Abstract
Background
Sedation is increasingly used to facilitate procedures on children in emergency departments (EDs). This overview of systematic reviews (SRs) examines the safety and efficacy of sedative agents commonly used for procedural sedation in children in the ED or similar settings.
Methods
We followed standard SR methods: comprehensive search; dual study selection, quality assessment, data extraction. We included SRs of children (1 month to 18 years) where the indication for sedation was procedure‐related and performed in the ED.
Results
Fourteen SRs were included (210 primary studies). The most data were available for propofol (six reviews/50,472 sedations) followed by ketamine (7/8,238), nitrous oxide (5/8,220), and midazolam (4/4,978). Inconsistent conclusions for propofol were reported across six reviews. Half concluded that propofol was sufficiently safe; three reviews noted a higher occurrence of adverse events, particularly respiratory depression (upper estimate 1.1%; 5.4% for hypotension requiring intervention). Efficacy of propofol was considered in four reviews and found adequate in three. Five reviews found ketamine to be efficacious and seven reviews showed it to be safe. All five reviews of nitrous oxide concluded it is safe (0.1% incidence of respiratory events); most found it effective in cooperative children. Four reviews of midazolam made varying recommendations. To be effective, midazolam should be combined with another agent that increases the risk of adverse events (upper estimate 9.1% for desaturation, 0.1% for hypotension requiring intervention).
Conclusions
This comprehensive examination of an extensive body of literature shows consistent safety and efficacy for nitrous oxide and ketamine, with very rare significant adverse events for propofol. There was considerable heterogeneity in outcomes and reporting across studies and previous reviews. Standardized outcome sets and reporting should be encouraged to facilitate evidence‐based recommendations for care.
Sedation is increasingly used to perform procedures on sick or injured children in emergency departments (EDs).1, 2, 3 Typical indications for procedural sedation include fracture/dislocation reduction, wound care, laceration repair, lumbar puncture, placement of a venous catheter, and diagnostic imaging.4
The American Academy of Pediatrics suggests five goals for sedating children during procedures: 1) to protect the patient's safety and welfare; 2) to minimize discomfort and pain; 3) to control anxiety, minimize psychological trauma, and maximize the potential for amnesia; 4) to control the patient's behavior and promote safe completion of the procedure; and 5) to return the patient to a state in which discharge from medical care is safe.5
Emergency providers have long sought an agent that is universally efficacious as well as safe. In searching for this ideal medication, healthcare practitioners have used many different agents (alone or in combination) at varied doses via multiple routes. Researchers have attempted to evaluate these sedatives and analgesics in clinical trials, with varied results. The purpose of this review was to conduct a comprehensive synthesis of existing evidence to evaluate the safety and efficacy of sedative agents commonly used for procedural sedation in children in the ED or similar settings.
Methods
In September 2013, a comprehensive search was conducted in the following biomedical databases: Medline (from 1946), Embase (from 1980), CDSR (from 2005), DARE (3rd Quarter 2013), HTA (3rd Quarter 2013), International Pharmaceutical Abstracts (from 1970) all via the Ovid platform, and CINAHL via EBSCO (from 1937; all databases in Data Supplement S1, available as supporting information in the online version of this paper). The Medline search was updated in November 2014, and PubMed (limited to the last 180 days) was also searched.
Reference lists of the included reports were scanned for potentially relevant reviews. Websites of relevant agencies were screened: Agency for Healthcare Research and Quality, Australian Government National Health and Medical Research Council, Canadian Agency for Drugs and Technologies in Health, Canadian Medical Association Infobase, National Institute for Health and Care Excellence, National Guidelines Clearinghouse, PROSPERO registry of systematic reviews, and Scottish Intercollegiate Guidelines Network. Citations of included reviews were forward searched in Web of Science and Google Scholar.
A priori we planned to include systematic reviews involving children (1 month to 18 years) where the indication for sedation was procedure‐related and performed in the ED. Children under continuous sedation while intubated were excluded. Interventions included propofol (+/− opioid), ketamine, ketamine/propofol combined, nitrous oxide, and midazolam, administered via any route of administration, using any dose. Our primary outcome was safety, defined broadly as any side effect, adverse effect, or adverse event. Secondary outcomes included serious intervention for an adverse event, efficacy (i.e., successful completion of the procedure, level/depth of sedation), length of sedation, and length of stay in the ED.
Adverse events were based on published recommendations from a consortium of North American clinicians:6 oxygenation (desaturation requiring an intervention), central apnea requiring intervention, obstructive apnea, laryngospasm, pulmonary aspiration, retching/vomiting, bradycardia requiring intervention, hypotension requiring intervention, excitatory movements (myoclonus, muscle rigidity or generalized motor seizure), behavioral reactions (paradoxic response to sedation, unpleasant recovery reaction), permanent neurologic injury, death, or any other effect not previously mentioned.
Two reviewers independently screened search results for potentially relevant reviews and examined the full text of these reviews to determine if they fulfilled our inclusion criteria. Discrepancies were resolved through discussion and where necessary a third reviewer.
The AMSTAR tool was used to assess the methodological quality of the included reviews (amstar.ca). Two reviewers assessed the studies independently and resolved discrepancies through discussion.
One reviewer extracted the characteristics, outcomes, and conclusions of the included systematic reviews into structured tables. A second reviewer verified data for completeness and accuracy. Disagreements were resolved through discussion.
Results are described narratively based on reporting in the systematic reviews. Data on adverse events are presented in tables based on how they were presented in the review, i.e., counts with number of patients or number of sedations as the denominator or rates per 10,000 patients.
Results
From 2,882 unique references, 14 completed systematic reviews7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 were included (Figure 1, Table 1). Three protocols for in‐process reviews were identified on propofol21 and midazolam22 for procedural sedation and structured sedation programs in acute care settings;23 since the protocols provide no data they are not included in this review. The number of primary studies across all reviews was 435; however, not all studies were relevant to our topic (e.g., some included both pediatric and adult populations or settings other than the ED). The following results pertain to the 210 relevant primary studies (Data Supplements S2 and S3, available as supporting information in the online version of this paper).
Figure 1.
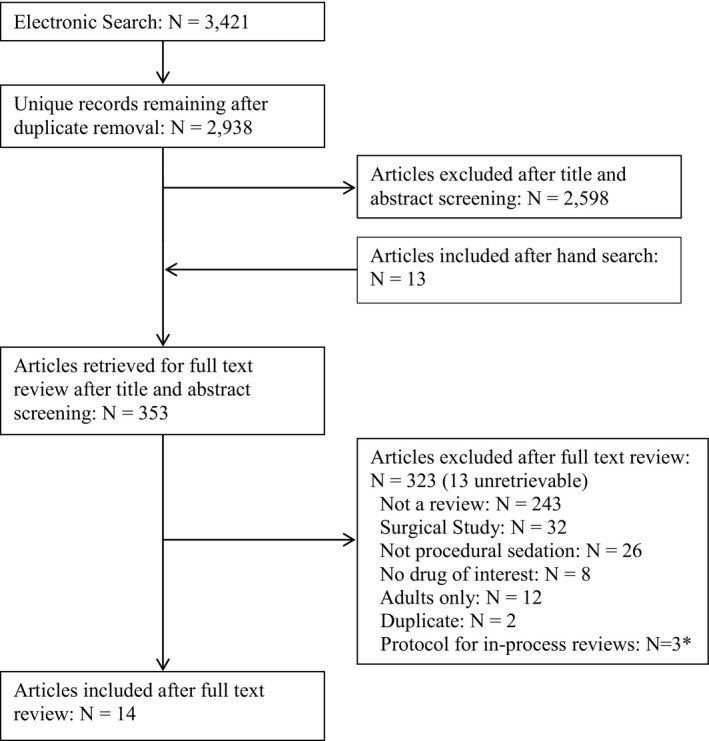
Flow diagram of articles through the review. *Protocols did not include any data for inclusion in the results of this review.
Table 1.
Description of Included Reviews by Sedative Agent
| Sedative agent | Publication Year of Reviews (Range) | No. of Reviews Included in Analysis | No. of Studies Included in Original Reviewa | No. of Studies Included in This Overviewa, b | No. of Sedations Included in Overviewa | AMSTAR Score (Maximum 11)a |
|---|---|---|---|---|---|---|
| Ketamine | 2005–2010 | 7 | 14 (12–99) | 14 (2–32) | 3,052 (2,604–8,238c) | 4 (2–7) |
| Midazolam | 2004–2011 | 4 | 57.5 (4–99) | 4.5 (4–13) | 1,857.5 (301–4,978) | 3.5 (2–7) |
| Nitrous oxide | 2005–2013 | 5 | 26 (12–99) | 9 (3–9) | 116 (58–8,220)d | 4 (1–7) |
| Propofol | 2004–2010 | 6 | 51.5 (8–99) | 4.5 (1–7) | 862 (89–50,472) | 3.5 (3–7) |
Data are reported as median (range).
Study involved children (1 month to 21 years) and indication for use of sedation was procedure‐related and was performed in the ED only.
Green reported different denominators for different outcomes; the highest denominator is reported here.
Does not include review by Pedersen as not all study sample sizes were reported.
Ten reviews specified they were interested in procedural sedation performed only in the ED or for emergency care,7, 9, 10, 11, 12, 13, 14, 15, 17, 18 while four included sedations carried out in a range of settings.8, 16, 19, 20 Laceration repair and fracture reduction were the most commonly specified procedures; however, most reviews included any procedure requiring pain control. The identified reviews investigated ketamine, midazolam, nitrous oxide, and propofol often in combination with another of the drugs of interest or with other sedatives or analgesics (e.g., fentanyl). No relevant reviews examining ketamine/propofol combined and dexmedetomidine were located.
Overall, the quality of the included reviews was poor with 12 of the 14 reviews scoring 4 or less out of 11 on the AMSTAR tool (Table 2). Two reviews scored 7 out of 11.12, 20 The majority of reviews met the criteria for appropriate methods used to combine studies (14/14) and reported characteristics of included studies (12/14). Fewer reviews met the criteria for incorporated scientific quality into the conclusions (9/14), performed a comprehensive search (5/14), assessed scientific quality of included studies (5/14), and provided an a priori design (3/14). Only one review satisfied the criteria for duplicate study selection and data extraction, searched for studies regardless of publication type, provided a list of included and excluded studies, and reported conflicts of interest. No review assessed for publication bias.
Table 2.
Methodologic Quality of Included Systematic Reviews
| AMSTAR Question | Deasy 20109 | Faddy 200513 | Green 2009a10 | Green 2009b11 | Howes 200415 | Lamond 20108 | Leroy 201019 | Mace 200417 | Migita 200612 | Mistry 200514 | NICE 201020 | Pedersen 201316 | Symington 200618 | Jameson 20117 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was an a priori design provided? | No | No | Yes | Yes | No | No | No | No | No | No | Yes | No | No | No |
| 2. Was there duplicate study selection and data extraction? | No | Can't answer | Can't answer | Can't answer | Can't answer | No | No | No | Yes | No | No | No | No | No |
| 3. Was a comprehensive literature search performed? | Yes | No | No | No | Yes | No | Yes | No | Yes | No | Yes | No | No | No |
| 4. Did the authors search for reports regardless of the publication status? | Can't answer | Can't answer | No | No | Can't answer | No | Can't answer | No | Yes | No | No | No | No | Can't answer |
| 5. Was a list of studies (included and excluded) provided? | No | No | No | No | No | No | No | No | Yes | No | No | No | No | No |
| 6. Were the characteristics of the included studies assessed and documented? | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| 7. Was the scientific quality of the included studies assessed and documented? | No | Yes | No | No | No | Yes | No | Yes | No | No | Yes | No | No | No |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | No |
| 9. Were the methods used to combine the findings of studies appropriate? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Was the likelihood of publication bias assessed? | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| 11. Was the conflict of interest stated? | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No |
| Total score | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 4 | 7 | 2 | 7 | 1 | 3 | 2 |
Adverse events are reported in Tables 3, 4, 5. Many systematic reviews reported on a variety of measures to reflect sedation efficacy. Due to the heterogeneity of methods used to report these outcomes, it was challenging to report efficacy data in a meaningful and concise manner; therefore, we summarized efficacy based on the conclusions of the reviews (Table 6). The following sections provide a synthesis based on the primary drug of interest.
Table 3.
Frequency of Respiratory‐related Events Reported in Included Reviews
| Reviews | O2 Desaturation | Apnea | Laryngospasm | Aspiration | Assisted Ventilation |
|---|---|---|---|---|---|
| Ketamine | |||||
| Deasy 20109 |
46/1,202 (3.8%) 6 studies |
2/1,022 (0.2%) 1 study |
4/1,523 (0.3%) 2 studies |
— |
1/418 (0.3%) 3 studies |
| Green 200911 | — |
63/8,353 (0.8%) 32 studies |
22/8,353 (0.3%) 32 studies |
— | — |
| Howes 200415 |
7/835 (0.8%) 3 studies |
3/1,178 (0.3%) 2 studies |
7/1,851 (0.4%) 4 studies |
— |
0/130 2 studies |
| Mace 200417 |
77/646 (11.9%) 4 studies |
— |
15/1,130 (1.3%) 2 studies |
0/3,154 12 studies |
8/353 (2.3%) 2 studies |
| Migita 200612 |
36/184 (19.6%) 2 studies |
— | — | — |
17/184 (9.2%) 2 studies |
| Mistry 200514 |
19/1,347 (1.4%) 3 studies |
4/1,288 (0.3%) 2 studies |
8/1,288 (0.6%) 2 studies |
— | — |
| NICE 201020 |
131/3,600 (3.6%) 9 studies |
— |
91/1,492 (6.1%) 1 study |
— |
6/1,178 (0.5%) 2 studies |
| Midazolam | |||||
| Jameson 20117 |
0/102 1 study |
— | — | — | — |
| Leroy 201019 |
341/63,765 (9.1%) 3 studies |
11/3,765 (0.3%) 3 studies |
1/1,180 (0.08%) 1 study |
— |
3/2,424 (0.1%) 2 studies |
| Mace 200417 |
12/1,180 (1.0%) 1 study |
— |
1/1,180 (0.08%) 1 study |
— | — |
| NICE 201020 |
27/836 (3.2%) 4 studies |
— | — |
0/288 4 studies |
4/807 (0.5%) 4 studies |
| Nitrous Oxide | |||||
| Faddy 200513 |
1/762 (0.1%) 1 study |
— | — | — | — |
| Leroy 201019 | — | — | — |
0/220 1 study |
— |
| Migita 200612 |
1/709 (0.1%) 1 study |
— | — | — |
0/7,511 1 study |
| NICE 201020 |
4/5,799 (0.07%) 1 study |
— | — | — | — |
| Pedersen 201316 | 1 study | — | — | — | — |
| Propofol | |||||
| Lamond 20108 |
92/1,003 (9.2%) 7 studies |
17/1,003 (1.7%) 7 studies |
30/17,066a (0.2%) 60 studies |
0 |
11/1,003 (1.1%) 7 studies |
| Leroy 201019 |
19/393 (4.8%); 154b
2 studies |
3/291 (1.0%); 575b
2 studies |
4b
1 study |
4b
1 study |
3/445 (0.7%) 2 studies |
| Mace 201017 |
54/582 (9.3%) 4 studies |
— | — | — |
5/519 (1.0%) 3 studies |
| Migita 200612 |
5/43 (11.6%) 1 study |
— | — | — | — |
| NICE 201020 |
736/50,228 (1.5%) 2 studies |
— | — |
4/50,228 (0.008%) 2 studies |
3/392 (0.8%) 1 study |
| Symington 200618 |
47/587 (8.0%) 5 studies |
4/587 (0.7%) 5 studies |
— | — | — |
Data are reported as n/N (%) and number of studies reported.
Denominator represents all sedations analyzed in the review, not just those that occurred in the ED.
Outcomes reported per 10,000 patients.
Table 4.
Frequency of Cardiac‐related Events Reported in Included Reviews
| Reviews | Bradycardia | Hypotension Requiring Interventiona | Death |
|---|---|---|---|
| Ketamine | |||
| Deasy 20109 | — | — | — |
| Green 200911 | — | — | — |
| Howes 200415 | — | — | — |
| Mace 200417 | — | — | — |
| Migita 200612 | — | — | — |
| Mistry 200514 | — | — | — |
| NICE 201020 | — | — | — |
| Midazolam | |||
| Jameson 20117 | — | — | — |
| Leroy 201019 | — |
2/135 (1.5%) 3 studies |
— |
| Mace 200417 |
24/393 (6%) 1 study |
— | — |
| NICE 201020 | — | — | — |
| Nitrous Oxide | |||
| Leroy 201019 | — | — | — |
| Migita 200612 | — | — | — |
| NICE 201020 | — | — | — |
| Pedersen 201316 | — | — | — |
| Faddy 200513 | — | — | — |
| Propofol | |||
| Lamond 20108 | — |
25/465 (5.4%) 4 studies |
0 |
| Leroy 201019 | — |
0/52 (0.0%) 1 study |
0b
1 study |
| Mace 201017 |
24/393 (6.1%) 1 study |
— | — |
| Migita 200612 | — | — | — |
| NICE 201020 | — | — | — |
| Symington 200618 |
24/393 (6%) 1 study |
25/465 (5.4%) 4 studies |
— |
Data are reported as n/N (%) and number of studies reported.
Clinically significant hypotension only (i.e. hypotension requiring intervention).
Outcomes reported per 10,000 patients.
Table 5.
Frequency of Other Adverse Events Reported in Included Reviews
| Reviews | Emesis Without Aspiration | Pain with Injection | Paradoxical Reactions | Unpleasant Recovery Reactionsa |
|---|---|---|---|---|
| Ketamine | ||||
| Deasy 20109 |
310/2,525 (12.3%) 13 studies |
— | — |
213/2,102 (14.8%) 8 studies |
| Green 200910 |
694/8,353 (8.3%) 32 studies |
— | — |
630/8282 (7.6%) 32 studies |
| Howes 200415 |
159/2,251 (7.1%) 8 studies |
— | — |
93/1,720 (5.4%) 6 studies |
| Mace 200417 |
192/2,148 (8.9%) 10 studies |
— |
7/1,180 (0.8%) 1 study |
230/1,499 (15.3%) 5 studies |
| Migita 200612 |
12/130 (9.2%) 1 study |
— | — |
7/130 (5.4%) 1 study |
| Mistry 200514 |
176/1,584 (11.1%) 8 studies |
— | — |
268/1,755 (15.3%) 7 studies |
| NICE 201020 |
428/3,624 (11.8%) 11 studies |
— | — |
183/1,178 (15.5%) 3 studies |
| Midazolam | ||||
| Jameson 20117 | — | — | — | — |
| Leroy 201019 |
5/2,424 (0.2%) 2 studies |
— | — |
9/1244 (0.7%) 1 study |
| Mace 200417 |
4/1,180 (0.3%) 1 study |
— | — | — |
| NICE 201020 |
45/1,748 (2.6%) 5 studies |
— | — | — |
| Nitrous Oxide | ||||
| Leroy 201019 |
59/982 (6.0%) 2 studies |
— | — | — |
| Migita 200612 | — | — | — | — |
| NICE 201020 |
30/709 (4.2%) 2 studies |
— | — | — |
| Pedersen 201316 |
127/5,779 (2.2%) 1 study |
— | — | — |
| Faddy 200513 | 1 study | — | — | — |
| Propofol | ||||
| Lamond 20108 |
24/17,066b (0.1%) 60 studies |
951/17,066b (5.6%) 60 studies |
— | — |
| Leroy 201019 |
49c
1 study |
— | — | — |
| Mace 200417 | — | — | — | — |
| Migita 200612 | — |
3/43 (7.0%) 1 study |
— | — |
| NICE 201020 |
49/49,836 (0.1%) 1 study |
— | — | — |
| Symington 200618 |
1/393 (0.3%) 1 study |
7/194 (3.6%) 4 studies |
— | — |
Data are reported as n/N (%) and number of studies reported.
These were measured variably across studies and include: dysphoria (or dysphoric reactions), agitation (any, mild, moderate, severe), emergence reaction.
Denominator represents all sedations analyzed in the review, not just those that occurred in the ED.
Outcomes reported per 10,000 patients.
Table 6.
Conclusions on Safety and Efficacy of the Included Reviews
| Sedative Agent Review, Year of Publication (Indications for Sedation Included in Review) | Safety (+/−) | Efficacy (+/−) | Summary of Conclusions (With Respect to the Agents for Procedural Sedation in Children) |
|---|---|---|---|
| Ketamine | |||
| Deasy 20109 (any procedural sedation) | + | + | IV ketamine appears to have a better AE profile and ashorter recovery period. IV ketamine should be administered if access is available or if staff is skilled at initiating IV access. IM administration may be preferable if IV access is difficult. Brief procedures are believed to have the best recovery from IV administration. |
| NICE 201020 (painful or nonpainful diagnostic or therapeutic procedures) | + | + | IV and IM ketamine were shown to be equally effective. Smaller doses may be titrated via IV, which reduces the chance of sedation outlasting the procedure. Compared with midazolam‐fentanyl, ketamine‐midazolam was associated with lower pain and distress scores. Similar results were found for comparisons with propofol‐fentanyl, although ketamine‐midazolam had longer recovery time. Ketamine‐midazolam was associated with fewer oxygen desaturations in both comparisons. |
| Green 200910, 11 (any procedural sedation) | + | NA | Risk factors for ketamine‐associated AEs are high IV doses, administration to children aged <2 or >13 years, and the use of coadministered anticholinergics or benzodiazepines. Risks are not altered by route, oropharyngeal procedures, or underlying physical illness. Risk factors for any recovery agitation are low IM dose and unusually highIV dose, with no important risk factors for clinically important recovery agitation. The data did not support the regular or routine use of anticholinergics or benzodiazepines. |
| Migita 200612 (fracture reduction) | + | + | Ketamine was found to be the most effective of the parenteral treatments examined, although it has consistently longer recovery times than other agents. Ketamine‐midazolam therapy is associated with fewer AEs than other parenteral drug combinations. |
| Mistry 200514 (any conscious sedation) | + | + | Compared with traditional agents, ketamine is an effective agent with minimal AEs and sequelae. Administration via IV and IM routes are considered equally safe. However, administering physicians should be adequately trained in the use of ketamine and in airway management and resuscitation. Additionally, sufficient support personnel are required for patient management. |
| Howes 200415 (any painful procedure) | + | NA | Ketamine is safe and acceptable. Rare occurrences of serious AEs require experienced staff skilled in advanced airway maintenance, with adequate monitoring and resuscitation equipment available. |
| Mace 200417 (any painful procedure) | + | + | For brief, painful procedures ketamine is effective as a sole agent or in combination with a benzodiazepine. Ketamine can be safely used, but may require head positioning, supplemental oxygen, occasional bag‐valve‐mask ventilatory support, and measures to address laryngospasm. The addition of midazolam to ketamine does not decrease the incidence of emergent reactions, but does decrease the incidence of emesis. |
| Midazolam | |||
| Jameson 20117 (simple lacerations requiring suturing) | NA | − | In a comparison of midazolam versus ketamine, ketamine was recommended as sedative of choice as it offers quick, reliable sedation with minimal AEs and has rapid onset and offset time. Ketamine can be delivered via IM if venous access is difficult. |
| Leroy 201019 (any procedural sedation) | − | NA | During PS and its subsequent recovery phase the use of benzodiazepines, chloral hydrate, barbiturates, opiates, or combinations of these medicines pose a variable risk of potentially serious AEs, especially for respiratory depression and/or airway obstruction. For medicines such as chloral hydrate, midazolam, barbiturates, opiates, or combinations, the depth of sedation, effectiveness and duration of sedation, and timing of AEs cannot be reliably predicted. |
| NICE 201020 (painful or nonpainful diagnostic or therapeutic procedures) | + (alone) − (combination) | − (alone) + (combination) | Midazolam was the most common sedative investigated; however, it is probably not an effective sedative drug on its own and can be combined with fentanyl, ketamine, propofol, or NO. When doses are limited, midazolam alone had a good safety profile. In combination with ketamine, NO, or opioids, midazolam can produce deep sedation, which may result in harms; therefore, the AEs of multidrug sedation should be weighed against the benefit of pain relief for a procedure. |
| Mace 200417 (any painful procedure) | − | + | Fentanyl and midazolam are effective agents. The efficacy of IV fentanyl and midazolam ranges from 91% to 100%, which is similar to alternative agents. The analgesic and sedative effects of fentanyl may be increased when combined with a benzodiazepine. The combination of fentanyl and midazolam appears to have a greater risk of respiratory depression; therefore, clinicians should monitor patients for signs of respiratory depression and have appropriate training and support to treat apnea. |
| Nitrous oxide | |||
| Pedersen 201316 (brief, painful minor procedures) | + | + (not equal for all children) | For minor painful procedures NO is a safe and effective method to use to achieve sedation. Onset is rapid, quickly reversible, does not have major AEs, and can be safely administered by a dedicated staff member trained in basic airway management. NO is not equally effective for all children, and another method of pain management should be prepared in case of treatment failure. |
| Leroy 201019 (any procedural sedation) | + | NA | NO is associated with an extremely low chance of serious AEs. Risks include: 1) <1 year old and 2) simultaneous use of other sedatives. No significant difference in median fasting time between patients with and without emesis was found. NO 70% causes significantly deeper sedation compared to NO 50%; however, there is no significant difference in AE rates between regimens. |
| NICE 201020 (painful or nonpainful diagnostic or therapeutic procedures) | + | + | NO was not found to be more effective than oxygen alone in young uncooperative children; however, when children were cooperative NO provided sufficient analgesia in a wide range of painful procedures. Overall, NO was well tolerated, short acting, and highly effective in selected patient groups and settings. Occasional AEs include dysphoria and vomiting, but this may be related to higher concentrations. |
| Migita 200612 (fracture reduction) | ? | ? | Data are too limited to support this intervention's effectiveness or to make conclusions on its safety. NO does, however, have significantly shorter treatment times than other modalities. |
| Faddy 200513 (any procedural sedation) | + | + | Previously, NO 50% has been shown to have similar efficacy for pain relief compared to IV administered conventional analgesia including opioid analgesia. Side effects are uncommon and AEs (hypotension, oxygen desaturation) could not be attributed to NO inhalation. Recovery from sedative effects of NO is faster compared with IV analgesia. The side effect profile of this agent suggests that it could be used safely by adequately trained lay persons in the prehospital setting. NO 50% is an effective and safe form of analgesia. |
| Propofol | |||
| Lamond 20108 (any procedural sedation) | + | NA | Propofol used for procedural sedation is associated with a low risk of minor AEs. Confounding variables that influence the likelihood of these events include: adjunct opiates, propofol dosing strategies, and supplemental oxygen. Minor AEs for propofol are similar to those found for other ED sedation agents. Capnography provides useful clinical feedback about impending hypoventilation and apnea. Therefore, AE data for pediatric propofol sedation supports its ongoing use in the ED. |
| Leroy 201019 (any procedural sedation) | − | NA | Use of propofol for PS presents a real risk of potentially serious AEs, especially respiratory depression and/or airway obstruction. PS with propofol is equally safe when conducted by anesthesiologists versus nonanesthesiologists if the latter are well trained and part of a dedicated sedation team. |
| Mace 201017 (any painful procedure) | + | + | Propofol combined with opiate agents is effective in producing cooperation for painful procedures, as is propofol when given alone. Propofol is safe when given in combination with opiates and alone, but may require head positioning, supplemental oxygen, and occasional bag‐valve‐mask ventilatory support. |
| NICE 201020 (painful or nonpainful diagnostic or therapeutic procedures) | – | + | Propofol can be titrated to achieve any level of sedation. In comparison to other drug combinations, unconsciousness and airway effects are more likely with propofol, but are brief. Recovery after propofol is more rapid and airway obstruction or apnea can be managed with appropriate skills and equipment. |
| Migita 200612 (fracture reduction) | − | − | Propofol is not as effective as ketamine therapy and is associated with more AEs, particularly respiratory events and hypotension than other parental agents. Recovery time and total sedation time are shorter with propofol than other treatment modalities. |
| Symington 200618 (any procedural sedation) | + | + | Propofol can be used safely and effectively in the ED. Many studies appear to use deep sedation or general anesthesia, which is not recommended for nonanesthetists in the United Kingdom, and could be considered dangerous when patients are not fasted or fully prepared preprocedure. |
AE = adverse event; IM = intramuscular; IV = intravenous; NA = not analyzed; NO = nitrous oxide; PS = procedural sedation.
Nitrous oxide was examined in five reviews (nine studies, 8,220 sedations).12, 13, 16, 19, 20 All five reviews concluded that nitrous oxide can be safely used in procedural sedation outside the operating room. Authors agreed that the occurrence of serious adverse events were uncommon and that onset and recovery times were faster than other treatment modalities. Three reviews commented on efficacy, and overall found it was an effective agent.13, 16, 20 Pedersen et al.16 noted that nitrous oxide may not be equally effective for all children. The NICE review stated that nitrous oxide was not more effective than oxygen alone in sedating uncooperative children, but could be successfully used in a wide range of procedures for cooperative children.20
Four reviews investigated midazolam (13 studies, 4,978 sedations).7, 17, 19, 20 Authors made varying recommendations for midazolam use in procedural sedation in children as follows. Jameson7 stated that although midazolam has been the traditional sedative of choice, ketamine should be preferred for its rapid onset time, minimal adverse events, and intramuscular delivery option. Similarly, one review found that although midazolam was the most frequently investigated drug,20 it was likely not a sufficient sedative on its own. To obtain sufficient sedation they recommend that midazolam be combined with fentanyl, ketamine, propofol, or nitrous oxide. Mace et al.17 concluded that intravenous midazolam, when combined with fentanyl, provides an efficacy range of 91% to 100%, which is similar to other agents. However, they noted that midazolam combined with fentanyl demonstrated a greater risk for respiratory depression, which matched the findings of Leroy et al.19
Six reviews provided evidence on propofol (seven studies, 50,472 sedations).8, 12, 17, 18, 19, 20 Conclusions regarding the safety and efficacy of propofol varied across reviews; this may be dependent on whether the primary studies evaluated propofol alone or in combination with an analgesic. Half of reviews concluded that propofol was sufficiently safe for use in the ED,8, 17, 18 while three noted a higher occurrence of adverse events, particularly respiratory depression.12, 19, 20 The efficacy of propofol was considered in four of the reviews and found to provide adequate sedation in three.17, 19, 20 Migita12 stated that propofol was not as effective as ketamine, although recovery time with propofol was shorter than other agents.
Ketamine was examined in seven reviews (32 studies, 8,238 sedations).9, 10, 11, 12, 14, 15, 17, 20 All reviews considered ketamine safe for use in children requiring procedural sedation. Four reviews compared ketamine delivered via intramuscular versus intravenous routes. Deasy and Babl9 reported that intravenous administration resulted in a better adverse event profile and shorter recovery time; the other three reviews stated that the two routes were equally effective or equally safe.10, 11, 14, 20 In terms of efficacy, five of the reviews agreed that ketamine was an effective drug for sedation,9, 12, 14, 17, 20 while two reviews only examined safety.10, 11, 15
Interpretation
The choice of sedative agent depends on the efficacy and safety profile of the agent, as well as its relative safety and efficacy compared to other medications. Other practical considerations include the indication for sedation and depth of sedation required (i.e., this may vary for nonpainful, minor, and major painful procedures) and patient characteristics (e.g., preprocedural fasting; ASA physical status; and other anatomic, physiological, and developmental factors). Safe administration of any agent requires a thorough understanding of its effects, interactions, and sedation time intervals.6 Such knowledge promotes appropriate drug administration, particularly when multiple agents are used, in titrated doses, and avoids the potential for oversedation.24 This review focused on sedative agents commonly used in North America for children undergoing procedures in the ED.
For minor painful procedures in some cooperative children nitrous oxide was found to be safe and effective to achieve sedation and analgesia;13, 16, 19, 20 however, another method of pain management should be prepared in case of treatment failure.16 Nitrous oxide has significantly shorter treatment times than other modalities with rapid onset12 and is quickly reversible.13, 16, 20 Reported minor effects (nausea/vomiting, dizziness, voice change, dysphoria) were uncommon and major adverse effects (hypotension, oxygen desaturation) could not be attributed to nitrous oxide inhalation.13, 16, 19 Risk factors for adverse effects included patients <1 year old, simultaneous use of other sedatives,19 and depth of sedation. Both a challenge and an advantage to sedation with nitrous oxide is that it may be safely given by the children themselves via a self‐administered demand valve.4
Midazolam, administered through various routes, is the most commonly used benzodiazepine for procedural sedation. Midazolam alone was found not to provide reliable sedation for procedures.7, 20 Its safety, effectiveness and duration of sedation, and the timing of adverse effects could not be reliably predicted.19 Midazolam has been combined with fentanyl, ketamine, propofol, or nitrous oxide to produce deep sedation with analgesia, but is associated with adverse effects including apnea,19 laryngospasm,17, 19 bradycardia,17 and/or hypotension.19
Propofol, alone18 or when combined with opiates, can be titrated to achieve varied levels of sedation.17, 20 In one review it was not as effective as ketamine.12 The use of propofol for procedural sedation presents a risk of potentially serious adverse effects, especially respiratory depression, airway obstruction,17, 18, 20 and hypotension.12 These may be exacerbated when used with opioids. Propofol is associated with a low risk of adverse effects, including assisted ventilation,8, 17, 19, 20 desaturation,8, 12, 17, 18, 19, 20 emesis,8, 19, 20 and pain with injections.8, 18, 19
The advantage of propofol is that recovery time and total sedation time are shorter than other treatment modalities.12, 20 Further, propofol may require only a single loading dose to produce sedation.2, 4, 25 When provided by an experienced individual, propofol appears safe with high satisfaction ratings from patients and parents.2, 4 Propofol has been known to cause pain on injections, which may be reduced by administering a small amount of lidocaine prior to the propofol or delivering the sedative via a large vein.26
Ketamine has been widely used since 1970. It provides a unique dissociative state and is well tolerated and effective and preserves upper‐airway reflexes, making it ideal for sedation in the ED.2, 4, 25, 27 Consistently found to be one of the most effective medications for procedural sedation,12, 14, 15, 17, 20 ketamine can be titrated intravenously, or administered as a bolus intramuscularly.14, 20
When administered with midazolam, ketamine had a longer recovery time than propofol‐fentanyl20 and midazolam with fentanyl.12 Ketamine‐midazolam therapy was associated with fewer adverse effects than other parenteral drug combinations,12, 14 including fewer oxygen desaturations than midazolam‐fentanyl and propofol‐fentanyl,20 but patients may require head positioning, supplemental oxygen, occasional bag‐valve‐mask ventilatory support, and measures to address laryngospasm. The concurrent administration of midazolam with ketamine was not found to decrease the incidence of emergent reactions, but did decrease the incidence of emesis.17 The data did not support the regular or routine use of anticholinergics or benzodiazepines.10, 11 While equally safe according to one review,14 intravenous ketamine appears to have a better adverse effect profile and a shorter recovery period than intramuscular ketamine, which should be reserved for patients in whom intravenous access is difficult.9
A combination of ketamine and propofol was first used in the operating room in the early 1990s; however, we found no relevant systematic reviews of this drug combination. These drugs can be delivered premixed or sequentially with ketamine administered first, which allows the analgesic effect of the ketamine to occur first as well as to decrease injection site pain from propofol.28 Case series have shown that when in combination, lower doses of each drug can be used to provide effective sedation compared to when given as a monotherapy of either agent. Additional benefits of this combination include cardiovascular stability, airway preservation, reduced recovery agitation, and antiemetic properties. A short recovery time and high provider satisfaction rates may also make this combination desirable for use in children in the ED.28, 29
Limitations
This overview of systematic reviews provides a comprehensive synthesis of the literature examining commonly used agents for procedural sedation in children in the ED setting. There were several limitations stemming largely from the heterogeneity in outcomes, inconsistency in outcome assessment, and unclear reporting across this body of literature. The heterogeneity in terms of how efficacy is measured and reported across primary studies severely limits the ability of reviewers to synthesize this literature, compare efficacy across studies, and come to aggregate conclusions. Standardized outcome sets and reporting in primary studies should be encouraged to assist with future syntheses, which are key to providing evidence‐based recommendations for care. The results of this overview are limited to the specific procedures, dosages, and settings of the studies that were reviewed. Many reviews and primary studies pool data across indications, which did not allow us to assess efficacy/safety by indication.
Conclusions
This overview shows that there are safe and effective options to sedate children for painful procedures in the ED. For minor painful procedures in some cooperative children nitrous oxide was found to be a safe and effective method to achieve minimal sedation and analgesia. Midazolam alone was found not to provide effective and reliable sedation for procedures and when combined with other agents is associated with adverse events. There is mixed evidence for the efficacy and safety of propofol largely driven by evaluation with and without analgesia; desirable features, in particular the rapid onset and recovery time, need to be balanced with potential for respiratory depression and hypotension. There is consistent evidence supporting the efficacy and safety of ketamine, which underscores its value and widespread use for sedation in the ED.
Supporting information
Data Supplement S1. Complete search strategies for the electronic database search.
Data Supplement S2. Overlap of primary studies examined in the included systematic reviews.
Data Supplement S3. Count of medications investigated in studies included in analysis.
Academic Emergency Medicine 2016;23:519–530 © 2016 by the Society for Academic Emergency Medicine26858095
This work was supported by the Networks of Centres of Excellence and the Women and Children's Health Research Institute. The funders played no role in formulating the question, collecting and interpreting data, or reporting results. Dr. Lisa Hartling holds a New Investigator Salary Award from the Canadian Institutes of Health Research (CIHR).
The authors have no potential conflicts to disclose.
References
- 1. Havidich JE, Cravero JP. The current status of procedural sedation for pediatric patients in out‐of‐operating room locations. Curr Opin Anaesthesiol 2012;25:453–60. [DOI] [PubMed] [Google Scholar]
- 2. Doctor K, Roback MG, Teach SJ. An update on pediatric hospital‐based sedation. Curr Opin Pediatr 2013;25:310–6. [DOI] [PubMed] [Google Scholar]
- 3. Godwin SA, Burton JH, Gerardo CJ, et al. Clinical policy: Procedural sedation and analgesia in the emergency department. Ann Emerg Med 2014;63:247–58. [DOI] [PubMed] [Google Scholar]
- 4. Krauss BS, Krauss BA, Green SM. Procedural sedation and analgesia in children. N Engl J Med 2014;371:91. [DOI] [PubMed] [Google Scholar]
- 5. American Academy of Pediatrics; American Academy of Pediatric Dentistry ; Coté CJ, Wilson S; Work Group on Sedation . Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics 2006;118:2587–602. [DOI] [PubMed] [Google Scholar]
- 6. Bhatt M, Kennedy RM, Osmond MH, et al. Consensus‐based recommendations for standardizing terminology and reporting adverse events for emergency department procedural sedation and analgesia in children. Ann Emerg Med 2009;53:426–35. [DOI] [PubMed] [Google Scholar]
- 7. Jameson E. Question 3 Ketamine or midazolam: does it matter which? Arch Dis Child 2011;96:106–8. [DOI] [PubMed] [Google Scholar]
- 8. Lamond DW. Review article: Safety profile of propofol for paediatric procedural sedation in the emergency department. Emerg Med Australas 2010;22:265–86. [DOI] [PubMed] [Google Scholar]
- 9. Deasy C, Babl FE. Intravenous vs intramuscular ketamine for pediatric procedural sedation by emergency medicine specialists: a review. Paediatr Anaesth 2010;20:787–96. [DOI] [PubMed] [Google Scholar]
- 10. Green SM, Roback MG, Krauss B, et al. Predictors of emesis and recovery agitation with emergency department ketamine sedation: an individual‐patient data meta‐analysis of 8,282 children. Ann Emerg Med 2009;54:171–80. [DOI] [PubMed] [Google Scholar]
- 11. Green SM, Roback MG, Krauss B, et al.; Emergency Department Ketamine Meta‐Analysis Study Group . Predictors of airway and respiratory adverse events with ketamine sedation in the emergency department: an individual‐patient data meta‐analysis of 8,282 children. Ann Emerg Med 2009;54:158–68. [DOI] [PubMed] [Google Scholar]
- 12. Migita RT, Klein EJ, Garrison MM. Sedation and analgesia for pediatric fracture reduction in the emergency department: a systematic review. Arch Pediatr Adolesc Med 2006;160:46–51. [DOI] [PubMed] [Google Scholar]
- 13. Faddy SC, Garlick SR. A systematic review of the safety of analgesia with 50% nitrous oxide: can lay responders use analgesic gases in the prehospital setting? Emerg Med J 2005;22:901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mistry RB, Nahata MC. Ketamine for conscious sedation in pediatric emergency care. Pharmacotherapy 2005;25:1104–11. [DOI] [PubMed] [Google Scholar]
- 15. Howes MC. Ketamine for paediatric sedation/analgesia in the emergency department. Emerg Med J 2004;21:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pedersen RS, Bayat A, Steen NP, Jacobsson ML. Nitrous oxide provides safe and effective analgesia for minor paediatric procedures – a systematic review. Dan Med J 2013;60:A4627. [PubMed] [Google Scholar]
- 17. Mace SE, Barata IA, Cravero JP, et al. Clinical policy: evidence‐based approach to pharmacologic agents used in pediatric sedation and analgesia in the emergency department. Ann Emerg Med 2004;44:342–77. [DOI] [PubMed] [Google Scholar]
- 18. Symington L, Thakore S. A review of the use of propofol for procedural sedation in the emergency department. Emerg Med J 2006;23:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leroy PL, Schipper DM, Knape HJ. Professional skills and competence for safe and effective procedural sedation in children: recommendations based on a systematic review of the literature. Int J Pediatr 2010;2010:934298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Clinical Guideline Centre (UK) . NICE Clinical Guidelines, No. 112. Sedation in Children and Young People: Sedation for Diagnostic and Therapeutic Procedures in Children and Young People. London: Royal College of Physicians (UK), 2010. [PubMed] [Google Scholar]
- 21. Wakai A, Staunton P, Cummins F, O'Sullivan R. The use of propofol for procedural sedation in emergency departments. Cochrane Database Syst Rev 2015;7:CD007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morao SR, Bernardo O, Santos H, Sampaio C. Midazolam for sedation before procedures (Protocol). Cochrane Database Syst Rev 2011;12:CD009491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCoy S, Wakai A, Blackburn C, et al. Structured sedation programmes in the emergency department, hospital and other acute settings: protocol for systematic review of effects and events. Syst Reviews 2013;2:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet 2006;367:766–80. [DOI] [PubMed] [Google Scholar]
- 25. Mahajan C, Dash HH. Procedural sedation and analgesia in pediatric patients. J Pediatr Neurosci 2014;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jalota L, Kalira V, George E, et al. Prevention of pain on injection of propofol: systematic review and meta‐analysis. BMJ 2011;342:d1110. [DOI] [PubMed] [Google Scholar]
- 27. Green SM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation in children. Ann Emerg Med 2004;44:460–71. [DOI] [PubMed] [Google Scholar]
- 28. Alletag MJ, Auerbach MA, Baum CR. Ketamine, propofol, and ketofol use for pediatric sedation. Pediatr Emerg Care 2012;28:1391–5. [DOI] [PubMed] [Google Scholar]
- 29. Green SM, Andolfatto G, Krauss B. Ketofol for procedural sedation? Pro and con. Ann Emerg Med 2011;57:444–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement S1. Complete search strategies for the electronic database search.
Data Supplement S2. Overlap of primary studies examined in the included systematic reviews.
Data Supplement S3. Count of medications investigated in studies included in analysis.


