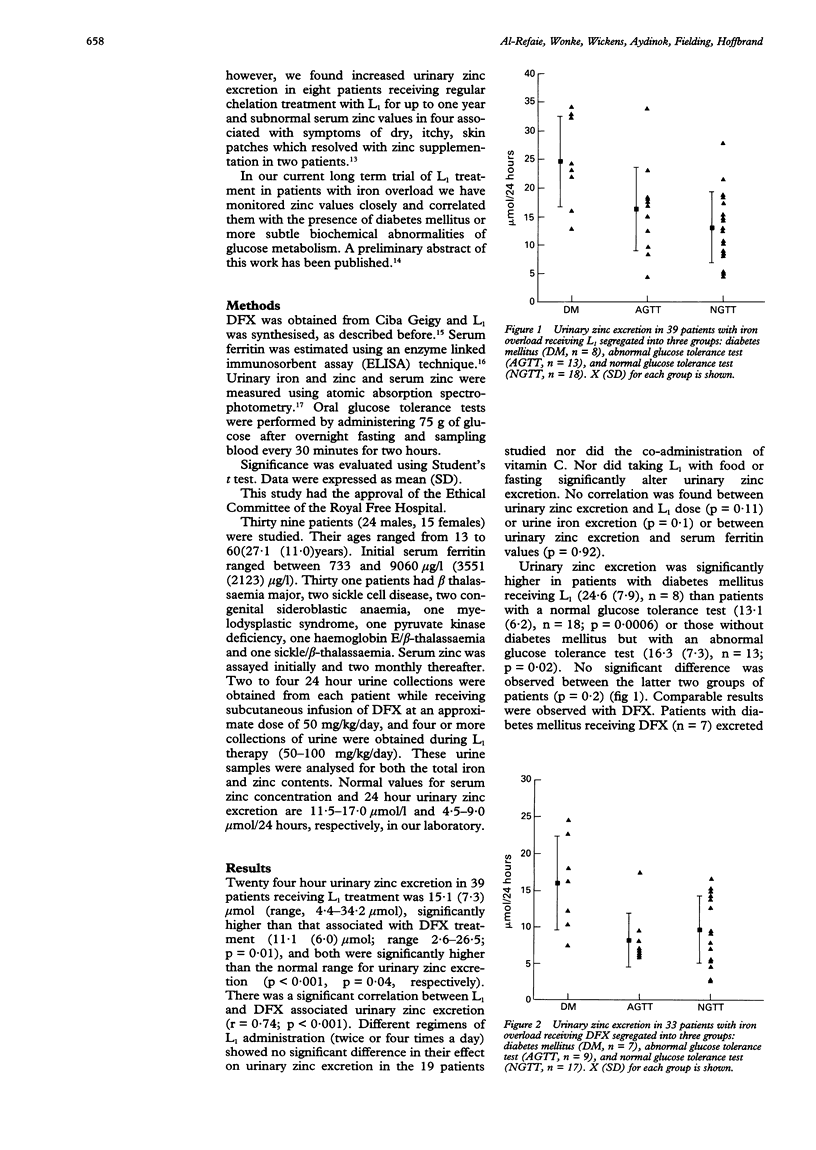
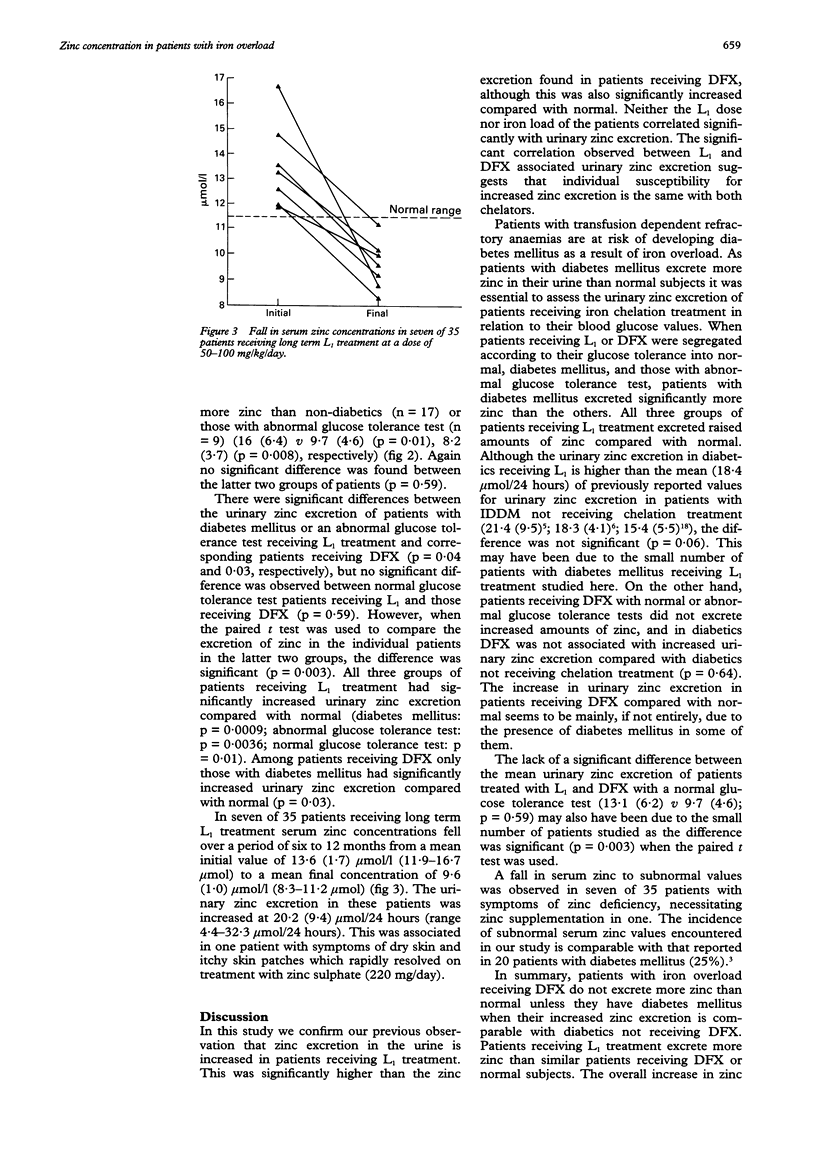
Abstract
AIMS--To determine the changes in serum zinc concentration and the extent of urinary zinc excretion in patients with iron overload receiving the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1) or desferrioxamine (DFX), and to correlate these results with blood glucose concentration. METHODS--Serum zinc and ferritin concentrations, urinary zinc and iron excretion were regularly assayed in 39 patients and the glucose tolerance test (GTT) was performed in each patient. Patients were segregated according to their GTT into normal, diabetic, and those with an abnormal GTT. The mean of L1- or DFX associated urinary zinc excretion for each group was determined and compared with the other two groups and with normal value. L1 associated urinary zinc excretion was also compared with L1 dose, serum ferritin values, and urinary iron excretion. RESULTS--Both DFX and L1 were associated with a significantly increased urinary zinc excretion (15.1 (7.3) mumol/24 hours, 11.1 (6.0) mumol/24 hours, respectively) compared with normal subjects. In patients receiving DFX this increase only occurred in patients with diabetes mellitus. Both diabetic and non-diabetic patients receiving L1 treatment excreted more zinc than normal. Diabetic patients receiving L1 or DFX excreted more zinc than non-diabetics receiving the same treatment. No correlation was found between urinary zinc excretion and L1 dose or patients' serum ferritin concentrations. In seven patients receiving long term L1 treatment a fall in serum zinc was observed from an initial 13.6 (1.6) mumol/l to a final 9.6 (0.8) mumol/l. In one patient this was associated with symptoms of dry skin and itchy skin patches requiring treatment with oral zinc sulphate. CONCLUSIONS--In contrast to DFX, L1 treatment is associated with increased zinc loss. This, however, is modest and does not lead in most patients to subnormal serum zinc concentrations. In a few patients whose negative zinc balance may give rise to symptoms, zinc supplementation rapidly corrects the deficit.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craft N. E., Failla M. L. Zinc, iron, and copper absorption in the streptozotocin-diabetic rat. Am J Physiol. 1983 Feb;244(2):E122–E128. doi: 10.1152/ajpendo.1983.244.2.E122. [DOI] [PubMed] [Google Scholar]
- Flowers C. A., Kuizon M., Beard J. L., Skikne B. S., Covell A. M., Cook J. D. A serum ferritin assay for prevalence studies of iron deficiency. Am J Hematol. 1986 Oct;23(2):141–151. doi: 10.1002/ajh.2830230209. [DOI] [PubMed] [Google Scholar]
- Honnorat J., Accominotti M., Broussolle C., Fleuret A. C., Vallon J. J., Orgiazzi J. Effects of diabetes type and treatment on zinc status in diabetes mellitus. Biol Trace Elem Res. 1992 Jan-Mar;32:311–316. doi: 10.1007/BF02784616. [DOI] [PubMed] [Google Scholar]
- Kiilerich S., Hvid-Jacobsen K., Vaag A., Sørensen S. S. 65 zinc absorption in patients with insulin-dependent diabetes mellitus assessed by whole-body counting technique. Clin Chim Acta. 1990 Jul;189(1):13–18. doi: 10.1016/0009-8981(90)90229-l. [DOI] [PubMed] [Google Scholar]
- Kinlaw W. B., Levine A. S., Morley J. E., Silvis S. E., McClain C. J. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med. 1983 Aug;75(2):273–277. doi: 10.1016/0002-9343(83)91205-6. [DOI] [PubMed] [Google Scholar]
- Mahajan S. K. Zinc in kidney disease. J Am Coll Nutr. 1989 Aug;8(4):296–304. doi: 10.1080/07315724.1989.10720305. [DOI] [PubMed] [Google Scholar]
- Maldonado Martín A., Gil Extremera B., Fernández Soto M., Ruiz Martínez M., González Jiménez A., Guijarro Morales A., de Dios Luna del Castillo J. Zinc levels after intravenous administration of zinc sulphate in insulin-dependent diabetes mellitus patients. Klin Wochenschr. 1991 Sep 16;69(14):640–644. doi: 10.1007/BF01649424. [DOI] [PubMed] [Google Scholar]
- Mooradian A. D., Morley J. E. Micronutrient status in diabetes mellitus. Am J Clin Nutr. 1987 May;45(5):877–895. doi: 10.1093/ajcn/45.5.877. [DOI] [PubMed] [Google Scholar]
- Pippard M. J., Jackson M. J., Hoffman K., Petrou M., Modell C. B. Iron chelation using subcutaneous infusions of diethylene triamine penta-acetic acid (DTPA). Scand J Haematol. 1986 May;36(5):466–472. doi: 10.1111/j.1600-0609.1986.tb02282.x. [DOI] [PubMed] [Google Scholar]
- Prasad A. S., Oberleas D. Thymidine kinase activity and incorporation of thymidine into DNA in zinc-deficient tissue. J Lab Clin Med. 1974 Apr;83(4):634–639. [PubMed] [Google Scholar]
- Scudder P. R., Al-Timimi D., McMurray W., White A. G., Zoob B. C., Dormandy T. L. Serum copper and related variables in rheumatoid arthritis. Ann Rheum Dis. 1978 Feb;37(1):67–70. doi: 10.1136/ard.37.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonke B., Hoffbrand A. V., Aldouri M., Wickens D., Flynn D., Stearns M., Warner P. Reversal of desferrioxamine induced auditory neurotoxicity during treatment with Ca-DTPA. Arch Dis Child. 1989 Jan;64(1):77–82. doi: 10.1136/adc.64.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Refaie F. N., Wonke B., Hoffbrand A. V., Wickens D. G., Nortey P., Kontoghiorghes G. J. Efficacy and possible adverse effects of the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1) in thalassemia major. Blood. 1992 Aug 1;80(3):593–599. [PubMed] [Google Scholar]


