Summary
Centrosomes are major microtubule organizing centers (MTOCs) that play an important role in chromosome segregation during cell division. Centrosomes provide a stable anchor for microtubules, constituting the centers of the spindle poles in mitotic cells, and determining the orientation of cell division. However, visualization of centrosomes is challenging because of their small size. Especially in mouse tissues, it has been extremely challenging to observe centrosomes belonging to a specific cell type of interest among multiple comingled cell types. To overcome this obstacle, we generated a tissue‐specific centrosome indicator. In this mouse line, a construct containing a floxed neomyocin resistance gene with a triplicate polyA sequence followed by an EGFP‐Centrin1 fusion cassette was knocked into the Rosa locus. Upon Cre‐mediated excision, EGFP‐Centrin1 was expressed under the control of the Rosa locus. Experiments utilizing mouse embryo fibroblasts (MEFs) demonstrated the feasibility of real‐time imaging, and showed that EGFP‐Centrin1 expression mirrored the endogenous centrosome cycle, undergoing precisely one round of duplication through the cell cycle. Moreover, experiments using embryo and adult mouse tissues demonstrated that EGFP‐Centrin1 specifically mirrors the localization of endogenous centrosomes. genesis 54:286–296, 2016. © 2016 The Authors. Genesis Published by Wiley Periodicals, Inc.
Keywords: centriole, centrin, cell division, cell cycle
INTRODUCTION
Centrosomes play a major role as microtubule organizing centers (MTOCs), affect cytoskeletal architecture, the positioning of cell organelles, and provide the basal structure for cilia in quiescent cells (Nigg and Stearns, 2011). In cycling cells, the centrosome undergoes precisely one round of duplication from S through G2. In mitosis, each centrosome, constituted by a pair of centrioles, is located at the center of each spindle pole, determines the orientation of cell division, and is allocated into each daughter cell, thus the number of centrosomes is strictly regulated (Nigg and Stearns, 2011). Extra centrosomes have been postulated to cause cancer (Nigg and Stearns, 2011).
Centrosomes were discovered more than a century ago, as structures in the cytoplasm where spindle poles arise (Szollosi et al., 1972). During the last two decades, the centrosome cycle has been clarified (Piel et al., 2000), and mechanisms by which the number of centrosomes is regulated are an active area of investigation. Major obstacles to investigating centrosome dynamics are their small size. Centrosomes are constituted by a pair of two barrel‐shaped centrioles 0.5µm long, 0.2µm in diameter, and surrounded by a cloud of amorphorous pericentriolar matrix (PCM) (Nigg and Stearns, 2011; Bornens, 2012).
Centrin is a small protein concentrated at the distal lumen of each centriole, appearing as centrioles form (Paoletti et al., 1996). Cells stably expressing Centrin‐EGFP in conjunction with time‐lapse imaging have clarified centrosome dynamics (Piel et al., 2000; White et al., 2000). Two transgenic mouse lines have been generated to facilitate centrosome visualization. One contains CAG promoter driven GFP‐Centrin2, with expression dependent on CAG promoter activity and positional effects of transgene integration (Higginbotham et al., 2004). In this line, lack of cell type‐specificity makes it difficult to define centrosomes within cells of interest among comingled cell types within tissues. Another line contains keratine14 promoter‐driven GFP‐Centrin1, expressed only in skin, salivary gland, or mammary gland epithelium (Lechler and Fuchs 2005), and therefore not useful for other cell types.
To facilitate visualization of centrosome dynamics in vivo in a cell type specific manner, we generated a ROSA‐Neo‐EGFP‐Cetn1 mouse line, knocking a floxed neomyocin resistance gene with a triplicate polyA cassette followed by a cDNA encoding EGFP‐Centrin1 into the ROSA locus. In this line EGFP‐Centrin1 is expressed only in cells that have expressed Cre or their descendents. Experiments using embryonic fibroblasts demonstrated that EGFP‐Centrin1 expression mirrored previously reported centrosome dynamics (Piel et al., 2000). In mouse embryos, EGFP‐Centrin1 began to be expressed at blastocyst stages. As expected, numerous EGFP‐Centrin1 signals were observed in multiciliated epithelial cells, however, the number of EGFP‐Centrin‐1 signals marking dividing cells was strictly regulated (Roszko et al., 2006). The ROSA‐Neo‐EGFP‐Cetn1 mouse line thus provides a valuable tool for a broad range of investigators to explore centrosome biology.
RESULTS AND DISCUSSION
Generation of a Tissue‐Specific Centrosome Indicator Targeted into the Rosa Locus
As EGFP‐Centrin1 allows for centrosome visualization in vitro and in vivo, we cloned a cDNA encoding EGFP‐Centrin1 after a floxed neomycin resistance gene followed by triplicated poly A derived from simian virus 40 (SV40) into a vector targeting the Rosa locus (Fig. 1A). Four positive ESC clones were obtained from 250 Neomycin resistant clones (Fig. 1B). Two of four positive ESC clones were injected into blastocysts. Targeting vectors were properly targeted to the Rosa locus, confirming successful germline transmission (Fig. 1C,D). By crossing ROSA‐Neo‐EGFP‐Cetn1 mice to cardiac‐specific Nkx2.5‐Cre mice (Moses et al., 2001), EGFP‐Centrin1 was expressed specifically in heart, as expected (Fig. 1E).
Figure 1.
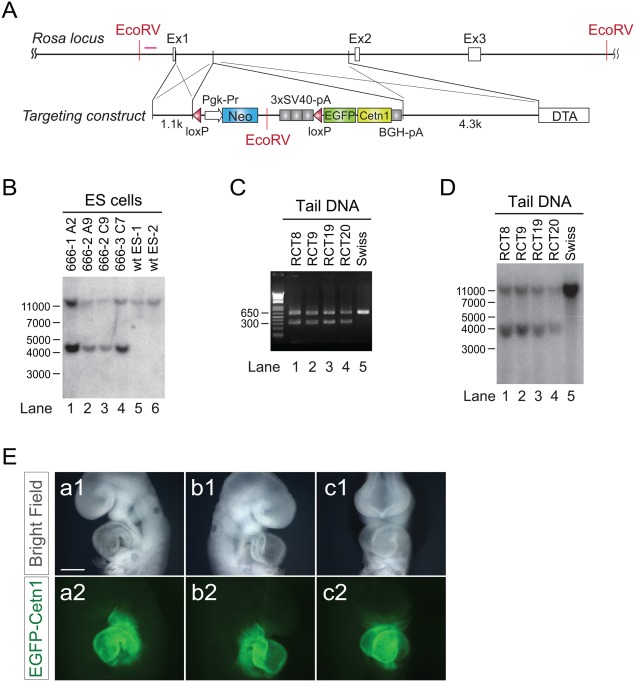
Generation of tissue‐specific centrosome indicator. A: Design of targeting vector for ROSA locus. Human Centrin1 cDNA fused with EGFP coding sequence at N‐terminus was placed after a floxed Neomycin resistant gene and triplicated SV40 poly A cassette. B: Southern blot for ES clones. C: PCR with mouse tail DNA after germ‐line transmission. D: Southern blot with mouse tail DNA, indicating successful germ‐line transmission. E: ED9.5 embryo of ROSA‐Neo‐EGFP‐Cetn1 mouse line after crossing with Nkx2.5‐Cre, indicating lineage specific expression of EGFP‐Centrin1. (a1, b1, c1): bright field, (a2, b2, c2): EGFP‐Centrin1. Scale bar: 1 mm.
EGFP‐Centrin1 Expression Mirrors Endogenous Duplication Cycle of Centrioles During Cell Cycle In Vivo
To examine how EGFP‐Centrin1 was regulated during cell cycle, a mouse line able to express EGFP‐Centrin1 in all cell types under control of the ROSA locus was generated utilizing Protamine Cre to ablate the Neo‐polyA cassette in ROSA‐Neo‐EGFP‐Cetn1 mice (O'Gorman et al., 1997). ROSA‐EGFP‐Cetn1 mice were maintained for more than 22 generations, bred and grew well, with no overt phenotypes. Mouse embryonic fibroblasts (MEFs) were harvested from ROSA‐EGFP‐Cetn1 embryos. MEFs were synchronized in G0 by serum starvation, then induced to re‐enter cell cycle by addition of 10% fetal bovine serum (FBS) (Fig. 2A). Cultures were fixed with 4% paraformaldehyde (PFA) every six hours, then immunostained with antibodies to α‐Tubulin and the G2/M phase specific marker, Serine 10 phospho‐Histone H3 (pH3). EGFP‐Centrin1 was well visualized without antibody staining.
Figure 2.
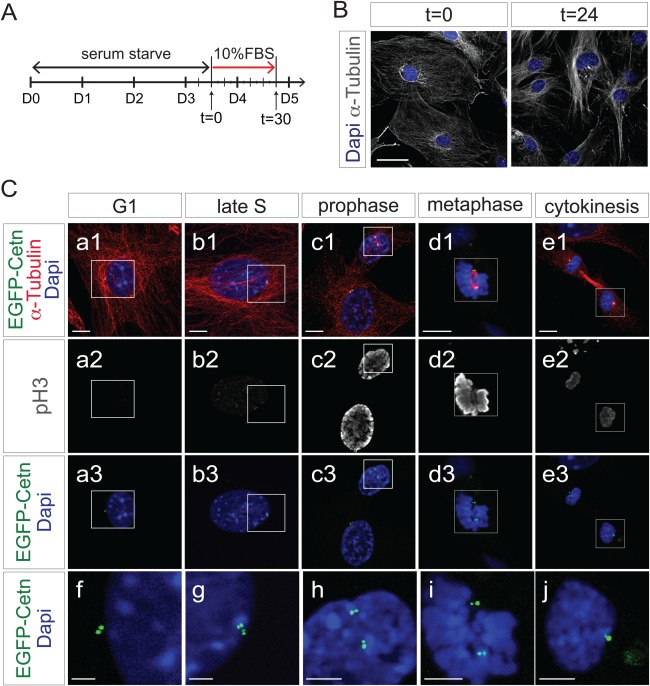
EGFP‐Centrin1 mirrors endogenous centrosome duplication cycle. A: Diagram of time‐course experiment shown in B and C. B: Fluorescence microscope images of ROSA‐EGFP‐Cetn1 MEFs in quiescence after serum starvation (t = 0), and in proliferation 24 h after serum induction (t = 24). Cells were immunostained with antibody to α‐Tubulin. Scale bar: 50 µm. C: Time‐course experiment following synchronization of ROSA‐EGFP‐Cetn1 MEFs. Cells were immunostained with antibodies to α‐Tubulin and Serine 10 phospho‐Histone H3 (pH3). (f, g, h, i, j) Magnified pictures of square area in of (a, b, c, d, e). Note centrosomes are duplicated by the end of S phase, and each centrosome is allocated into each dividing cell. Scale bars: 10 µm (a, b, c, d, e), 5µm (f, g, h, i, j).
MEFs exhibited two EGFP‐Centrin1 signals in close proximity, representing mother and daughter centrioles (Fig. 2C,f). Once procentioles are formed from each centriole in S phase, each procentriole is elongated throughout S and G2 phase, resulting in structures containing four centrioles (Nigg and Stearns, 2011). Consistent with this centriole duplication cycle, four EGFP‐Centrin1 signals were seen in proximity at late S/G2 phase (Fig. 2C,g). At prophase, two pairs of EGFP‐Centrin1 signals were segregated to opposite poles to configure the spindle poles (Fig. 2C,h,i). After cytokinesis, each pair of centrioles, as represented by EGFP‐Centrin1 was partitioned to each of the daughter cells.
EGFP‐Centrin1 Colocalizes with γ‐Tubulin and Golgi Apparatus In Vivo
As cells become spherical in mitosis, it is difficult to capture two segregated EGFP‐Centrin1 centrosomal signals with a single z‐plane (Fig. 3A). For proper visualization of both EGFP‐Centrin1 centrosomal signals, captured z‐stack images need to be 3D reconstructed (Fig. 3C, a1‐a4), sometimes additionally needing to be tilted when the EGFP‐Centrin1 signal is obscured by DAPI staining (Fig. 3C,b). Time‐lapse imaging of ROSA‐EGFP‐Cetn1 MEFs, demonstrated that EGFP‐Centrin1 signals predict the axis of cell division (Fig. 3B). Occasionally, ROSA‐EGFP‐Cetn1 MEFs evidenced aberrant cell division, such as chromosomes segregating into three distinct directions led by three centrosomes (Fig. 3D, b1‐b4), or aberrant bi‐nucleation led by multiple centrosomes (Fig. 3D, c1‐c4).
Figure 3.
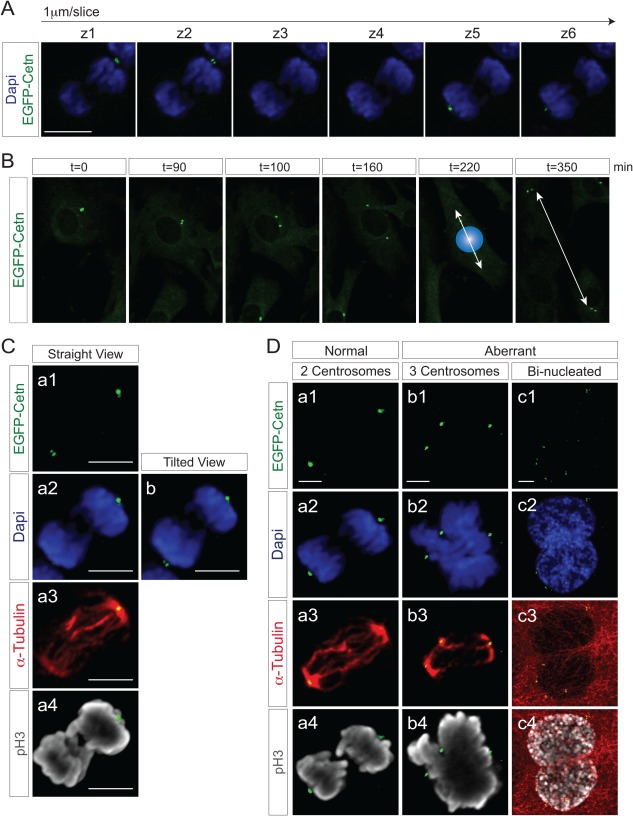
Z‐stack/3D reconstitution and live imaging with ROSA‐EGFP‐Cetn1 MEFs. A: Z‐stack images of a ROSA‐EGFP‐Cetn1 MEF undergoing mitosis. Z‐stack images of were captured at the interval of 1 µm. Note EGFP‐Centrin1 at each spindle pole can be seen at distinct plane. Scale bar: 10 µm. B: Time‐lapse imaging with ROSA‐EGFP‐Cetn1 MEFs. Blue sphere represents nucleus right before cell division. C: Fluorescence microscope images of a mitotic ROSA‐EGFP‐Cetn1 MEF. Cells were immunostained with antibodies to α‐Tubulin and Serine 10 phospho‐Histone H3 (pH3). (a1‐a4): A 3D reconstructed image from straight view. Note one of EGFP‐Centrin1 signals at spindle poles was obscured (a2 and a4), but both EGFP‐Centrin1 signals can be clearly seen with tilted view (b). Scale bars: 5 µm. D: Normal cell division and aberrant cell division. (a) The number of centrosomes is strictly regulated in normal cell division. (b, c) Aberrant cell division is accompanied by extra centrosomes. Scale bars: 5 µm.
The PCM surrounding the centriole contains γ‐Tubulin ring complexes (γTuRCs), which nucleate microtubules, acting as microtubule organizing centers (MTOCs) (Moritz et al., 1995; Zheng et al., 1995). To examine colocalization of EGFP‐Centrin1 with γ‐Tubulin, ROSA‐EGFP‐Cetn1 MEFs were immunostained with antibodies to γ‐Tubulin and α‐Tubulin. EGFP‐Centrin1 co‐localized with γ‐Tubulin throughout M phase (Fig. 4A).
Figure 4.
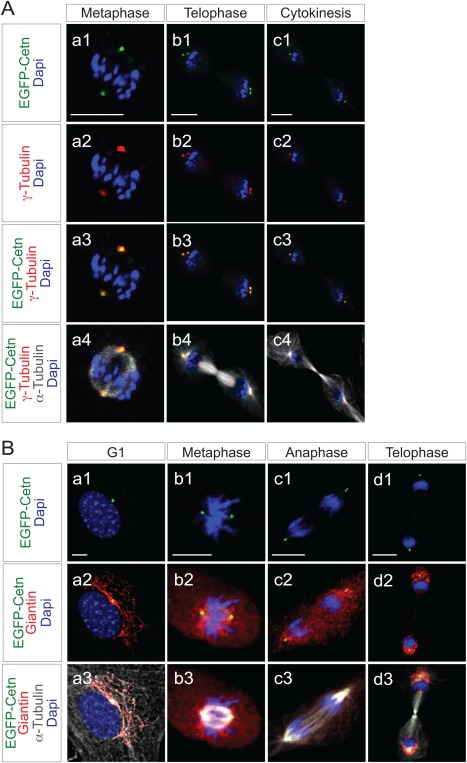
Correlation of EGFP‐Centrin1 with γ‐Tubulin or Gogi apparatus in ROSA‐EGFP‐Cetn1‐MEFs. A: Fluorescence microscope images of ROSA‐EGFP‐Cetn1 MEFs in metaphase (a1‐4), telophase (b1‐4), or cytokinesis (c1‐4). Cells were immunostained with antibodies to γ‐Tubulin and α‐Tubulin. Note EGFP‐Centrin1 co‐localizes with γ‐Tubulin. Scale bars: 10 µm. B: Fluorescence microscope images of ROSA‐EGFP‐Cetn1 MEFs in G1 phase (a1‐3), metaphase (b1‐3), anaphase (c1‐2), or cytokinesis (d1‐3). Cells were immunostained with antibodies to Giantin and α‐Tubulin. Note Golgi apparatus localizes adjacent to EGFP‐Centrin1 although Golgi apparatus dispersed throughout the cytosol in metaphase and anaphase (b2 and c2). Scale bars: 10 µm.
Centrosomes are adjacent to Golgi in interphase (Sutterlin and Colanzi, 2010), while Golgi membranes are fragmented and dispersed throughout the cytoplasm during mitosis (Sutterlin and Colanzi, 2010). To examine the correlation between EGFP‐Centrin1 and the Golgi through the cell cycle, ROSA‐EGFP‐Cetn1 MEFs were immunostained with antibodies to Giantin and α‐Tubulin. EGFP‐Centrin1 colocalized with Giantin in G0, as expected (Fig. 4B, a1‐a3). In metaphase and anaphase, the Golgi apparatus was dispersed throughout the cytosol, however, Golgin was more concentrated around EGFP‐Centrin1 (Fig. 4B, b1‐b3, c1‐c3). In telophase, the Golgi apparatus had reformed in each daughter cell, and again co‐localized with EGFP‐Centrin1 (Fig. 4B, d1‐d3).
EGFP‐Centrin1 Expression Mirrors Endogenous Centrosomes in Mouse Embryos
Centrosomes play a key role in mitotic spindle formation and cell division in early mouse embryos, however, there are no centrioles until the 16‐cell stage (Calarco‐Gillam et al., 1983). Consistent with this, EGFP‐Centrin1 signal was not observed in 2‐ to 4‐cell stage embryos (Fig. 5A1 and A2), while EGFP‐Centrin1 signals were observed in early blastocysts at ED3.5 (Fig. 5B1‐B3).
Figure 5.
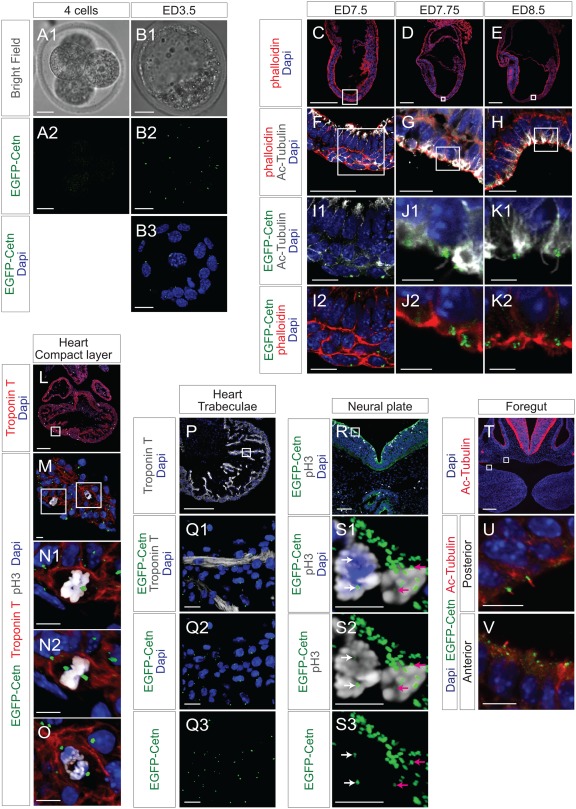
EGFP‐Centrin1 in developing mouse embryo. A, B: Bright field and fluorescence microscope images of ED2.5 and ED3.5 ROSA‐EGFP‐Cetn1 mouse embryos. Note there is no significant EGFP‐Centrin1 signal on ED2.5. Scale bars: 20 µm. C, D, E, F, G, H, I, J, and K. Fluorescence microscope images of ROSA‐EGFP‐Cetn1 mouse embryo sections of ED7.5, ED7.75, and ED8.5. Embryo sections were immunostained with antibody to acetylated‐Tubulin, and stained with Phalloidin and DAPI. (I, J, K): Magnified images of square regions of (F, G, H). Note cilia formation at node (J1 and K1) and EGFP‐Centrin1 at the base of cilia (J2 and K2). Scale bars: 200µm (C, D, E), 20µm (F, G, H), 5µm (I, J, K). L, M, N, O. ED10.5 ROSA‐EGFP‐Cetn1 embryo heart sections were immunostained with antibodies to Troponin T and pH3. (M): A magnified picture of the square area of (L). (N and O): Magnified pictures of square area of (M). (N1 and O): Straight view. (N1): Note one of EGFP‐Centrin1 signals is obscured by DAPI staining. (N2): Tilted view of (N1). Note both EGFP‐Centrin1 signals can be seen. Scale bars: 200µm (L), 10µm (M, N, O). P. ED10.5 ROSA‐EGFP‐Cetn1 embryo heart sections were immunostained with antibody to Troponin T. Scale bars: 200µm (P), 20µm (Q). R and S. ED10.5 ROSA‐EGFP‐Cetn1 embryo tissue sections were immunostained with antibody to pH3. (S1‐3): Magnified pictures of square area of (R). White and pink arrows indicate a pair of centrosomes at spindle poles in each dividing cell. Scale bars: 200µm (R), 10µm (S1‐3). T, U, V. ED10.5 ROSA‐EGFP‐Cetn1 embryo tissue sections were immunostained with an antibody to acetylated‐Tubulin. Scale bars: 10µm. (U and V): Magnified pictures of square regions of (T). Scale bars: 200 µm (T), 10 µm (U, V).
Distal appendages of mother centrioles function as basal bodies for primary cilia (Nigg and Stearns, 2011). To examine cilia, mouse tissue sections were immunostained with antibody to acetylated‐Tubulin, and stained with Phalloidin and DAPI. With acetylated‐Tubulin, cilia were at the node of mouse embryos at ED7.75 or ED8.5 (Fig. 5J1 and K1). As expected, EGFP‐Centrin1 was found at the base of each cilium (Fig. 5J2 and K2).
To examine EGFP‐Centrin1 in dividing cells, ED10.5 ROSA‐EGFP‐Cetn1 heart sections were immunostained with antibodies to Troponin T and phospho‐Histone H3 (pH3). EGFP‐Centrin1 was seen at spindle poles of dividing cardiomyocytes in the compact layer (Fig. 5N1 and O). As EGFP‐Centrin1 signals were not on a single Z plane, 3D reconstructed images were tilted to observe EGFP‐Centrin1 at both spindle poles simultaneously (Fig. 5N2), demonstrating that EGFP‐Centrin1 indicated the orientation of cell division. In trabeculae, EGFP‐Centrin1 localized to the inner side of trabeculae, opposite to endocardium, demonstrating polarization of trabecular myocytes (Fig. 5Q1‐Q3).
In neural plate at ED10.5, multiple EGFP‐Centrin1 signals were observed at the side of the neural groove, suggesting the presence of multiple cilia within these neuroepithelial cells (Fig. 5S1‐S3). However, even in the presence of multiple EGFP‐Centrin1 signals within a cell, only a pair of centrosomes expressing EGFP‐Centrin1 in each cell were observed associated with dividing chromosomes, playing a role as MTOCs of the spindle poles. (Fig 5S1‐S3, see a pair of white arrows and pink arrows)
To examine cilia in foregut endoderm, sections of ED10.5 Rosa‐EGFP‐Cetn1 embryos were immunostained with antibody to acetylated‐Tubulin. As shown in Fig. 5U and 5V, EGFP‐Centrin1 was at the base of cilia labeled by acetylated‐Tubulin.
EGFP‐Centrin1 Expression Is Evident in Each Organ of Adult Mouse
Tissue sections of intestine and skin of two month old ROSA‐EGFP‐Cetn1;ROSA‐mTmG mice without Cre were obtained. ROSA‐mTmG mice (Muzumdar et al., 2007) were utilized to delineate outlines of each cell. As expected, signals from Rosa‐mTomato were observed on membranes of all cells. However, fluorescent intensity was markedly variable depending on cell type, making it sometimes challenging to delineate outlines of each cell unequivocally. Therefore, to observe the outlines of each cell, tissue sections were additionally immunostained with antibody to E‐cadherin. As shown in Figure 6A–C, fluorescent EGFP‐Centrin1 was mainly localized at the luminal side.
Figure 6.
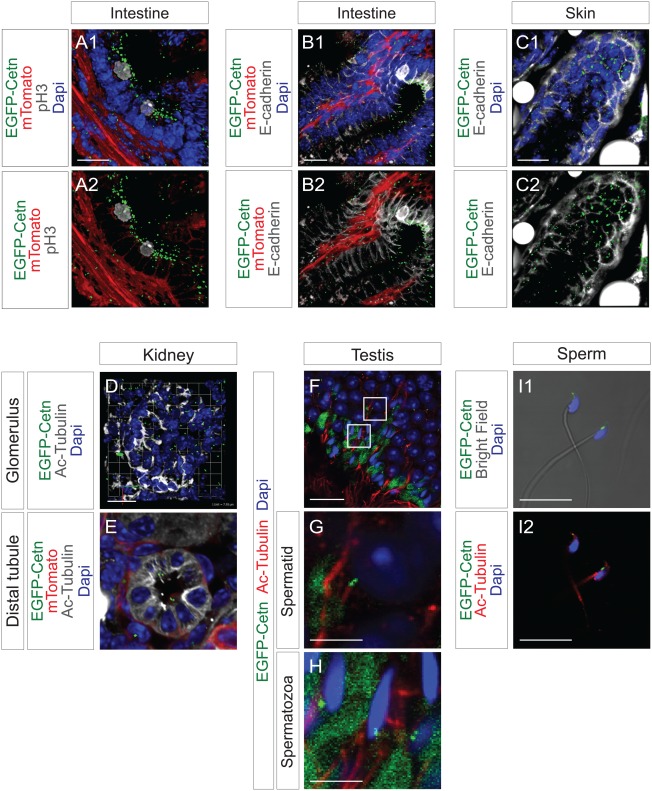
EGFP‐Centrin1 in adult mouse. A, B: Intestine tissue sections of ROSA‐EGFP‐Cetn1;Rosa‐mTmG were immunostained with the antibody to pH3 or E‐cadherin. C: Skin tissue sections of ROSA‐EGFP‐Cetn1;Rosa‐mTmG were immunostained with antibody to E‐cadherin. D, E, F, G, H: Kidney (D, E) and testis (F, G, H) tissue sections of ROSA‐EGFP‐Cetn1 were immunostained with antibody to acetylated‐Tubulin. (G, H): Magnified pictures of square areas of (F). H: Extensive green signal was observed in extracellular matrices, presumably caused by auto‐fluorescence. I: Isolated sperm of ROSA‐EGFP‐Cetn1 was immunostained with antibody to acetylated Tubulin. Note EGFP‐Centrin1 signal is located at the base of flagella. Scale bars: 20 µm (A, B, C, D, F), 5 µm (G, H, I).
To examine cilia in adult mouse, tissue sections of kidney, testis, and sperm were immunostained with antibody to acetylated‐Tubulin. In kidney, cilia were in the distal tubule, not in the glomerulus, and EGFP‐Centrin1 was at the base of cilia (Fig. 6D,E). In testis, flagella labeled by acetylated‐Tubulin were both in spermatids and spermatozoa (Fig. 6G,H), and EGFP‐Centrin1 was at the base of flagella. In isolated mature sperm, EGFP‐Centrin1 was at the base of flagella (Fig. 6I1, I2).
From these results, EGFP‐Centrin1 was specifically expressed in each centriole, gave high resolution, and closely mirrored behavior of endogenous centrosomes. Additionally, lineage specific visualization of centrosomes can facilitate understanding centrosome biology in the complex context of mouse tissues.
METHODS
Mice
All animals were maintained and experiments performed in accordance with institutional guidelines at University of California, San Diego. Protamine‐Cre (O'Gorman et al., 1997), Nkx2.5‐Cre Troponin T‐Cre (Jiao et al., 2003), and R26‐mTmG (Muzumdar et al., 2007) were purchased from Jackson Laboratories. ROSA‐Neo‐EGFP‐Cetn1 line have been bred into a Black Swiss outbred background for more than 6 generations, did not show any overt difference from wild‐type mouse. ROSA‐EGFP‐Cetn1 line was generated by crossing ROSA‐Neo‐EGFP‐Cetn1 into Protamine‐Cre. Genotyping for transgene was performed by PCR, using following primers, forward primer (P1): 5′‐ AAAGTCGCTCTGAGTTGTTAT‐3′, reverse primer (P2) 5′‐ GCGAAGAGTTTGTCCTCAACC‐3′, P3 reverse primer (P3): 5′‐ GGAGCGGGAGAAATGGATATG ‐3′. P1 and P2 give rise to 313bp amplicon for knock‐in allele, while P1 and P3 give rise to 626 bp amplicon for wild‐type allele. The conditional Cre‐dependent and the recombined Rosa‐EGFP‐Centrin1 lines will be available to the research community upon acceptance of the manuscript.
GENERATION OF ROSA‐NEO‐EGFP‐CETN1 MICE
cDNA coding human Centrin1 fused with EGFP at N‐terminus in‐frame was kindly provided by Dr. Fuchs E (The Rockfeller Univeristy). EGFP‐Centrin1 cDNA was amplified with PCR, cloned into pBigT containing a loxP‐flanked cassette with a PGK‐neo resistant gene with a triplicated polyA, then cloned into pROSA26PA (Srinivas et al., 2001). pBigT and pROSA26PA were kindly provided by Dr. Costantini F (Columbia University). After linearization with SacII, targeting vector was applied to pronuclear injection.
Southern Blotting
Genomic DNA was purified from tail tip biopsies, followed by digestion with EcoRV. Digested genomic DNA was separated by agarose gel electrophoresis. The probe was amplified by PCR using following primers, forward primer: 5′‐ AAGGTAATGTCTTTGGTGTGG GAA‐3′, reverse primer: 5′‐ CTTTTCGTCTTCTCAGCTA CCTTTAC‐3′. The probe gives rise to a 4.1kb band for knock‐in allele and a 11.5kb band for wild‐type allele.
Cell Culture and Cell Synchronization
Mouse embryonic fibroblasts (MEFs) were isolated by trypsinization of skin tissues of ED14.5 mouse embryos. MEFs were maintained in DMEM (Thermo Fisher Scientific), 10% FBS at 37˚C in 5% CO2. MEFs were synchronized in G0 phase by culturing in DMEM, 0.1% FBS at 37˚C in 5% CO2 for 72 hours.
IMMUNOFLUORESCENCE
Cells were fixed in 4% paraformaldehyde (PFA) for 10 min at room temperature, or in methanol (MtOH) for 20 min at −20˚C. Embryos were fixed in 4% PFA, and embedded in Tissue‐Tek OCT after sucrose gradient treatment. Primary antibodies used for immunohistochemistry were anti‐α−Tubulin mouse monoclonal (DM1A, Abcam, 1 : 200), anti‐γ‐Tubulin rabbit polyclonal (T5192, Sigma‐Aldrich, 1 : 100), anti‐Acetylated α‐Tubulin mouse monoclonal (6‐11B‐1, Abcam, 1 : 100), anti‐Giantin rabbit polyclonal (ab24586, Abcam), anti‐Integrin‐β1 rat monoclonal (MAB1997, Millipore, 1 : 200), anti‐E‐cadherin rat monoclonal (DECMA‐1, Sigma, 1 : 200), Serine 10 phospho‐Histone H3 rabbit polyclonal (06‐570, Millipore, 1:200), anti‐TroponinT mouse monoclonal (13‐11, Thermo Fisher Scientific, 1 : 200), or anti‐Numb rabbit monoclonal (C29G11, Cell Signaling, 1 : 50). Secondary antibodies used were Alexa 555, or 647 anti‐rabbit, mouse, or rat IgG (Life Technologies), followed by nuclear staining with DAPI. Stained sections were mounted with Dako fluorescence mounting medium, and visualized using an Olympus confocal microscope (FV1000). Z‐stack images were reconstructed into 3D with Volocity software.
ACKNOWLEDGMENT
The authors thank E Fuchs (Rockfeller University) for providing EGFP‐Centrin1 cDNA, F Costantini (Columbia University) for pBigT and pROSA‐PA.
LITERATURE CITED
- Bornens M. 2012. The centrosome in cells and organisms. Science 335:422–426. [DOI] [PubMed] [Google Scholar]
- Calarco‐Gillam PD, Siebert MC, Hubble R, Mitchison T, Kirschner M. 1983. Centrosome development in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell 35:621–629. [DOI] [PubMed] [Google Scholar]
- Higginbotham H, Bielas S, Tanaka T, Gleeson JG. 2004. Transgenic mouse line with green‐fluorescent protein‐labeled Centrin 2 allows visualization of the centrosome in living cells. Transgenic Res 13:155–164. [DOI] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. 2003. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev 17:2362–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. 2005. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. 1995. Microtubule nucleation by gamma‐tubulin‐containing rings in the centrosome. Nature 378:638–640. [DOI] [PubMed] [Google Scholar]
- Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. 2001. Embryonic expression of an Nkx2‐5/Cre gene using ROSA26 reporter mice. Genesis 31:176–180. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. 2007. A global double‐fluorescent Cre reporter mouse. Genesis 45:593–605. [DOI] [PubMed] [Google Scholar]
- Nigg EA, Stearns T. 2011. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 13:1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman S, Dagenais NA, Qian M, Marchuk Y. 1997. Protamine‐Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci USA 94:14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti A, Moudjou M, Paintrand M, Salisbury JL, Bornens M. 1996. Most of centrin in animal cells is not centrosome‐associated and centrosomal centrin is confined to the distal lumen of centrioles. J Cell Sci 109:3089–3102. [DOI] [PubMed] [Google Scholar]
- Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. 2000. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J Cell Biol 149:317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszko I, Afonso C, Henrique D, Mathis L. 2006. Key role played by RhoA in the balance between planar and apico‐basal cell divisions in the chick neuroepithelium. Dev Biol 298:212–224. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterlin C, Colanzi A. 2010. The Golgi and the centrosome: Building a functional partnership. J Cell Biol 188:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szollosi D, Calarco P, Donahue RP. 1972. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci 11:521–541. [DOI] [PubMed] [Google Scholar]
- White RA, Pan Z, Salisbury JL. 2000. GFP‐centrin as a marker for centriole dynamics in living cells. Microsc Res Tech 49:451–457. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchison T. 1995. Nucleation of microtubule assembly by a gamma‐tubulin‐containing ring complex. Nature 378:578–583. [DOI] [PubMed] [Google Scholar]


