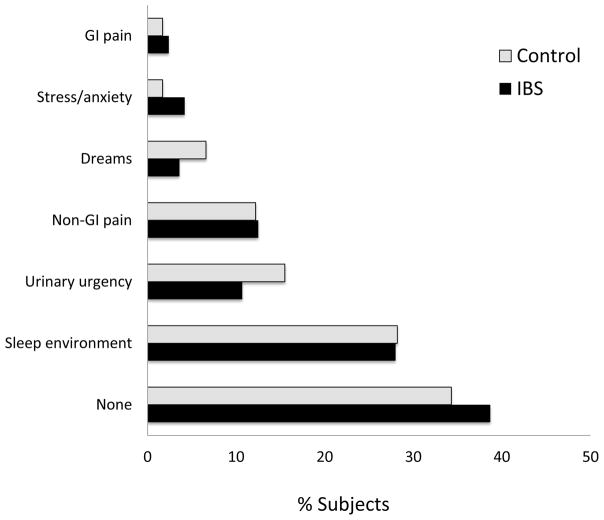
Fewer IBS patients reported waking up feeling ‘refreshed’ or ‘somewhat refreshed’, (72.6% of controls vs 55.8% of IBS patients, p=0.01). The most commonly cited reason for disturbed sleep was the sleep environment (Figure 2). Only 2.4% of IBS patients attributed their sleep disturbances to GI symptoms, most attributed sleep disturbances to nocturia and non-GI pain such as back pain, headaches and arthralgias. IBS patients perceived similar sleep duration per night as healthy individuals (approximately 7 hours), but reported having significantly increased number of awakenings per night when compared to controls (2.3 ±0.2 vs. 1.7 ±0.1, p<0.001; Table 2). Using the validated PSQI questionnaire, there were significant differences in subjective sleep quality between the two groups (p=0.025).
Figure 2.
Subjective etiologies of sleep disturbances by study group.
Table 2.
Subjective Sleep Ratings and Activity Prior to Sleep
| Irritable bowel syndrome (n=24) | Healthy controls (n=26) | p value | |
|---|---|---|---|
| Subject estimates of sleep | |||
| Mean sleep duration, hours | 6.9 ±0.3 | 6.9 ±0.1 | 0.99 |
| Mean time to fall asleep, min | 23.6±2.9 | 19.1±1.5 | 0.16 |
| Mean number of awakenings per night | 2.3 ±0.2 | 1.7±0.1 | <0.001 |
| Subjective sleep quality (%) | |||
| ’Refreshing’ | 19.6% | 21.1% | 0.01 |
| ’Somewhat refreshing’ | 36.2% | 51.5% | |
| ’Not refreshing (fatigued)’ | 44.2% | 27.5% | |
| Sleep disturbance experienced (%) | 61.3% | 66.7% | |
| Etiology of sleep disturbance | χ2=5.7, 0.45 | ||
| None | 38.7% | 34.3% | |
| Sleep environment | 28.0% | 28.2% | |
| Urinary urgency | 10.7% | 15.5% | |
| Non-GI pain | 12.5% | 12.2% | |
| Dreams | 3.6% | 6.6% | |
| Stress/anxiety | 4.2% | 1.7% | |
| GI pain | 2.4% | 1.7% | |
| Pittsburgh Sleep Quality Index (PSQI) | |||
| PSQI Total Score* | 8.5 ±1.1 | 5.4 ±0.8 | 0.025 |
| Sleep Disturbance+† | 2 (1–3) | 1 (0–3) | 0.08 |
| Days dysfunction+† | 1 (0–3) | 1 (1–3) | 0.07 |
| Sleep latency+† | 1 (1–3) | 1 (0–3) | 0.22 |
| Sleep Quality+† | 1 (1–3) | 1 (0–3) | 0.046 |
| Activities prior to retiring to bed (% total nights) | |||
| Physical activity/exercise | 15.5% | 13.7% | 0.54 |
| Consumed caffeine | 21.4% | 15.4% | 0.22 |
Score range: minimum score of 0 (better) to maximum score of 21 (worse); > 5 associated with poor sleep quality;
Score ranges: minimum Score of 0 (better) to Maximum Score of 3 (worse).
Score reported as median (range) values with significance determined using non-parametric (Mann Whitney U) testing
Actigraphy Sleep Data
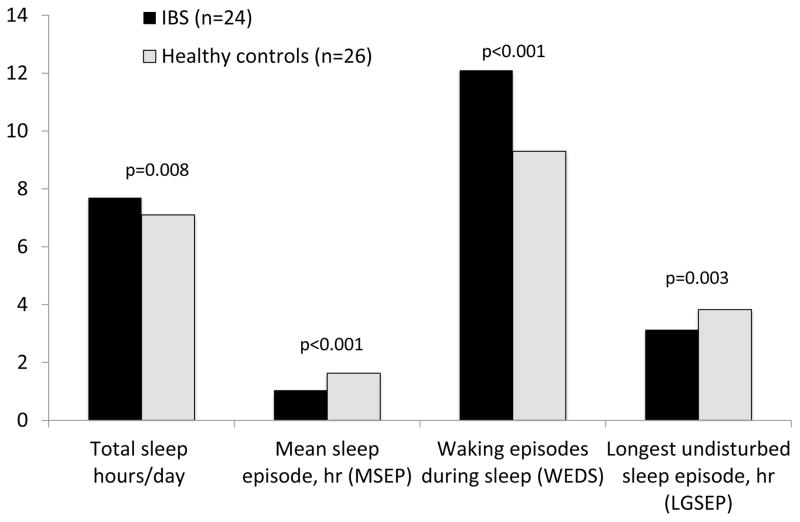
In contrast to subjective daily log reports, objective actigraph data revealed IBS patients spent significantly greater amount of time sleeping each day compared to controls (7.7 ±0.2 vs. 7.1 ±0.1 respectively, p=0.008). IBS patients also had a greater number of waking episodes during sleep than controls (12.1 ±0.6 vs. 9.3 ±0.5, p<0.001). As a result, the IBS group had significantly shorter mean sleep episode duration and longest undisturbed sleep episode (p<0.001 and p=0.003, respectively; Figure 3). Actigraph measures of disturbed sleep correlated well with total Pittsburgh Sleep Quality (r=0.43, p<0.001) in addition to several PSQI subdomains; however, actigraphy did not correlate with self-reported sleep on daily logs (hours of sleep or sleep awakenings, r<0.12, p>0.15 for each).
Figure 3. Actigraphy parameters by study group.
Actigraphy parameters of particular interest included mean sleep episode duration (hours) waking episodes during sleep, longest undisturbed sleep episode (hours) and total hours of sleep per day.
Given the known potential influences of tricyclic antidepressants (TCAs) on sleep, a post hoc analysis of IBS patients on and off tricyclic medications was performed. Thus, we first compared the sleep parameters among IBS patients on TCA (n=6) compared to those not on TCA (n=18). No significant differences were found in the IBS subgroups on- or off TCAs in terms of sleep quality (number of waking episodes, sleep duration, or longest undisturbed sleep; p>0.30 for each). However, significant differences in actigraphy sleep measures persisted when IBS patients not taking TCAs (n=18) were compared to healthy controls (p<0.03 for each).
Relationship of Disturbed Sleep and GI Symptom Data in IBS
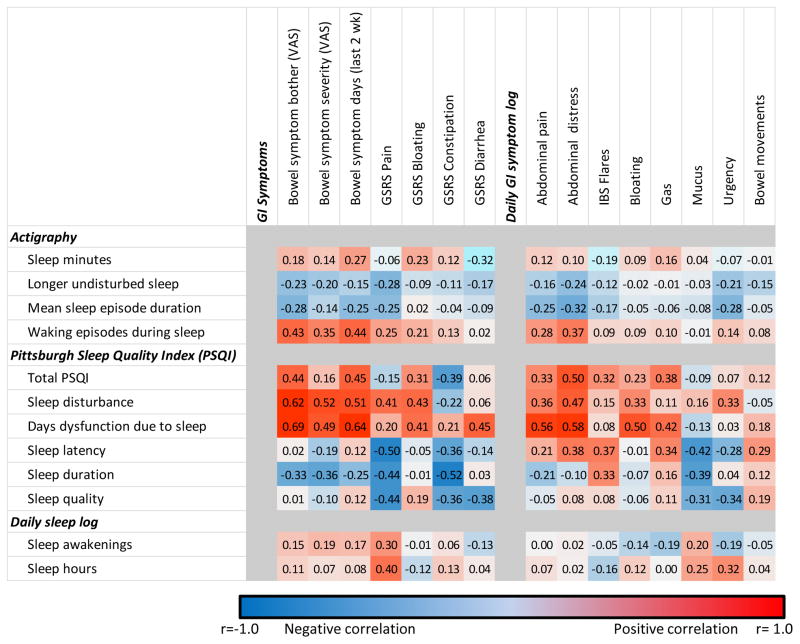
Number of waking episodes during sleep ( ‘waking episodes’) was utilized as a representative measure of sleep disturbance (correlations between waking episodes and longest- and mean sleep episodes were r= −0.50, r= −0.62 respectively, p<0.001 for each). Significant relationships were found between waking episodes and abdominal pain ratings (VAS, GSRS, and daily symptom log all (r ≥0.25, p≤0.01 for each) as well as GI Bother (r=0.43, p<0.001) and daily abdominal distress (r=0.37, p<0.001) as shown in Figure 4. Greater number of symptomatic days also were related to disturbed sleep (p<0.001). Sleep disturbances did not, however, have significant relationships with bowel pattern (Constipation or Diarrhea on the GSRS; p>0.18 for each) or with other bowel symptoms such as daily reports of bloating, mucus, gas or urgency (p>0.3 for each). Similar results were obtained when comparing waking episodes during sleep and GI symptoms. In contrast to what was seen with IBS patients, healthy controls had no significant relationship with sleep disturbances, any of the measures of abdominal pain or bowel pattern (data not shown). Self-reported sleep on the Pittsburgh Sleep Quality Index (PSQI), including the total PSQI score, sleep disturbance, and sleep latency domains correlated well with self-reported GI pain ratings, including bloating and daily rating of abdominal distress. As with PSQI data, self-reported sleep disturbances correlated with abdominal pain on the GSRS (r=0.29, p=0.003), but demonstrated much weaker associations with other GI symptom domains, including bowel symptom Bother and Frequency as well as GSRS non-pain symptoms (p>0.05 for each).
Figure 4. Correlation matrix between GI symptoms and actigraphy, subjective sleep report in IBS patients.
Correlations are color coded as indicated by the legend, with positive correlations shown in red, and negative correlations in blue. The color intensity reflects the strength of the correlation. GSRS = Gastrointestinal Symptom Rating Scale.
Relationship of Disturbed Sleep on Health-Related Quality of Life (HRQOL) in IBS
Actigraphic measured waking episodes demonstrated robust relationships with patient reports of both general- and IBS-specific HRQOL in the IBS cohort (r= −0.58 and −0.52 respectively, p<0.001 for each) (Figure 5). Similarly, several Pittsburg Sleep Quality domains correlated well with HRQOL, including total PSQI, sleep disturbances, and days of dysfunction due to sleep (SF-36: r=−0.58 to −0.65, p<0.001; IBS-QOL: r=−0.53 to −0.56, p<0.001). Daily sleep log reports of sleep quality were not significantly associated with HRQOL measures.
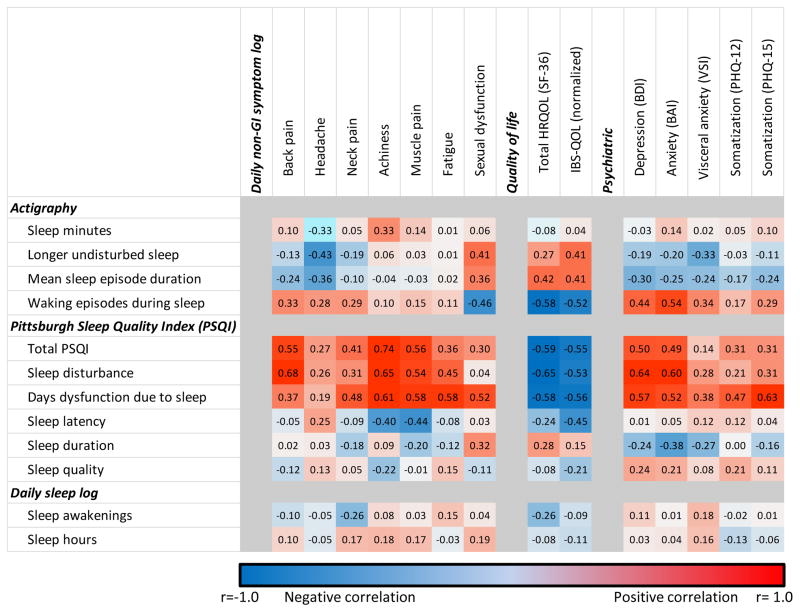
Figure 5. Correlation matrix between non-GI symptoms, quality of life, psychatric symptoms and actigraphy, subjective sleep report in IBS patients.
Correlations are color coded as indicated by the legend, with positive correlations shown in red, and negative correlations in blue. The color intensity reflects the strength of the correlation.
Relationship of Disturbed Sleep and Non-GI Symptoms and Psychiatric Features in IBS
Greater numbers of waking episodes detected on actigraphy significantly correlated with several non-GI pain symptoms (back pain, headache, neck pain; r≥0.28, p≤0.01 for each) and somatoform complaints (PHQ-12: r=0.17, p=0.036). Total PSQI also correlated well with these specific pain symptoms and somatoform complaints (r=0.27 to 0.54, p≤0.05 for each) as well as for the more general symptoms of achiness, muscle pain, and fatigue (≥0.35, p<0.005 for each). Daily sleep logs did not exhibit statistical relationships with these non-GI symptoms.
Mood measures, including depression, anxiety and GI specific-anxiety all correlated significantly with waking episodes on actigraphy (r≥0.34, p<0.001 for each), and several PSQI measures (total score, sleep disturbance, and days dysfunction due to sleep; r≥0.28, p≤0.009 for each, the exception being VSI, r=0.14, p=0.2). Linear regression models established that mood measures influenced following-day abdominal pain independently of the effects of sleep (waking episodes) in the IBS patients (B≥0.097, p ≥0.03 for each). Mediation analysis exploring the relationship of sleep disturbances and mood suggested that sleep effects on abdominal pain symptoms may be partially mediated by the influence of disordered sleep on mood, particularly depression and visceral specific anxiety (Supplemental figure 1).
Discussion
Our findings reveal objective sleep disturbances to be a key factor in the expression IBS symptoms. Abdominal pain and distress were perturbed by disordered sleep the preceding night, while bowel pattern and accessory IBS symptoms seem less affected by sleep quality. Sleep quality similarly was associated with general and IBS-specific HRQOL. We found the influences of sleep disturbances to extend beyond the gut, with relationships observed between sleep disruption and somatic pain (i.e., back pain, headache, neck pain), depression, and anxiety (generalized and visceral-specific). To our knowledge, this is the first study to prospectively assess the broad influence of sleep disturbances on the symptoms and function of the IBS patient using both objective and subjective measures.
Sleep disorders are very common, with symptoms affecting as many as 70 million Americans8, 35. One in three individuals reporting sleep disturbances meet clinical criteria for IBS36. As many as 74% of IBS patients characterize themselves as “poor sleepers”37; polysomnography has shown that IBS patients experience shallow, non-restorative sleep38. In our study, we found that IBS patients had similar sleep latency patterns and sleep duration as healthy controls, but had poorer sleep quality, with greater numbers of awakenings during sleep. Intuitively, it might be presumed that the poor sleep quality among IBS sufferers results from nighttime GI symptoms, preventing the onset of restorative sleep cycles, or leading to arousal from sleep. However, our data did not suggest either to be the case: IBS patients infrequently (less than 5% of the time) identified their GI symptoms to be a factor leading to sleep disruption. Longitudinal studies suggest that self-reported daytime IBS symptoms do not predict sleep quality the subsequent night10. Observational evidence suggests that circadian disturbances instead may have a causative role in GI symptoms. Nojkov et al. found that nurses working rotating shifts had a significantly higher prevalence of IBS diagnoses and greater abdominal pain compared to their peers working fixed schedules39. Similar data among medical residents revealed a 30% increased likelihood of an IBS diagnosis for every additional hour of on-call sleep deprivation.40. In the clinical laboratory, Schey and colleagues were able to induce visceral hypersensitivity in GERD patients following sleep deprivation11. Collectively, these studies offer supportive evidence of a causal relationship of disturbed sleep augmenting visceral hypersensitivity in IBS.
In the current study, we observe that disturbed sleep, particularly waking episodes after sleep onset, strongly correlated with worse abdominal pain the following day in IBS patients. Additionally, sleep quality predicted IBS patient reports of GI symptom Bother, Severity, and number of symptomatic days. However, we did not find sleep to be a significant factor in bowel pattern or accessory IBS symptoms (e.g., bloating, mucus, and urgency). These findings align with a previous exploratory study by Buchanan et al which found that measured sleep quality did not predict following-day reports of non-pain GI symptoms12. Sleep disturbances also were associated with a variety of non-GI pain complaints, including headache, back pain, and neck pain in our IBS participants.
Sleep disturbances are known to have profound physiologic consequences, including increases in pro-inflammatory cytokines and cortisol levels, while at the same time diminishing parasympathetic tone41. Such physiologic effects are particularly relevant to inflammatory bowel conditions, such as Crohn’s disease, where patients exhibit poorer sleep quality. 42 Downstream enhancements in nociception across a variety of noxious stimuli (e.g., heat, pressure) following sleep deprivation have been demonstrated in both animal models and healthy subjects43. Disruptions in sleep also affect somatic pain syndromes such as fibromyalgia, where sleep complaints and abnormal sleep physiology commonly is detected44. These physiologic and clinical observations suggest that a generalized hyperalgesia may result from sleep disturbances, perhaps as a result of central sensitization of spinal sensory neurons or more proximal sensory neurocircuitry45. Awakenings during sleep, as were observed prominently among our IBS group, are known to be detrimental to pain-inhibitory function46. In IBS, several other factors have been demonstrated as relevant to visceral hypersensitivity, including alterations in neurotransmitters (e.g., serotonin), intestinal permeability, microinflammation, and the bacterial milieu47. The IBS patient population thus might be particularly susceptible to the untoward effect of sleep on sensitivity as a “second hit,” further exacerbating these gut-based derangements.
A multitude of correlational studies highlight the relationship of poor sleep and mood disturbances15, 48. From a physiologic view, sleep deprivation results in a diminished capacity to regulate emotional reactivity, leading to enhanced susceptibility to depression and/or anxiety49. This relationship bears mention as psychological comorbidities, including depression, anxiety, and somatisation all are common to IBS13 and are associated with more severe GI symptoms in affected individuals; 50, 51 moreover, mood and sensory function have shared representation in the emotional-arousal and cognitive modulation brain neurocircuitry. Though it was not the intent of this study to decipher the complex interplay of these factors, we found statistical evidence that the negative effects of sleep on abdominal pain may be partially explained by the influence of disturbed sleep on mood, particularly depression and bowel-centric anxiety.
Simple self-report instruments, such as the Pittsburgh Sleep Quality Index, which correlated well with actigraphic sleep measures in our study and demonstrated significant associations with reports of GI and non-GI pain symptoms. When detected, sleep disturbances may serve as a critical therapeutic target in the management of IBS, and could be easily measured using such questionnaires in the clinic setting. Previous work has suggested that a common treatment for insomnia, melatonin, may be helpful in decreasing IBS symptoms,52–54 the value of other insomnia therapies in IBS remain uninvestigated. Nevertheless, various treatment options, in particular non-pharmacologic approaches such as cognitive behavioral therapy, exercise, and meditation have shown promise in improving sleep hygiene and symptoms in patients with other chronic pain conditions55.
Strengths of this study included the prospective assessment of sleep quality using both objective (actigraphic) and validated self-report measures in a clinically-defined IBS population, and the implementation of psychiatric measures deemed potentially relevant in understanding the relationship of sleep and IBS. In studying a real-world referral IBS population, efforts were not undertaken to exclude patients on TCAs or hypnotics; importantly, we did not observe any differences in the key findings of the study when excluding these cases. We acknowledge this tertiary IBS population to be more severely symptomatic and burdened with greater psychological comorbidity than community-based IBS populations; while limiting somewhat our ability to generalize these findings, we feel that the pervasiveness of disordered sleep in the general population compels a broad interest in these observations. Lifestyle factors potentially relevant to sleep, such as caffeine and alcohol intake and physical activity also were examined; these activities did not appear to conspicuously affect our sleep results, and were reported by the minority of participants. Though patients endorsing sleep diagnoses, such as obstructive sleep apnea, were excluded from the study, we acknowledge that without formal polysomnographic studies it is possible that patients harboring undiagnosed sleep conditions could have been included in the study.
In summary, our findings suggest that sleep disturbances lead to greater abdominal and somatic pain reporting in IBS patients. Mood symptoms and health related quality of life also are negatively impacted by poor sleep quality in this population. Though additional work is required to elucidate the physiologic basis for these findings, the clinical detection of sleep issues using self-report measures may identify IBS patients who might benefit from targeted sleep interventions.
Supplementary Material
Sobel analyses suggest significant partial mediation of disturbed sleep effects on GI pain via increased depression and visceral specific anxiety.
Acknowledgments
Research support: National Institute of Health (NIDDK) K23 DK84113 (GSS) and Washington University School of Medicine Mentors in Medicine (MiM) Program (AP and BC)
Footnotes
Podium presentation in preliminary form at the Digestive Diseases Week San Diego, California May 2012
Roles: A Patel, M Kumar, GS Sayuk: study concept and design; data analysis and interpretation; drafting and revision of manuscript; study supervision; CP Gyawali: study concept, data interpretation; drafting and revision of manuscript; B Cassell, M Ciorba, E Vivio: data collection and analysis. Dr. Sayuk is the guarantor of the article.
None of the authors have any conflicts of interest to report. No writing assistance was obtained.
References
- 1.Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: a systematic review. The American journal of gastroenterology. 2002;97(8):1910–5. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 2.Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119(3):654–60. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- 3.de Roest RH, Dobbs BR, Chapman BA, et al. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. International journal of clinical practice. 2013;67(9):895–903. doi: 10.1111/ijcp.12128. [DOI] [PubMed] [Google Scholar]
- 4.Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2015 doi: 10.1111/apt.13167. [DOI] [PubMed] [Google Scholar]
- 5.Levy RL, Linde JA, Feld KA, Crowell MD, Jeffery RW. The association of gastrointestinal symptoms with weight, diet, and exercise in weight-loss program participants. Clin Gastroenterol Hepatol. 2005;3(10):992–6. doi: 10.1016/s1542-3565(05)00696-8. [DOI] [PubMed] [Google Scholar]
- 6.Johannesson E, Ringstrom G, Abrahamsson H, Sadik R. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World journal of gastroenterology: WJG. 2015;21(2):600–8. doi: 10.3748/wjg.v21.i2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolan-Sewell RT, Riley WT, Hunt CE. NIH State-of-the-Science Conference on Chronic Insomnia. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2005;1(4):335–6. [PubMed] [Google Scholar]
- 8.Khoury J, Doghramji K. Primary Sleep Disorders. Psychiatr Clin North Am. 2015;38(4):683–704. doi: 10.1016/j.psc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Fass R, Fullerton S, Tung S, Mayer EA. Sleep disturbances in clinic patients with functional bowel disorders. Am J Gastroenterol. 2000;95(5):1195–2000. doi: 10.1111/j.1572-0241.2000.02009.x. [DOI] [PubMed] [Google Scholar]
- 10.Jarrett M, Heitkemper M, Cain KC, Burr RL, Hertig V. Sleep disturbance influences gastrointestinal symptoms in women with irritable bowel syndrome. Digestive diseases and sciences. 2000;45(5):952–9. doi: 10.1023/a:1005581226265. [DOI] [PubMed] [Google Scholar]
- 11.Schey R, Dickman R, Parthasarathy S, et al. Sleep deprivation is hyperalgesic in patients with gastroesophageal reflux disease. Gastroenterology. 2007;133(6):1787–95. doi: 10.1053/j.gastro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Buchanan DT, Cain K, Heitkemper M, et al. Sleep measures predict next-day symptoms in women with irritable bowel syndrome. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2014;10(9):1003–9. doi: 10.5664/jcsm.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140–56. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 14.Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. The American journal of psychiatry. 2005;162(1):50–7. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- 15.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Jama. 1989;262(11):1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 16.Larsson MB, Tillisch K, Craig AD, et al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142(3):463–472. e3. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsenbruch S. How positive and negative expectations shape the experience of visceral pain. Handbook of experimental pharmacology. 2014;225:97–119. doi: 10.1007/978-3-662-44519-8_6. [DOI] [PubMed] [Google Scholar]
- 18.Van Oudenhove L, Aziz Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10(3):158–67. doi: 10.1038/nrgastro.2013.10. [DOI] [PubMed] [Google Scholar]
- 19.Drossman DACE, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead W, editors. Rome III: The Functional Gastrointestinal Disorders. McLean: Degnon Associates, Inc; 2006. [Google Scholar]
- 20.Sperber AD, Shvartzman P, Friger M, Fich A. A comparative reappraisal of the Rome II and Rome III diagnostic criteria: are we getting closer to the ‘true’ prevalence of irritable bowel syndrome? European journal of gastroenterology & hepatology. 2007;19(6):441–7. doi: 10.1097/MEG.0b013e32801140e2. [DOI] [PubMed] [Google Scholar]
- 21.Svedlund J, Sjodin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Digestive diseases and sciences. 1988;33(2):129–34. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 22.Bengtsson M, Ohlsson B, Ulander K. Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS-IBS) BMC gastroenterology. 2007;7:16. doi: 10.1186/1471-230X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riegler G, Esposito I. Bristol scale stool form. A still valid help in medical practice and clinical research. Techniques in coloproctology. 2001;5(3):163–4. doi: 10.1007/s101510100019. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64(2):258–66. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of general psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of consulting and clinical psychology. 1988;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 27.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Alimentary pharmacology & therapeutics. 2004;20(1):89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 28.Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69(1):89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- 29.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Digestive diseases and sciences. 1998;43(2):400–11. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 30.Reilly MC, Bracco A, Ricci JF, Santoro J, Stevens T. The validity and accuracy of the Work Productivity and Activity Impairment questionnaire--irritable bowel syndrome version (WPAI:IBS) Aliment Pharmacol Ther. 2004;20(4):459–67. doi: 10.1111/j.1365-2036.2004.02091.x. [DOI] [PubMed] [Google Scholar]
- 31.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Garratt AM, Ruta DA, Abdalla MI, Buckingham JK, Russell IT. The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? Bmj. 1993;306(6890):1440–4. doi: 10.1136/bmj.306.6890.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26(1):81–5. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 35.Johnson DA. Gastroesophageal reflux disease and sleep disorders: a wake-up call for physicians and their patients. Rev Gastroenterol Disord. 2005;5(Suppl 2):S3–11. [PubMed] [Google Scholar]
- 36.Vege SS, Locke GR, 3rd, Weaver AL, Farmer SA, Melton LJ, 3rd, Talley NJ. Functional gastrointestinal disorders among people with sleep disturbances: a population-based study. Mayo Clinic proceedings. 2004;79(12):1501–6. doi: 10.4065/79.12.1501. [DOI] [PubMed] [Google Scholar]
- 37.Goldsmith G, Levin JS. Effect of sleep quality on symptoms of irritable bowel syndrome. Digestive diseases and sciences. 1993;38(10):1809–14. doi: 10.1007/BF01296103. [DOI] [PubMed] [Google Scholar]
- 38.Rotem AY, Sperber AD, Krugliak P, Freidman B, Tal A, Tarasiuk A. Polysomnographic and actigraphic evidence of sleep fragmentation in patients with irritable bowel syndrome. Sleep. 2003;26(6):747–52. doi: 10.1093/sleep/26.6.747. [DOI] [PubMed] [Google Scholar]
- 39.Nojkov B, Rubenstein JH, Chey WD, Hoogerwerf WA. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol. 2010;105(4):842–7. doi: 10.1038/ajg.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells MM, Roth L, Chande N. Sleep disruption secondary to overnight call shifts is associated with irritable bowel syndrome in residents: a cross-sectional study. The American journal of gastroenterology. 2012;107(8):1151–6. doi: 10.1038/ajg.2011.486. [DOI] [PubMed] [Google Scholar]
- 41.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism: clinical and experimental. 2006;55(10 Suppl 2):S20–3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 42.van Langenberg DR, Papandony MC, Gibson PR. Sleep and physical activity measured by accelerometry in Crohn’s disease. Aliment Pharmacol Ther. 2015;41(10):991–1004. doi: 10.1111/apt.13160. [DOI] [PubMed] [Google Scholar]
- 43.Schuh-Hofer S, Wodarski R, Pfau DB, et al. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain. 2013;154(9):1613–21. doi: 10.1016/j.pain.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 44.Diaz-Piedra C, Di Stasi LL, Baldwin CM, Buela-Casal G, Catena A. Sleep disturbances of adult women suffering from fibromyalgia: A systematic review of observational studies. Sleep medicine reviews. 2015;21:86–99. doi: 10.1016/j.smrv.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Chee MW, Chuah LY. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Current opinion in neurology. 2008;21(4):417–23. doi: 10.1097/WCO.0b013e3283052cf7. [DOI] [PubMed] [Google Scholar]
- 46.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 47.Mertz H. Review article: visceral hypersensitivity. Alimentary pharmacology & therapeutics. 2003;17(5):623–33. doi: 10.1046/j.1365-2036.2003.01447.x. [DOI] [PubMed] [Google Scholar]
- 48.Ford DE, Cooper-Patrick L. Sleep disturbances and mood disorders: an epidemiologic perspective. Depression and anxiety. 2001;14(1):3–6. doi: 10.1002/da.1041. [DOI] [PubMed] [Google Scholar]
- 49.Gruber R, Cassoff J. The interplay between sleep and emotion regulation: conceptual framework empirical evidence and future directions. Current psychiatry reports. 2014;16(11):500. doi: 10.1007/s11920-014-0500-x. [DOI] [PubMed] [Google Scholar]
- 50.Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. The American journal of gastroenterology. 2011;106(10):1749–59. doi: 10.1038/ajg.2011.201. quiz 1760. [DOI] [PubMed] [Google Scholar]
- 51.Vu J, Kushnir V, Cassell B, Gyawali CP, Sayuk GS. The impact of psychiatric and extraintestinal comorbidity on quality of life and bowel symptom burden in functional GI disorders. Neurogastroenterol Motil. 2014;26(9):1323–32. doi: 10.1111/nmo.12396. [DOI] [PubMed] [Google Scholar]
- 52.Lu WZ, Gwee KA, Moochhalla S, Ho KY. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2005;22(10):927–34. doi: 10.1111/j.1365-2036.2005.02673.x. [DOI] [PubMed] [Google Scholar]
- 53.Mozaffari S, Rahimi R, Abdollahi M. Implications of melatonin therapy in irritable bowel syndrome: a systematic review. Current pharmaceutical design. 2010;16(33):3646–55. doi: 10.2174/138161210794079254. [DOI] [PubMed] [Google Scholar]
- 54.Siah KT, Wong RK, Ho KY. Melatonin for the treatment of irritable bowel syndrome. World journal of gastroenterology: WJG. 2014;20(10):2492–8. doi: 10.3748/wjg.v20.i10.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roehrs TA, Workshop P. Does effective management of sleep disorders improve pain symptoms? Drugs. 2009;69(Suppl 2):5–11. doi: 10.2165/11531260-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sobel analyses suggest significant partial mediation of disturbed sleep effects on GI pain via increased depression and visceral specific anxiety.